Introduction
Mammary tumors in dogs occur at a frequency of 3
times the incidence of mammary tumors in humans and have recorded a
higher incidence rate than other livestock (1). Mammary tumors are the most frequent
cutaneous neoplasms in female dogs, and approximately 50% are
diagnosed as malignant tumors (2).
Following surgical excision, approximately 48% of the affected dogs
die or are euthanized within 1 year due to tumor recurrence or
metastasis (3). Canine mammary
tumors are heterogeneous, and the different clinical and biological
features (4) make it difficult to
determine a prognosis and treatment for affected dogs, as is often
the case in humans. Additional and reliable tools for effective
therapy are required in veterinary medicine. Furthermore, a recent
study revealed clear evidence of a similarity between human and dog
tumors in regard to the deregulation of several cancer-related
genes, including PI3K/Akt, PTEN and Wnt-β catenin and
MAPK signaling (5). Accordingly,
canine mammary tumors can also provide a suitable natural model for
the comparative study of human breast cancer (5–7).
Hyaluronan (HA) is a non-sulfated linear
glycosaminoglycan that consists of repeating disaccharide subunits
of glucuronic acid and N-acetylglucosamine (8,9). It is
ubiquitously distributed in the extra- and pericellular spaces of
most animal tissues (8,9). HA plays a critical role in regulating
matrix assembly, cell migration, differentiation and proliferation
(9–11). Thus, production of HA increases
during active tissue remodeling, e.g., during embryonic development
and wound healing (12). In cancer,
previous studies have shown that high stromal HA levels correlate
with tumor aggressiveness and low survival rates in patients with
breast, ovarian and prostate cancer (13–15).
Similarly, in vitro studies have demonstrated that the
inhibition of HA synthesis correlates with the downregulation of
proliferative, invasive and metastatic potential in cancer cells
(16–18).
In vertebrates, HA is synthesized at the plasma
membrane by 3 HA synthases, HAS1, 2 and 3, which couple glucuronic
acid (GlcUA) and N-acetylglucosamine (GlcNAc) into a linear polymer
using the corresponding UDP-sugars (UDP-GlcUA and UDP-GlcNAc) as
substrates. HA is secreted as a free glycosaminoglycan and tethered
at the cell surface via several HA receptors including as CD44 and
RHAMM (receptor for HA-mediated motility) (19,20).
Increasing levels of pericellular HA may help maintain the
malignant phenotype of cancer cells by providing a suitable
environment (21). Interaction of
HA with HA receptors stimulates signaling events such as
anchorage-independent tumor cell growth, survival and migration,
thereby increasing metastatic spread (22). Furthermore, CD44 is overexpressed in
several human types of cancer and previous reports have shown that
an HA-CD44 interaction causes activation of EGFR (epidermal growth
factor receptor)-, PI3 kinase-, MAP kinase- and Rho-mediated
signaling pathways that promote tumor growth, migration and
chemotherapeutic resistance. Expression of CD44 has also been
confirmed in canine mammary and melanocytic tumor tissues by
immunohistochemistry (23,24). Therefore, as in humans, the
inhibition of the HA-CD44 interaction might be regarded as a novel
therapeutic target in mammary tumors in dogs.
4-Methylumbelliferone (4-MU:
7-hydroxy-4-methyl-2H-1-benzopyran-2-one) was previously reported
to specifically inhibit synthesis of HA in cultured human skin
fibroblasts (25). Studies of the
mechanism of action revealed that 4-MU induced inhibition of HA
synthesis involving the glucuronidation of 4-MU by endogenous
UDP-glucuronyltransferases (UGT) resulting in a depletion of
UDP-GlcUA (26). Moreover, it is
also known that 4-MU can act by reducing levels of HAS mRNA
in breast, melanoma and ovarian cancer cells (27). Some studies have shown anticancer
effects of 4-MU through decreased HA synthesis in vitro and
in vivo(28,29). However, to our knowledge, no study
examining the anticancer effect of 4-MU on canine mammary tumors
has been published.
In this study, we hypothesized that inhibition of HA
production by 4-MU would negatively regulate the tumor growth of
canine mammary tumors. To assess our hypothesis, we analyzed the
effects of 4-MU on cell growth and apoptosis in CF33 canine mammary
tumor cells. Moreover, in this study, we also assessed whether or
not dogs are suitable research models for functional analysis of HA
in human breast cancer.
