Introduction
Peripheral T-cell lymphomas (PTCLs) constitute a
rare, heterogeneous class of non-Hodgkin lymphomas (NHLs)
characterised by generally poor prognoses. The most common PTCL
subtypes are angioimmunoblastic T-cell lymphoma (AITL), peripheral
T-cell lymphoma not-otherwise-specified (PTCL-NOS), and
anaplastic-lymphoma-kinase (ALK)-positive or ALK-negative
anaplastic large-cell lymphomas (ALCLs) (1).
PTCL patients are more likely to present with
aggressive clinical features such as bone-marrow involvement, B
symptoms, or other comorbidities (1,2).
Moreover, most patients are elderly (median age: >60 years),
usually presenting with extra-nodal lymphoma sites and high serum
LDH levels (1,2).
A previously established, standard treatment for
B-cell lymphomas is the R-CHOP combination chemotherapy, which
includes rituximab, cyclophosphamide, doxorubicin, vincristine, and
prednisone (3,4). Although the CHOP regimen is the widely
used first-line treatment for PTCLs, its effectiveness has not been
established prospectively. Previous studies including relatively
large numbers of cases have reported unfavourable prognoses with
5-year overall survival (OS) rates of <40% (1,9–11) with
the exception of ALK+-ALCL and primary cutaneous T-cell
lymphomas (CTCLs). ALK+-ALCLs yield a more favourable
outcome following conventional chemotherapy (5,6) and
primary CTCLs mostly take an indolent clinical course (7,8).
Because of reportedly low PTCL OS rates, many physicians strive to
improve the results of CHOP or other comparable regimens, hoping to
achieve better patient outcomes.
To establish effective treatment protocols for PTCLs
and to improve patient outcomes, multiple clinical trials have been
undertaken. For example, the SWOG clinical trials, which compared
CHOP to second- or third-generation dose-intensive regimens
indicated in aggressive lymphomas, established that CHOP was less
toxic than, but as efficacious as, the new-generation intensive
regimens. However, routine immunophenotyping was not performed in
that study (12).
Autologous stem-cell transplantation (ASCT) is a
promising, intensive, consolidating treatment option for
refractory/relapsing patients who underwent salvage therapies
(13,14). However, ASCT findings are
controversial (15–19). It has been difficult to ascertain
the significance of ASCT because i) many relevant studies have used
relatively small study case numbers; ii) some have included
ALK+-ALCL, which are known to lead to better outcomes
than other T-cell lymphomas; and iii) ASCT has been performed in
relatively younger PTCL patients.
In this study, we treated consecutive PTCL patients
with our original dose-intensified CHOP, Double-CHOP. Patients
achieving complete remission (CR) were subsequently treated with
high-dose therapy (HDT)/ASCT, or high-dose methotrexate (HDMTX)
treatment. Herein, we present our Double-CHOP and ASCT treatment
outcomes.
Patients and methods
Patients
From December 1996 to February 2012, newly diagnosed
adult PTCL patients, aged 15–69 years, including those with
ALK−-ALCL, AITL, PTCL-NOS, and γ/δ T-cell lymphoma were
included in this study. Patients with other subtypes of T-cell
lymphomas, including ALK+-ALCL, CTCLs, adult T-cell
lymphoma (ATL), and those with low-grade IPI risk or with localised
lesions were excluded. Other exclusion criteria included Eastern
Cooperative Oncology Group (ECOG) performance status (PS) of 4;
markedly impaired cardiac, renal, or pulmonary functions; and
positivity for hepatitis-B surface antigen. All patients underwent
systemic computed tomography (CT) scans, bone-marrow aspiration
and/or biopsy, and either gallium scintigraphy or positron-emission
tomography (PET) for lymphoma staging. The study was approved by
the institutional review board of our hospital.
Treatment
The Double-CHOP regimen consisted of 3 courses of
intravenous (i.v.) administration of cyclophosphamide (750
mg/m2, days 1–2, over 2 h), doxorubicin (50
mg/m2, days 1–2, >30 min), vincristine (1.4
mg/m2, day 1, max 2 mg/body), and per os (p.o.)
prednisone (50 mg/m2, days 1–5). For patients aged
>60, cyclophosphamide dose was modified as follows: course 1,
750 mg/m2, day 1; course 2, 500 mg/m2, days
1–2; and course 3, 750 mg/m2, days 1–2. Treatment
intensity was augmented during every course unless leukocyte
recovery (WBC count, <3,000 on day 23) was delayed or an adverse
event (grade ≥3) other than haematological toxicities developed.