Materials and methods
Materials
4-Methylumbelliferone (4-MU) was purchased from Wako
Pure Chemicals (Osaka, Japan). An anti-BAX antibody (ABC11) was
obtained from Millipore (Bedford, MA, USA). An anti-β-actin
antibody (AC-15) was purchased from Sigma (St. Louis, MO, USA).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
for the MTT assay and hyaluronidase were purchased from Sigma.
Cell culture
Cells from the canine breast cancer cell line CF33
were obtained from the American Type Culture Collection (Manassas,
VA, USA). The cells were grown as monolayers, cultured in DMEM
(Nissui, Tokyo, Japan) supplemented with 10% heat-inactivated fetal
bovine serum (FBS), 4 mM L-glutamine, 10 mg/ml streptomycin and
10,000 =U/ml penicillin G. The cells were maintained at 37°C in a
5% CO2 incubator. The 4-MU stock solution for
experiments was dissolved in DMSO and the final concentration of
DMSO in the medium was adjusted to 0.1%.
Quantification of HA
To determine the effect of 4-MU on CF33 cells, we
quantified HA in conditioned medium using a commercially available
competitive enzyme-linked immunosorbent assay kit (ELISA) (Echelon
Biosciences, Salt Lake City, UT, USA), in which the colorimetric
signal was inversely proportional to the amount of HA present in
the sample.
Particle exclusion assays
To analyze the effect of 4-MU on the areas of the
HA-rich pericellular matrices, we used a particle exclusion assay.
Fixed sheep erythrocytes (Sigma) were reconstituted in
phosphate-buffered saline (PBS) to a density of 5.4×108
cells/ml and used for the particle exclusion assay as previously
described (30). HA matrices were
visualized by adding 6.0×107 erythrocytes to the growth
medium and viewing under an All-in-One Fluorescence Microscope
BZ-9000 (Keyence Corp., Osaka, Japan). The HA-rich pericellular
matrix-to-cell-area ratios were determined by image analysis using
ImageJ. All measurements were made by tracing the digitized images.
Non-treated medium and hyaluronidase-containing medium served as
negative and positive controls, respectively.
MTT assay
The MTT assay, which is widely used to measure cell
proliferation and to screen for anticancer drugs, is based on the
reduction of a tetrazolium salt (31–33).
We used the MTT assay to evaluate the effects of the 4-MU on cell
proliferation. CF33 cells were plated in 96-well plates at
1×103 cells/well. At each time point (days 0–4), 20
μl of MTT reagent was added and the plates were incubated
for 4 h. Subsequently, 100 μl of 10% SDS and 0.01 N HCl were
added to each well. Following overnight incubation, the absorbances
were measured at 570 nm using a Model 550 microplate reader
(Bio-Rad Laboratories, Tokyo, Japan). In these experiments, 5
replicate wells were used for each time point and the results were
calculated as the means ± SD.
Cell cycle and apoptosis analysis
Cells were harvested and washed with PBS,
resuspended in 70% ethanol in distilled water and kept at −30°C
overnight. Prior to analysis, cells were mixed and incubated for 30
min in PBS containing 0.05 mg/ml propidium iodide (PI) and 100 U/ml
RNase A. The suspension was then passed through a nylon mesh filter
and analyzed by FACSCalibur (Becton-Dickinson, Franklin Lakes, NJ,
USA). Furthermore, these data were analyzed using FlowJo 7 (Tree
Star, Inc., Ashland, OR, USA).
Real-time RT-PCR
Total-RNA was extracted from cells using the TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) and cDNAs were synthesized
with a PrimeScript™ RT reagent kit (Takara Bio, Inc., Shiga, Japan)
according to the manufacturer’s protocols. Real-time PCR was
performed using SYBR Premix Ex Taq™ (Takara Bio) and an ABI Prism
7500 Real-Time PCR system (Applied Biosystems, Foster City, CA,
USA) under the following conditions: 30 sec at 95°C; 40 cycles of 5
sec at 95°C and 34 sec at 60°C. Specific primer sets for BAX
(forward, 5′-CGCATCGGAGATGAACTGGA-3′; and reverse,
5′-ACCAGTTTGCTGGCAAAGTAGAAG-3′) were purchased from Takara Bio,
Inc. HAS1 (forward, 5′-GGACTACG TGCAGGTGTGTG-3′; reverse,
5′-CTCACCTAGGGGAC CACTGA-3′), HAS2 (forward,
5′-CTTAGAGCACTGGGA-3′; reverse, 5′-TCTAAAACTTTCACCA-3′),
HAS3 (forward, 5′-AAGTAGGGGGAGTTGG-3′; reverse, 5′-CCCAGAGGC
CCACTAA-3′), and GAPDH (forward, 5′-AAGGCTGAGA ACGGGA-3′; reverse,
5′-GGAGGCATTGCTGACA-3′) were obtained from Operon Biotechnologies
(Tokyo, Japan). The specificity of each amplification was confirmed
by a dissociation curve consisting of a single peak. All samples
were amplified in triplicate in each experiment. The values were
normalized by the expression of GAPDH.
Western blotting
Whole cell lysates were prepared with ice-cold RIPA
buffer (50 mM Tris-HCl pH 7.5, 1% nonidet P-40, 150 mM NaCl, 0.1%
SDS, 0.5% deoxycholic acid) containing 1 μg/ml leupeptin, 1
μg/ml pepstatin, 1 μg/ml aprotinin, 1 mM DTT, 1 mM
NaVO4 and 0.5 mM PMSF. The supernatants were saved as
total cell lysates following centrifugation. Aliquots of the cell
lysates (20 μg of protein) were separated by 12% SDS-PAGE
and transferred to PVDF membranes (ATTO, Tokyo, Japan). Primary
antibodies that bound to their antigens on the membranes were
detected using appropriate HRP-conjugated secondary antibodies
(Amersham Biosciences, Piscataway, NJ, USA) and SuperSignal
Chemiluminescence or a WesternBright Sirius western blotting kit
(Advansta, Menlo Park, CA, USA) according to the manufacturer’s
instructions.
Statistical analysis
Statistical comparisons between two groups were made
using an unpaired Student’s t-test. Values of P<0.05 were
considered to indicate a statistically significant difference.
Results
Inhibitory effect of 4-MU on HA in medium
and cell-associated matrix of CF33 cells
4-MU was originally found to inhibit HA synthesis in
cultured human skin fibroblasts (25). To determine the effect of 4-MU on HA
synthesis in the CF33 cells, we quantified the levels of HA in
conditioned medium using the ELISA assay. As shown in Fig. 1, 4-MU was associated with decreased
levels of HA production in a dose-dependent manner at all time
points, whereas there was no change in the HA levels between parent
cells and vehicle (DMSO)-treated controls. It was previously
reported that the cell-associated HA matrix plays a critical role
in the progression of several malignancies (13,34).
We confirmed the influence of 4-MU on HA-matrix formation by
particle exclusion assay. Abundant accumulation of the
cell-associated HA matrix was shown in CF33 cells following
incubation with control medium with or without DMSO (Fig. 2A and C). The HA matrix disappeared
after treatment with Streptomyces hyaluronidase, indicating
that these matrices were comprised of retained HA (Fig. 2B). Cells treated with 4-MU showed a
significant dose-dependent decrease in the area of the
cell-associated HA matrix and ratio of matrix area to cell area at
each time point (Fig. 2D–G).
Furthermore, 4-MU treatment caused a change in cell morphology. As
seen in Fig. 2C–F, within 72 h of
4-MU treatment, CF33 cells tended to become flat in form and there
was an expansion of the cell area compared with control. These
findings suggested that 4-MU inhibited HA synthesis and weakened
the cell-associated HA matrix. The observations that 4-MU
effectively reduced HA production in canine mammary tumor cells
were comparable to those previously reported in human and mouse
cells.