When patients developed grade ≥3 neutropenia, granulocyte
colony-stimulating factor (G-CSF) was administered until neutrophil
counts recovered. To administer subsequent courses of Double-CHOP,
an absolute leukocyte count ≥3.0×109/l, neutrophil count
≥1.0×109/l, and platelet count ≥1×1011/l were
required. If patients were i) 65 years or younger, ii) had an
acceptable ECOG PS, and iii) achieved complete remission (CR) or
unconfirmed CR (CRu) within 3 courses of Double-CHOP, their
peripheral-blood stem-cells were collected and subsequent HDT and
ASCT indicated. The third cycle of Double-CHOP regimen was used for
stem cell mobilization. Consolidating HDT regimen consisted of
cyclophosphamide (60 mg/kg, day -7 and -6, i.v. >3 h), etoposide
(500 mg/m2; day -6, -5, and -4; i.v. >6–8 h), and
ranimustine (250 mg/m2, day -3 and -2, i.v. >1 h).
ASCT was performed on day 0 and G-CSF administered from day 1 until
neutrophil engraftment. As an alternative to HDT/ASCT, HDMTX (8
g/m2, day 1, i.v. >4 h) was indicated for patients
who could not yield a sufficient number of stem cells or were
ineligible for HDC.
Treatment response and toxicity
Response to Double-CHOP regimen was assessed by
physical examination, CT scanning, and either gallium scintigraphy
or PET before ASCT indication. Patients were classified based on
achieving CR/CRu, partial response (PR), stable disease (SD), or
progressive disease (PD), according to the criteria previously
reported by Cheson et al(20). Patients remained in the hospital
until achieving haematological recovery in each chemotherapy cycle.
Blood and physical examinations were performed more than twice per
week. Toxicity related to the Double-CHOP regimen was evaluated
according to the National Cancer Institute Common Terminology
Criteria for Adverse Events, version 4.0.
Statistical analyses
The patients OS was calculated from treatment
initiation to the date of death or until follow-up termination.
Relapse-free survival (RFS) for patients who achieved CR was
calculated from the date of the last treatment or ASCT to either
the recurrence date or the follow-up termination. OS and RFS were
calculated according to the Kaplan-Meier method. Prognostic factors
likely affecting clinical outcomes were analysed by univariate Cox
proportional-hazard regression model. Statistical analyses were
performed using the JMP software, version 8.0.1 (SAS Institute
Inc., Cary, NC, USA).
Results
Patients
Twenty-eight consecutive PTCL patients, including 13
PTCL-NOS, 11 AITL, 3 ALK−-ALCL patients, and 1 γ/δ
T-cell lymphoma patient, were enrolled in this study. Misdiagnosed
patients, patients with other T-cell lymphoma subtypes, or those
treated with alternative regimens, were excluded. Patients were
mostly male (20 men and 8 women) and aged 17–69 years (median: 58
years). Regarding IPI and PIT scores, 11 (39%), 10 (36%), and 7
(25%) patients were at low-intermediate, high-intermediate, and
high IPI risk, respectively. Seven (25%) patients had ≥3 PIT
scores. Patients who required immediate treatment because of a poor
ECOG PS likely caused by an underlying disease or rapid disease
progression, were indicated to receive the standard CHOP regimen
before Double-CHOP treatment. Similarly, because many patients
required immediate administration of therapeutic agents, we
modified the treatment protocol to initiate standard CHOP followed
with 3 courses of Double-CHOP, in 2003. Overall, 23 patients
received standard CHOP before Double-CHOP. Patients characteristics
are reported in Table I.
 | Table IPatients characteristics. |
Table I
Patients characteristics.