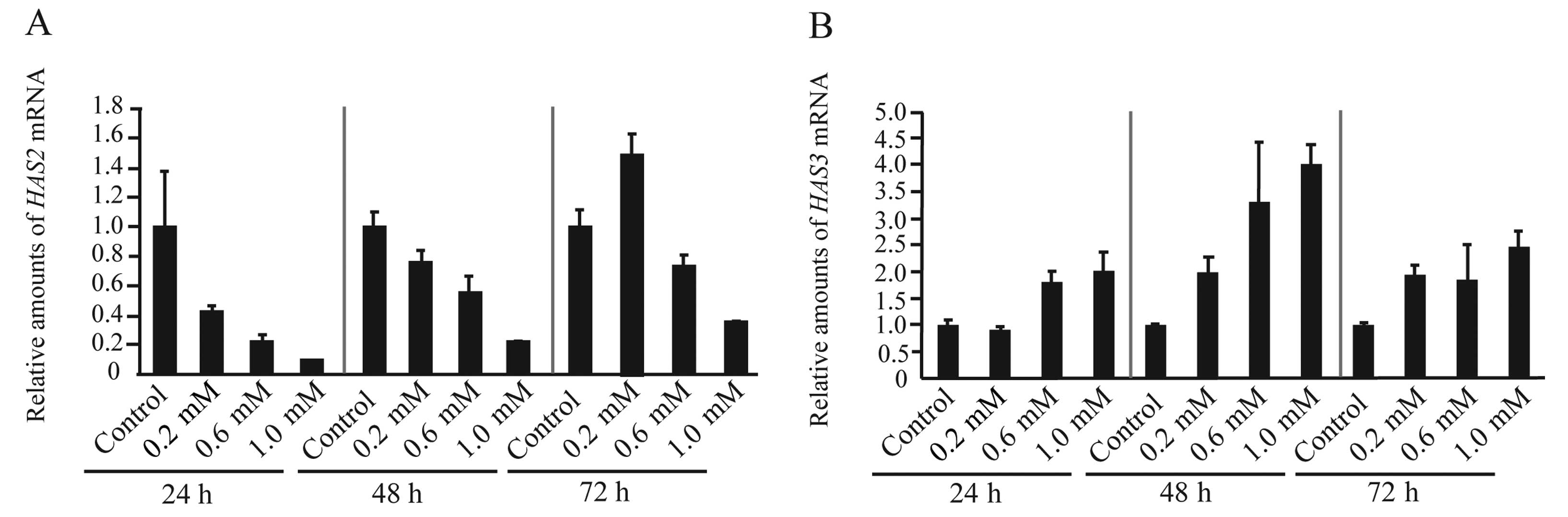
HAS2 mRNA levels are downregulated in
CF33 cells treated with 4-MU, while HAS3 mRNA levels are
upregulated
Three HAS isoforms (HAS1, HAS2, and HAS3) have been
identified in mammalian cells. It has been reported that inhibition
of HA synthesis by treatment with 4-MU reduced mRNA expression by
HAS2, HAS3, or both, in various cell lines, including
melanoma, breast and ovarian cancers (27). To assess the effect of 4-MU on the
expression of the HAS genes in CF33 cells, we investigated
the expression of HAS using real-time RT-PCR. We found that
the expression of HAS1 mRNA was undetectable in the CF33
cells (data not shown), which mainly expressed HAS2 and
HAS3 mRNA. CF33 cells treated with 4-MU showed a tendency to
decrease levels of HAS2 mRNA, while the expression of
HAS3 was increased (Fig. 3A and
B). These data indicated that 4-MU-related inhibition of HA
synthesis was mainly associated with downregulation of HAS2.
The increase in the levels of HAS3 mRNA may signify a
back-up system for HA production and these events are likely to
occur in HA-related genes (17,35).
4-MU markedly inhibits proliferation of
CF33 cells
The rate of cell proliferation is often associated
with that of HA synthesis (35).
Furthermore, it was previously reported that cell proliferation is
inhibited by 4-MU in various cancer cells (27,28).
Cell proliferation was assayed in the CF33 cells by MTT assay upon
addition of 4-MU and at 0, 1, 2, 3 and 4 days later (Fig. 4). The number of cells in control
cultures increased steadily during the days following plating,
while the proliferation rate was dose-dependently suppressed by
0.2, 0.6 and 1.0 mM concentrations of 4-MU (Fig. 4). Notably, by day 4 of the 1.0 mM
4-MU treatment, proliferation was completely blocked (Fig. 4). This result demonstrated that 4-MU
was able to inhibit the growth of canine mammary tumor cells as it
has in the several other cancer cell types mentioned.
4-MU induces G2/M phase cell cycle arrest
and subsequent apoptosis
4-MU caused marked inhibition of the proliferation
of CF33 cells in our experiments, and to determine whether 4-MU is
associated with alteration of cell cycle progression in the CF33
cells, cells were grown to 70% confluence and the cell cycle
distribution was analyzed by flow cytometry after 24-, 48- and 72-h
exposure to 4-MU (0.2, 0.6 and 1.0 mM). Among the cells treated
with 1.0 mM 4-MU at each time point, the percentage of cells in the
S phase was lower than the corresponding value for the control
cells, and the percentage of cells in the G2/M phases in cultures
treated with 1.0 mM 4-MU was significantly higher than in the
corresponding control cells (Fig.
5A–C). Following G2/M arrest, several cancer cell lines,
notably certain breast cancer cell lines, exhibit morphologic
changes consistent with apoptosis, i.e., plasma membrane blebbing,
the appearance of a rounded morphology and eventual detachment from
the surface of the tissue culture dish where the epithelial cancer
cell lines normally grow as an adherent monolayer (36). The percentage of apoptotic cells in
our specimens was quantified with PI staining and flow cytometric
analysis, with the sub-G0/G1 peak representing apoptotic cells. As
shown in Fig. 5D, apoptosis was
induced more frequently in cells treated with 4-MU compared to
control cells at all time points examined. The percentages of cells
treated with 1 mM 4-MU in the sub-G0/G1 phase were 4.04, 9.44, and
12.27% after 24, 48 and 72 h of incubation, respectively (Fig. 5D). In particular, samples with cells
treated with 1 mM 4MU showed percentages of apoptotic cells that
were approximately 24 times higher than control samples (Fig. 5D). Next, to clarify the effect of
4-MU on apoptosis-related proteins, we measured BAX mRNA and
protein expression levels using real-time RT-PCR and western
blotting. The 4-MU-treated cells demonstrated higher levels of
expression of BAX mRNA than control cells at all-time points
examined (Fig. 6A). In addition, we
observed that 4-MU (0.6 mM and 1.0 mM) caused dose-dependent
increases in BAX protein levels at 48 and 72 h (Fig. 6C and D). These results indicated
that 4-MU decreased cell survival mainly through an extrinsic
apoptosis pathway. It is conceivable that the 4-MU-treated cells
showed no change in the levels of BAX protein at 24 h (Fig. 6B) as a reflection of a lapse in time
from mRNA expression to protein synthesis.
Discussion
In recent studies, 4-MU has shown anticancer effects
involving apoptotic, anti-invasive and anti-angiogenic pathways
in vitro and in vivo(28,29,36,37).
We hypothesized that treatment of canine CF33 mammary tumor cells
with 4-MU might decrease the HA matrix, leading to the inhibition
of tumor cell growth and the induction of apoptosis. Our
experiments revealed that 4-MU-associated inhibition of HA
synthesis was accompanied by a reduction of HAS2 mRNA
levels, as well as marked growth retardation and apoptosis
associated with increases in BAX mRNA and protein
expression. Our findings suggest that 4-MU has potent anticancer
effects in breast cancer cells in dogs.
Previous studies have reported that HA plays a
critical role in the proliferation, progression, angiogenesis,
invasion and metastasis of several types of cancer. HA is a
ubiquitous extracellular matrix (ECM) component of the tumor
environment, particularly in the stroma, where its accumulation is
observed in various types of cancer, and it has been known to be
linked to tumor progression and poor survival of patients with
cancer (9,13–15).
Increased concentrations of HA indicate that its metabolism is
altered in cancer cells, and the imbalance of HA synthesis and/or
degradation may play an important role in tumor progression
(33). HAS2 mainly produces high
molecular weight HA (200–2000 kDa) and it is involved in a variety
of both pathological and physiological activities, including
proliferation, differentiation, inflammation, epithelial to
mesenchymal transition (EMT) and cancer progression (38–40).
Furthermore, it was recently reported that HAS2 plays an important
prometastatic role by generating a favorable microenvironment of
cancer stem-like cells (41). In
human breast cancer cells, HAS2 is increasingly recognized as a key
molecule for maintenance of the malignant phenotype (17,35).
In this study, we showed that 4-MU treatment in CF33 cells resulted
in inhibition of cell growth associated with downregulation of
HAS2 mRNA expression. Our data are consistent with a recent
report showing that HAS2 is related to inhibition of cell
proliferation, migration and metastasis in human breast cancer
cells (35). Therefore, 4-MU may
also serve as a potential therapeutic agent for breast cancer in
dogs. Moreover, our findings suggest that the canine mammary tumor
may be a suitable model for the study of the function of HA in
human breast cancer cells.
Newly synthesized HA molecules are extruded onto the
cell surface for assembly into pericellular or extracellular
matrices, and they can also be retained on the cell surface by
binding to HA receptors (CD44, RHAMM, Toll-like receptor-4)
(9,42). In particular, it is well known that
an HA-CD44 interaction can lead to the activation of intracellular
signaling pathways that affect the proliferation, survival,
migration and invasion of cancer cells (43,44).
Recent studies have shown that the HA-CD44 interaction activates
the PI3K-Akt and Ras-MAPK pathways, leading to cell motility and
cell survival-signaling pathways (45). In prostate cancer cells, 4-MU
induced a dose-dependent decrease in phosphorylated Akt levels,
indicating that Akt signaling is a critical mechanism in the
antitumor activity (28). Our
results suggested that 4-MU may indirectly inhibit activation of
the PI3K-Akt and Ras-MAPK pathways via suppression of HA synthesis
and perturbation of the HA-CD44 interaction.