| n=28 | % |
|---|
| Median age
(range) | 58 (17–69) | |
| Gender
(male/female) | 20/8 | |
| PTCL type |
| PTCL-NOS | 13 | 46 |
| AITL | 11 | 39 |
|
ALK−-ALCL | 3 | 11 |
| γ/δ T-cell
lymphoma | 1 | 4 |
| ECOG performance
status |
| 0–1 | 11 | 39 |
| 2–3 | 17 | 61 |
| LDH |
| Normal | 5 | 18 |
| High | 23 | 82 |
| Stage |
| 1–2 | 2 | 7 |
| 3–4 | 26 | 93 |
| Extranodal
sites |
| 0–1 | 23 | 82 |
| ≥2 | 5 | 18 |
| Bone-marrow
involvement |
| Yes | 8 | 29 |
| No | 20 | 71 |
| IPI risk
factor |
| Low-intermediate
(2) | 11 | 39 |
| High-intermediate
(3) | 10 | 36 |
| High (4–5) | 7 | 25 |
| PIT score |
| Group 1 | 7 | 25 |
| Group 2 | 14 | 50 |
| Group 3–4 | 7 | 25 |
CR rate and consolidation therapy
After Double-CHOP treatment, 19 (68%) patients
achieved CR/CRu, 4 PR, 1 NC, and 3 PD. Unfortunately, 1 patient
died of septicaemia during the treatment term. Of 19 patients with
CR/CRu, 10 were given G-CSF to allow harvesting of CD34-positive
cells. Eight (80%) patients yielded high numbers of CD34-positive
stem cells (>2.0×106/kg) after Double-CHOP. Two
patients were poorly mobilised by G-CSF, but yielded acceptable
numbers of stem cells by a harvest regimen using VP-16. Overall,
all patients aged ≤65 years underwent CR/CRu and received ASCT. For
patients aged >65 years, HDMTX was administered instead of ASCT
(n=7). Two patients with unacceptable ECOG PS experienced early
relapse after CR and were excluded from HDMTX or ASCT
treatment.
OS and risk factor
The follow-up term ranged from 4 to 123 months
(median, 31 months). Since treatment initiation, the 3- and 5-year
OS were 68 and 63%, respectively (Fig.
1A). No significant differences in OS were observed between
low-intermediate and high-intermediate to high IPI-risk groups
(Fig. 1B). Moreover, the PIT score
did not affect OS or IPI risk factors (Fig. 1C). When comparing different PTCL
subtypes, AITL tended to show a more favourable prognosis than did
other subtypes, but this difference was statistically insignificant
(Fig. 1D). The major cause of death
was disease progression or treatment-related toxicity. One case
treated with HDT/ASCT developed a secondary malignancy (acute
leukaemia) after experienced lymphoma relapse and received salvage
chemotherapy, and the patient died of leukaemia.
RFS
Among 19 patients in complete remission, the
calculated 3- and 5-year RFS were 60 and 43% at treatment
termination, respectively (Fig.
2A). No treatment-related mortality cases following HDMTX or