There are at least 2 different steps in the
inhibitory effect of 4-MU on HA synthesis. First, 4-MU results in
depletion of UDP-GlcUA (26).
Second, expression levels of HAS mRNAs are downregulated by
4-MU, resulting in the inhibition of HA synthesis (27). Kultti et al reported that
levels of HAS2 and/or HAS3 mRNA were downregulated in
all cancer cell lines examined (27), which is not consistent with our
findings, in which levels of HAS3 mRNA increased after
treatment with 4-MU. A possible explanation for the results of our
study is that 4-MU causes positive feedback for HAS3 mRNA
expression to compensate for the inhibition of HA synthesis. These
observations are likely to occur in HA-associated genes (17,35).
Our results further showed that the downregulation of HAS2
after treatment with 4-MU induced marked growth retardation and
apoptosis in CF33 cells. This observation supports the notion that
HAS2 preferentially promotes cancer progression compared
with HAS3 in CF33 cells, since the levels of HAS2,
but not HAS3, expression were correlated with the rate of
induction of growth inhibition and apoptosis.
Our present study is the first to show that 4-MU
might be a potential agent for improved chemotherapy against breast
cancer in dogs. The actions of 4-MU in the CF33 cells were similar
to those shown in humans, suggesting that dogs may be suitable as
an animal models for analyzing the role of HA in human breast
cancer.
Acknowledgements
The authors thank T. Oyama, M. Higashi and K. Azuma
for their critical discussions. This study was supported in part by
a grant-in-aid from the Life Science Research Center Nihon
University (To T.S.), a grant-in-aid from Nihon University (To
T.S.) and funds from the Laboratory of Veterinary Pharmacology,
Nihon University College of Bioresource Sciences.
References
|
1
|
Priester WA and Mantel N: Occurrence of
tumors in domestic animals. Data from 12 United States and Canadian
colleges of veterinary medicine. J Natl Cancer Inst. 47:1333–1344.
1971.
|
|
2
|
Sorenmo K: Canine mammary gland tumors.
Vet Clin North Am Small Anim Pract. 33:573–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Graham JC and Myers RK: The prognostic
significance of angiogenesis in canine mammary tumors. J Vet Intern
Med. 13:416–418. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto A, Pena L, Perez-Alenza MD, Sanchez
MA, Flores JM and Castano M: Immunohistologic detection of estrogen
receptor alpha in canine mammary tumors: clinical and pathologic
associations and prognostic significance. Vet Pathol. 37:239–247.
2000. View Article : Google Scholar
|
|
5
|
Uva P, Aurisicchio L, Watters J, Loboda A,
Kulkarni A, Castle J, Palombo F, Viti V, Mesiti G, Zappulli V,
Marconato L, Abramo F, Ciliberto G, Lahm A, La Monica N and de
Rinaldis E: Comparative expression pathways analysis of human and
canine mammary tumors. BMC Genomics. 10:1352009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Standberg JD and Goodman DG: Animal model
of human disease: canine mammary neoplasia. Am J Pathol.
75:225–228. 1974.PubMed/NCBI
|
|
7
|
Pinho SS, Carvalho S, Cabral J, Reis CA
and Gartner F: Canine tumors: a spontaneous animal model of human
carcinogenesis. Transl Res. 159:165–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weismann B, Rapport MM, Linker A and Meyer
K: Isolation of the aldobionic acid of umbilical cord hyaluronic
acid. J Biol Chem. 205:205–211. 1953.PubMed/NCBI
|
|
9
|
Toole BP: Hyaluronan: from extracellular
glue to pericellular cue. Nat Rev Cancer. 4:528–539. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turley EA, Noble PW and Bourguignon LY:
Signaling properties of hyaluronan receptors. J Biol Chem.
277:4589–4592. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fraser JR, Laurent TC and Laurent UB:
Hyaluronan: its nature, distribution, functions and turnover. J
Intern Med. 242:27–33. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Menzel E and Farr C: Hyaluronidase and its
substrate hyaluronan: biochemistry, biological activities and
therapeutic uses. Cancer Lett. 131:3–11. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Auvinen P, Tammi RH, Parkinen J, Tammi M,
Agren U, Johansson R, Hirvikoski P, Eskelinen M and Kosma VM:
Hyaluronan in peritumoral stroma and malignant cells associates
with breast cancer spreading and predicts survival. Am J Pathol.