HDT/ASCT ensued. RFS data for patients who underwent HDT/ASCT
(n=10) or HDMTX (n=7) are shown in Fig.
2B. Three- and 5-year RFS estimations in the former and latter
group were 68 and 58%, and 53 and 40%, respectively. No significant
differences were observed between these 2 groups; however, the
HDMTX group, including patients aged >65 years, likely relapsed
earlier than those who received HDT/ASCT.
Prognosis for relapsing patients
It has been reported that in patients not receiving
upfront ASCT, salvage regimen followed by ASCT is often a promising
treatment strategy (13,14). However, patients who relapsed after
treatment with intensive chemotherapy and upfront ASCT or HDMTX in
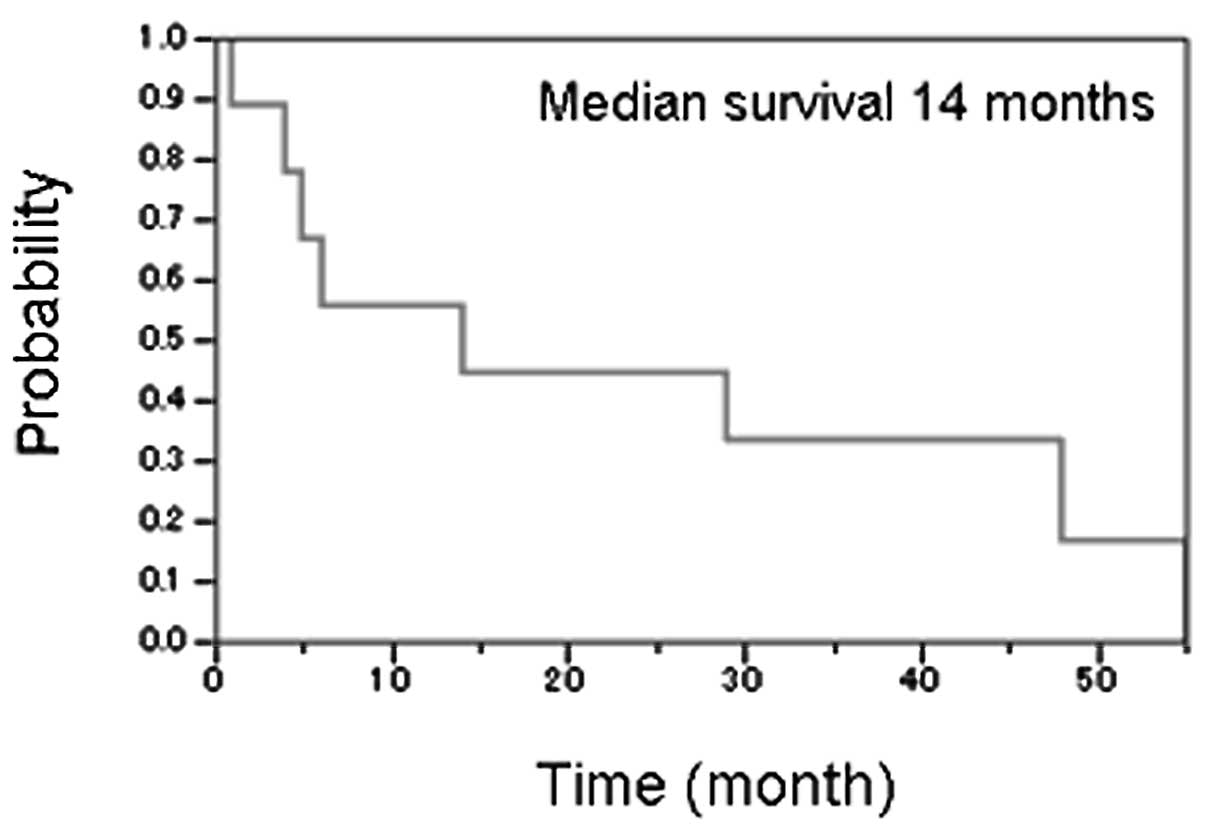
our study showed poor prognoses (Fig.
3). Of 9 relapsing patients, 8 received salvage chemotherapy
and 2 were subsequently treated with cord-blood transplantation
(CBT). Nevertheless, of those undergoing CBT, 1 died of fungal
infection and a second patient died of disease progression within 3
months. Only 1 of 9 relapsing cases was still alive with disease
after follow-up termination.
Prognosis for non-CR patients
Apart from 19 patients achieving CR and a single
case of treatment-related mortality, 4 underwent PR, 1 NC, and 3
experienced PD. One patient received ASCT after PR, but did not
achieve CR after treatment completion while experiencing early PD
(within 2 months). In contrast, 1 patient who received ASCT
following CR after salvage therapy achieved RFS of over 2 years. Of
4 patients who received allogeneic hematopoietic cell
transplantation (HSCT), 3 underwent BMT and 1 CBT. Three of 4 did
not achieve CR following transplantation; however, all patients
achieved and maintained CR after HSCT. Our results are particularly
noteworthy because we showed clinical efficacy for allogeneic HSCT
in PTCL patients who did not achieve CR after initial
treatment.
Toxicity
Treatment-related toxicity was monitored in all
patients throughout the Double-CHOP duration. The major
Double-CHOP-related toxicity events were bone marrow suppression
and infection. Grade 3 or grade 4 neutropaenia, anaemia, and
thrombocytopaenia were also common. Thus, all patients required
G-CSF administration. Some patients experienced febrile
neutropaenia (n=18), pneumonia (n=3), or septicaemia (n=2), along
with haematological toxicity. All but one septicaemic patient
recovered after receiving wide-spectrum antibiotics.