156:529–536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anttila MA, Tammi RH, Tammi MI, Syrjanen
KJ, Saarikoski SV and Kosma VM: High levels of stromal hyaluronan
predict poor disease outcome in epithelial ovarian cancer. Cancer
Res. 60:150–155. 2000.PubMed/NCBI
|
|
15
|
Lipponen P, Aaltomaa S, Tammi R, Tammi M,
Agren U and Kosma VM: High stromal hyaluronan level is associated
with poor differentiation and metastasis in prostate cancer. Eur J
Cancer. 37:849–856. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Golshani R, Lopez L, Estrella V, Kramer M,
Iida N and Lokeshwar VB: Hyaluronic acid synthase-1 expression
regulates bladder cancer growth, invasion, and angiogenesis through
CD44. Cancer Res. 68:483–491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Udabage L, Brownlee GR, Waltham M, Blick
T, Walker EC, Heldin P, Nilsson SK, Thompson EW and Brown TJ:
Antisense-mediated suppression of hyaluronan synthase-2 inhibits
the tumorigenesis and progression of breast cancer. Cancer Res.
65:6139–6150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu N, Gao F, Han Z, Xu X, Underhill CB
and Zhang L: Hyaluronan synthase 3 overexpression promotes the
growth of TSU prostate cancer cells. Cancer Res. 61:5207–5214.
2001.PubMed/NCBI
|
|
19
|
Cichy J and Pure E: The liberation of
CD44. J Cell Biol. 161:839–843. 2003. View Article : Google Scholar
|
|
20
|
Cheung WF, Cruz TF and Turley EA: Receptor
for hyaluronan-mediated motility (RHAMM), a hyaladherin that
regulates cell responses to growth factors. Biochem Soc Trans.
27:135–142. 1999.PubMed/NCBI
|
|
21
|
Itano N, Zhuo L and Kimata K: Impact of
the hyaluronan-rich tumor microenvironment on cancer initiation and
progression. Cancer Sci. 99:1720–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gotte M and Yip GW: Heparanase,
Hyaluronan, and CD44 in cancers: a breast carcinoma perspective.
Cancer Res. 66:10233–10237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serra M, Rabanal RM, Miquel L, Domenzain C
and Bassols A: Differential expression of CD44 in canine
melanocytic tumours. J Comp Path. 130:171–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paltian V, Alldinger S, Baumgartner W and
Wohlsein P: Expression of CD44 in canine mammary tumours. J Comp
Path. 141:237–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura T, Funahashi M, Takagaki K,
Munakata H, Tanaka K, Saito Y and Endo M: Effect of
4-methylumbelliferone on cell-free synthesis of hyaluronic acid.
Biochem Mol Biol Int. 43:263–268. 1997.PubMed/NCBI
|
|
26
|
Kakizaki I, Kojima K, Takagaki K, Endo M,
Kannagi R, Ito M, Maruo Y, Sato H, Yasuda T, Mita S, Kimata K and
Itano N: A novel mechanism for the inhibition of hyaluronan
biosynthesis by 4-Methylumbelliferone. J Biol Chem.
279:33281–33289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kultti A, Pasonen-Seppanen S, Jauhiainen
M, Rilla KJ, Karna R, Pyoria E, Tammi RH and Tammi MI:
4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of
cellular UDP-glucuronic acid and downregulation of hyaluronan
synthase 2 and 3. Exp Cell Res. 315:1914–1923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lokeshwar VB, Lopez LE, Munoz D, Chi A,
Shirodkar SP, Lokeshwar SD, Escudero DO, Dhir N and Altman N:
Antitumor activity of hyaluronic acid synthesis inhibitor
4-methylumbelliferone in prostate cancer cells. Cancer Res.
70:2613–2623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshihara S, Kon A, Kudo D, Nakazawa H,
Kakizaki I, Sasaki M, Endo M and Takagaki K: A hyaluronan synthase
suppressor, 4-methylumbelliferone, inhibits liver metastasis of
melanoma cells. FEBS Lett. 579:2722–2726. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knudson W and Knudson CB: Assembly of a
chondrocyte-like pericellular matrix on non-chondrogenic cells.
Role of the cell surface hyaluronan receptors in the assembly of a
pericellular matrix. J Cell Sci. 99:227–235. 1991.PubMed/NCBI
|
|
31
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Twentyman PR and Luscombe M: A study of
some variables in a tetrazolium dye (MTT) based assay for cell
growth and chemosensitivity. Br J Cancer. 56:279–285. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saito T, Kawana H, Azuma K, Toyoda A,
Fujita H, Kitagawa M and Harigaya K: Fragmented hyaluronan is an
autocrine chemokinetic motility factor supported by the
HAS2-HYAL2/CD44 system on the plasma membrane. Int J Oncol.