Non-haematological toxicities such as nausea, diarrhoea,
electrolyte abnormalities, or liver dysfunctions were also
reported, but these were reversible. Patients toxicity records are
shown in Table II.
 | Table IISignificant toxicities associated
with D-CHOP regimen. |
Table II
Significant toxicities associated
with D-CHOP regimen.
| Total (n=28) | % | Age ≥60 (n=10) | % |
|---|
| Leukopaenia | | | | |
| Grade 4 | 28 | 100 | 10 | 100 |
| Neutropaenia | | | | |
| Grade | 28 | 100 | 10 | 100 |
| Anaemia | | | | |
| Grade 3 | 24 | 86 | 10 | 100 |
| Grade 4 | 0 | 0 | 0 | 0 |
|
Thrombocytopaenia | | | | |
| Grade 3 | 11 | 39 | 5 | 50 |
| Grade 4 | 13 | 46 | 4 | 40 |
| Liver dysfunction
(AST/ALT/Total Bilirubin elevation) | | | | |
| Grade 3 | 5 | 18 | 1 | 10 |
| Grade 4 | 0 | 0 | 0 | 0 |
| Electrolyte
abnormality (serum Na and/or K) | | | | |
| Grade 3 | 6 | 21 | 2 | 20 |
| Grade 4 | 1 | 4 | 1 | 10 |
| Febrile
neutropaenia | | | | |
| Grade 3 | 18 | 64 | 4 | 40 |
| Grade 4 | 0 | 0 | 0 | 0 |
| Septicaemia | | | | |
| Grade 4 | 1 | 4 | 0 | 0 |
| Grade 5 | 1 | 4 | 0 | 0 |
| Pneumonia | | | | |
| Grade 3 | 3 | 11 | 0 | 0 |
| Grade 4 | 0 | 0 | 0 | 0 |
| Gastrointestinal
disorder | | | | |
| Grade 3 | 2 | 7 | 1 | 10 |
| Grade 4 | 0 | 0 | 0 | 0 |
| Peripheral
neuropathy | | | | |
| Grade 3 | 3 | 10 | 1 | 11 |
| Grade 4 | 0 | 0 | 0 | 0 |
Discussion
CHOP or CHOP-like therapy regimes have previously
been used to treat PTCLs. However, patients outcomes, particularly
in high-intermediate or high-risk cases as defined by age-adjusted
IPI, have reportedly been a poor 5-year OS of <40% (10,21).
Furthermore, despite multiple retrospective studies, improving
patients prognoses remains an eminent challenge. Major treatment
strategies attempting to improve patient outcome have been
classified into 4 groups: i) intensive treatment, ii) upfront ASCT,
iii) allogeneic HSCT, and iv) use of novel agents. ASCT is often
more effective when patient’s clinical status is documented and
controlled well before therapy initiation (15–18,22,23),
indicating that chemotherapy-sensitive disease can be suppressed
effectively with intensive consolidation therapies. Highly improved
RFS and OS reported in our study support the fact that a
well-documented disease status is quintessential for improving CR
rates by administering Double-CHOP and intensive high-dose therapy
regimens.
Contrarily, a previous German High-Grade Non-Hodgkin
Lymphoma Study Group (DSHNHL) report demonstrated that a
dose-intensified CHOEP (CHOP plus etoposide) regimen followed by
ASCT resulted in poor survival rates compared with a standard-dose
CHOEP regimen (24). Although our
protocol was similar to that used in this study, our recorded OS
results appear to be better than those reported for standard CHOP
regimens. The reasons for discordant results between this study and
ours remain elusive, while case numbers in both studies were
relatively small. Therefore, conducting large-scale studies is
required to ascertain the efficacy of intensified regimens better.
Our results also emphasise that the efficacy of dose-intensified
regimen(s) for PTCLs should be re-evaluated to improve patients
prognoses.
Our patients inclusion criteria are noteworthy
because we included a broad age group (<70 years; median: 58
years) and excluded low-risk IPI and ALK+-ALCL patients.
As shown in Table I, our patients
clinical characteristics were worse than those in previous reports,
which used multi-agent chemotherapy with or without ASCT (16,18).
Dose-modified CHOP was well tolerated and effective in elderly
patients included in our study, indicating the importance of
individualised dose modifications or intensifications. Although our
study could not determine an association between prognosis and IPI
risk factor and PIT score, patients outcomes are altered expectedly
with distinct treatment protocols. Consequently, the Double-CHOP
regimen may be more effective for PTCL patients with likely poor
prognostic factors.
We did not find a significant difference in relapse
rate or other events between ASCT- and HDMTX-treated groups, which
included patients younger or older than 65 years of age.
Nevertheless, patients who received ASCT tended to relapse later
than those who received HDMTX instead of ASCT. These different
trends suggest that a more intensive consolidation therapy may be
the means to sustain a prolonged disease-free status. On the
contrary, it is uncertain whether an intensified treatment may
increase the curative rates as shown by plateauing Kaplan-Meier
curve in Figs. 1 and 2. In addition, an optimised treatment
strategy should follow relapse when an intensified treatment
regimen, including upfront ASCT, was previously applied. ASCT after
disease control by salvage therapy has been shown to be a
favourable treatment strategy in both B- and T-cell lymphomas
(13,14). However, prognoses in our relapsing
patients who received intensified treatment were unfavourable,
showing a median 14-month OS following relapse. Most cases
receiving salvage chemotherapy were refractory and finally died of
disease progression. In such cases, rapid preparation for
allogeneic HSCT should be explored. In addition, these patients,
especially elderly patients who are not good HSCT candidates (e.g.
over 65 years), are ideal candidates for trialling new agents.
We have previously demonstrated that refractory
diffuse large B-cell lymphoma (DLBCL) is less favourable than
relapsing DLBCL (25). Reportedly,
the primary refractory and non-CR patients are likely better off
than relapsing patients. Four of 7 patients received allogeneic
HSCT on CR or non-CR and resulted only in a single case of
treatment-related mortality while 3 survived. Despite a relatively
small number of successful cases, we could surmise that rapid
decision-making may significantly improve patients outcomes, if
they were clinically resistant to chemotherapy. Previous studies
demonstrated results of allogeneic HSCT for PTCLs showing that
donor lymphocyte infusion was effective, indicating a graft versus
T-cell lymphoma effect (26,27).
Our cases achieved CR after allogeneic HSCT underscoring the
presence of a graft versus T-cell lymphoma effect, and the need for
lowering the decision-making threshold to indicate allogeneic HSCT
under refractory settings. If patient’s status was well sustained
and a donor was available, allogeneic HSCT may be a curative
strategy for refractory PTCLs. Of note, no recommendations can be
made regarding alternative donor transplants due to data paucity.
Therefore, alternative donor transplants should be considered as a
clinical trial.
In conclusion, our data suggest that 3 cycles of
Double-CHOP regimen may improve complete remission in patients with
PTCLs. According to our experience involving DLBCL (28,29),
Double-CHOP followed by ASCT/HDMTX consolidations is safe and may
achieve prolonged RFS, especially in patients with poor prognostic
factors. Since case numbers are relatively small, we cannot confirm
the ASCT impact. Moreover, prospective PTCL studies involving ASCT
and allogeneic HSCT are needed. To address these issues further,
well-designed, large-scale, prospective studies are required.
References
|
1
|
Coiffier B, Brousse N, Peuchmaur M, Berger
F, Gisselbrecht C, Bryon PA and Diebold J: Peripheral T-cell
lymphomas have a worse prognosis than B-cell lymphomas: a
prospective study of 361 immunophenotyped patients treated with the
LNH-84 regimen. The GELA (Groupe d’Etude des Lymphomes Agressives).
Ann Oncol. 1:45–50. 1990.
|
|
2
|
Vose J, Armitage J and Weisenburger D:
International T-Cell Lymphoma Project: International peripheral
T-cell and natural killer/T-cell lymphoma study: pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sehn LH, Donaldson J, Chhanabhai M,
Fitzgerald C, Gill K, Klasa R, MacPherson N, O’Reilly S, Spinelli
JJ, Sutherland J, Wilson KS, Gascoyne RD and Connors JM:
Introduction of combined CHOP plus rituximab therapy dramatically
improved outcome of diffuse large B-cell lymphoma in British
Columbia. J Clin Oncol. 23:5027–5033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feugier P, Van Hoof A, Sebban C,
Solal-Celigny P, Bouabdallah R, Ferme C, Christian B, Lepage E,
Tilly H, Morschhauser F, Gaulard P, Salles G, Bosly A, Gisselbrecht
C, Reyes F and Coiffier B: Long-term results of the R-CHOP study in
the treatment of elderly patients with diffuse large B-cell
lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte.
J Clin Oncol. 23:4117–4126. 2005.PubMed/NCBI
|
|
5
|
Gascoyne RD, Aoun P, Wu D, Chhanabhai M,
Skinnider BF, Greiner TC, Morris SW, Connors JM, Vose JM,
Viswanatha DS, Coldman A and Weisenburger DD: Prognostic
significance of anaplastic lymphoma kinase (ALK) protein expression
in adults with anaplastic large cell lymphoma. Blood. 93:3913–3921.