39:1311–1320. 2011.PubMed/NCBI
|
|
34
|
Simpson M, Reiland J, Burger S, Furcht L,
Spicer A, Oegema T and MaCarthy JB: Hyaluronan synthase elevation
in metastatic prostate carcinoma cells correlates with hyaluronan
surface retention, a prerequisite for rapid adhesion to bone marrow
endothelial cells. J Biol Chem. 276:17949–17957. 2001. View Article : Google Scholar
|
|
35
|
Li Y, Li L, Brown TJ and Heldin P:
Silencing of hyaluronan synthase 2 suppresses the malignant
phenotype of invasive breast cancer cells. Int J Cancer.
120:2557–2567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia W, Spector S, Hardy L, Zhao S, Saluk
A, Alemane L and Spector NL: Tumor selective G2/M cell cycle arrest
and apoptosis of epithelial and hematological malignancies by
BBL22, a benzazepine. Proc Natl Acad Sci USA. 97:7494–7499. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arai E, Nishida Y, Wasa J, Urakawa H, Zhuo
L, Kimata K, Kozawa E, Futamura N and Ishiguro N: Inhibition of
hyaluronan retention by 4-methylumbelliferone suppresses
osteosarcoma cells in vitro and lung metastasis in vivo. Br J
Cancer. 105:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Urakawa H, Nishida Y, Wasa J, Arai E, Zhuo
L, Kimata K, Kozawa E, Futamura N and Ishiguro N: Inhibition of
hyaluronan synthesis in breast cancer cells by
4-methylumbelliferone suppresses tumorigenicity in vitro and
metastatic lesions of bone in vivo. Int J Cancer. 130:454–466.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Camenisch TD, Spicer AP, Brehm-Gibson T,
Biesterfeldt J, Agustine ML, Calabro A Jr, Kubalak S, Klewer SE and
McDonald JA: Disruption of hyaluronan synthase-2 abrogates normal
cardiac morphogenesis and hyaluronan-mediated transformation of
epithelium to mesenchyme. J Clin Invest. 106:349–360. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vigetti D, Genasetti A, Karousou E, Viola
M, Moretto P, Clerici M, Deleonibus S, De Luca G, Hascall VC and
Passi A: Proinflammatory cytokines induce hyaluronan synthesis and
monocyte adhesion in human endothelial cells through hyaluronan
synthase 2 (HAS2) and the nuclear factor-kappaB (NF-kappaB)
pathway. J Biol Chem. 285:24639–24645. 2010. View Article : Google Scholar
|
|
40
|
Itano N and Kimata K: Mammalian hyaluronan
synthases. IUBMB Life. 54:195–199. 2002. View Article : Google Scholar
|
|
41
|
Okuda H, Kobayashi A, Xia B, Watabe M, Pai
SK, Hirota S, Xing F, Liu W, Pandey PR, Fukuda K, Modur V, Ghosh A,
Wilber A and Watabe K: Hyaluronan synthase HAS2 promotes tumor
progression in bone by stimulating the interaction of breast cancer
stem-like cells with macrophages and stromal cells. Cancer Res.
72:537–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sironen RK, Tammi M, Tammi R, Auvinen PK,
Anttila M and Kosma VM: Hyaluronan in human malignancies. Exp Cell
Res. 317:383–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bourguignon LY, Zhu H, Chu A, Iida N,
Zhang L and Hung MC: Interaction between the adhesion receptor,
CD44, and the oncogene product, p185HER2, promotes human ovarian
tumor cell activation. J Biol Chem. 272:27913–27918. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ouhit A, Abd Elmageed ZY, Abdraboh ME,
Lioe TF and Raj MH: In vivo evidence for the role of CD44s in
promoting breast cancer metastasis to the liver. Am J Pathol.
171:2033–2039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sohara Y, Ishiguro N, Machida K, Kurata H,
Thant AA, Senga T, Matsuda S, Kimata K, Iwata H and Hamaguchi M:
Hyaluronan activates cell motility of v-Src-transformed cells via
Ras-mitogen-activated protein kinase and phosphoinositide
3-kinase-Akt in a tumor-specific manner. Mol Biol Cell.
12:1859–1868. 2001. View Article : Google Scholar : PubMed/NCBI
|