1999.PubMed/NCBI
|
|
6
|
Falini B, Pileri S, Zinzani PL, Carbone A,
Zagonel V, Wolf-Peeters C, Verhoef G, Menestrina F, Todeschini G,
Paulli M, Lazzarino M, Giardini R, Aiello A, Foss HD, Araujo I,
Fizzotti M, Pelicci PG, Flenghi L, Martelli MF and Santucci A:
ALK+ lymphoma: clinico-pathological findings and
outcome. Blood. 93:2697–2706. 1999.PubMed/NCBI
|
|
7
|
Willemze R, Jaffe ES, Burg G, Cerroni L,
Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL,
Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler
R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH,
Wechsler J, Whittaker S and Meijer CJ: WHO-EORTC classification for
cutaneous lymphomas. Blood. 105:3768–3785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jenni D, Karpova MB, Seifert B, Golling P,
Cozzio A, Kempf W, French LE and Dummer R: Primary cutaneous
lymphoma: two-decade comparison in a population of 263 cases from a
Swiss tertiary referral centre. Br J Dermatol. 164:1071–1077. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abouyabis AN, Shenoy PJ, Lechowicz MJ and
Flowers CR: Incidence and outcomes of the peripheral T-cell
lymphoma subtypes in the United States. Leuk Lymphoma.
49:2099–2107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weisenburger DD, Savage KJ, Harris NL,
Gascoyne RD, Jaffe ES, MacLennan KA, Rüdiger T, Pileri S, Nakamura
S, Nathwani B, Campo E, Berger F, Coiffier B, Kim WS, Holte H,
Federico M, Au WY, Tobinai K, Armitage JO and Vose JM:
International Peripheral T-cell Lymphoma Project: Peripheral T-cell
lymphoma, not otherwise specified: a report of 340 cases from the
International Peripheral T-cell Lymphoma Project. Blood.
117:3402–3408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gisselbrecht C, Gaulard P, Lepage E,
Coiffier B, Brière J, Haioun C, Cazals-Hatem D, Bosly A, Xerri L,
Tilly H, Berger F, Bouhabdallah R and Diebold J: Prognostic
significance of T-cell phenotype in aggressive non-Hodgkin’s
lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood.
92:76–82. 1998.
|
|
12
|
Fisher RI, Gaynor ER, Dahlberg S, Oken MM,
Grogan TM, Mize EM, Glick JH, Coltman CA Jr and Miller TP:
Comparison of a standard regimen (CHOP) with three intensive
chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J
Med. 328:1002–1006. 1993.PubMed/NCBI
|
|
13
|
Kewalramani T, Zelenetz AD,
Teruya-Feldstein J, Hamlin P, Yahalom J, Horwitz S, Nimer SD and
Moskowitz CH: Autologous transplantation for relapsed or primary
refractory peripheral T-cell lymphoma. Br J Haematol. 134:202–207.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song KW, Mollee P, Keating A and Crump M:
Autologous stem cell transplant for relapsed and refractory
peripheral T-cell lymphoma: variable outcome according to
pathological subtype. Br J Haematol. 120:978–985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosing C and Champlin RE: Stem-cell
transplantation in T-cell non-Hodgkin’s lymphomas. Ann Oncol.
22:1471–1477. 2011.
|
|
16
|
Casulo C and Horwitz S: Should eligible
patients with T-cell lymphoma receive high-dose therapy and
autologous stem cell transplant in the upfront setting? Curr Oncol
Rep. 12:374–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savage KJ: Therapies for peripheral T-cell
lymphomas. Hematology Am Soc Hematol Educ Program. 2011:515–524.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reimer P: Impact of autologous and
allogeneic stem cell transplantation in peripheral T-cell
lymphomas. Adv Hematol. 2010:3206242010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moskowitz AJ and Moskowitz CH:
Controversies in the treatment of lymphoma with autologous
transplantation. Oncologist. 14:921–929. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheson BD, Horning SJ, Coiffier B, Shipp
MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A,
Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino
R, Harris NL, Armitage JO, Carter W, Hoppe R and Canellos GP:
Report of an international workshop to standardize response
criteria for non-Hodgkin’s lymphomas. NCI Sponsored International
Working Group. J Clin Oncol. 17:1244–1253. 1999.PubMed/NCBI
|
|
21
|
Ansell SM, Habermann TM, Kurtin PJ, Witzig
TE, Chen MG, Li CY, Inwards DJ and Colgan JP: Predictive capacity
of the International Prognostic Factor Index in patients with
peripheral T-cell lymphoma. J Clin Oncol. 15:2296–2301.
1997.PubMed/NCBI
|
|
22
|
Yang DH, Kim WS, Kim SJ, Bae SH, Kim SH,
Kim IH, Yoon SS, Mun YC, Shin HJ, Chae YS, Kwak JY, Kim H, Kim MK,
Kim JS, Won JH, Lee JJ and Suh CW: Prognostic factors and clinical
outcomes of high-dose chemotherapy followed by autologous stem cell
transplantation in patients with peripheral T cell lymphoma,
unspecified: complete remission at transplantation and the
prognostic index of peripheral T cell lymphoma are the major
factors predictive of outcome. Biol Blood Marrow Transplant.
15:118–125. 2009.
|
|
23
|
Corradini P, Tarella C, Zallio F, Dodero
A, Zanni M, Valagussa P, Gianni AM, Rambaldi A, Barbui T and
Cortelazzo S: Long-term follow-up of patients with peripheral
T-cell lymphomas treated up-front with high-dose chemotherapy
followed by autologous stem cell transplantation. Leukemia.
20:1533–1538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pfreundschuh M, Zwick C, Zeynalova S,
Dührsen U, Pflüger KH, Vrieling T, Mesters R, Mergenthaler HG,
Einsele H, Bentz M, Lengfelder E, Trümper L, Rübe C, Schmitz N and
Loeffler M: German High-Grade Non-Hodgkin’s Lymphoma Study Group
(DSHNHL): Dose-escalated CHOEP for the treatment of young patients
with aggressive non-Hodgkin’s lymphoma: II. Results of the
randomized high-CHOEP trial of the German High-Grade Non-Hodgkin’s
Lymphoma Study Group (DSHNHL). Ann Oncol. 19:545–552. 2008.
|
|
25
|
Miura K, Takei K, Kobayashi S, Kiso S,
Hirabayashi Y, Hojo A, Kodaira H, Yagi M, Kurita D, Kobayashi Y,
Tanaka T, Iriyama N, Hatta Y, Kura Y, Yamazaki T, Sawada U and
Takeuchi J: An effective salvage treatment using ifosfamide,
etoposide, cytarabine, dexamethasone, and rituximab (R-IVAD) for
patients with relapsed or refractory aggressive B-cell lymphoma.
Int J Hematol. 94:90–96. 2011. View Article : Google Scholar
|
|
26
|
Corradini P, Dodero A, Zallio F,
Caracciolo D, Casini M, Bregni M, Narni F, Patriarca F, Boccadoro
M, Benedetti F, Rambaldi A, Gianni AM and Tarella C:
Graft-versus-lymphoma effect in relapsed peripheral T-cell
non-Hodgkin’s lymphomas after reduced-intensity conditioning
followed by allogeneic transplantation of hematopoietic cells. J
Clin Oncol. 22:2172–2176. 2004.
|
|
27
|
Wulf GG, Hasenkamp J, Jung W, Chapuy B,
Truemper L and Glass B: Reduced intensity conditioning and
allogeneic stem cell transplantation after salvage therapy
integrating alemtuzumab for patients with relapsed peripheral
T-cell non-Hodgkin’s lymphoma. Bone Marrow Transplant. 36:271–273.
2005.PubMed/NCBI
|
|
28
|
Yamazaki T, Sawada U, Kura Y, Ito T,
Kaneita Y, Yasukawa K and Horie T: Dose-intensified CHOP
(double-CHOP) followed by consolidation with high-dose chemotherapy
for high and high-intermediate risk aggressive non-Hodgkin’s
lymphomas. Leuk Lymphoma. 43:2117–2123. 2002.PubMed/NCBI
|
|
29
|
Yamazaki T, Sawada U, Kura Y, Ito T,
Takeuchi J, Hatta Y, Aikawa S, Takei K, Ishizuka H, Saiki M and
Uenogawa K: Treatment of high-risk peripheral T-cell lymphomas
other than anaplastic large-cell lymphoma with a dose-intensified
CHOP regimen followed by high-dose chemotherapy. A single
institution study. Acta Haematol. 116:90–95. 2006. View Article : Google Scholar : PubMed/NCBI
|