Introduction
Esophageal cancer (EC) is the eighth most common
cancer worldwide (1), while
esophageal squamous cell carcinoma (ESCC) is the predominant
histological subtype in Asia, especially in China. It is
characterized by high incidence and mortality rate (1,2). We
found that stathmin is a differentially expressed protein between
cancer and adjacent normal tissues in ESCC using proteomic
technology.
Stathmin is an important protein which destabilizes
microtubules (3,4). Microtubules are essential for many
cellular processes, including mitosis, intracellular transport,
supportment of cell shape and cell motility, and stathmin playes an
important role in the regulation of microtubule which was involved
in the construction and function of the mitotic spindle (5). Phosphorylation of stathmin led to a
loss of the microtubule-destabilizing activity (6–8).
Inhibition of stathmin phosphorylation produced strong mitotic
phenotypes characterized by disassembly and disorganization of
mitotic spindles and abnormal chromosome distributions (6). Stathmin phosphorylation gradient was
necessary for correct spindle formation. Gradients of diffusible
morphogens are known to be crucial for the supracellular
self-organization of tissues and organisms (9).
Many studies had reported that stathmin was
overexpressed across a broad range of human cancers, including
acute leukemia, lymphoma, neuroblastoma and ovarian, prostate,
breast and lung cancer (10–12).
The upregulation of stathmin in ESCC was reported
and associated with differentiation degree, lymph node metastasis,
invasive depth and TNM stage (13,14).
Wang et al demonstrated that knockdown of stathmin by
antisense oligonucleotide can inhibit the proliferation of ECa109
cells (15). The expression and
exact biological function of stathmin in ESCC, especially motility,
remained largely unclear.
Materials and methods
Cell culture
ESCC cell lines EC0156 was established by our
laboratory (16). KYSE30, KYSE140,
KYSE150, KYSE170, KYSE180, KYSE410 and KYSE510 were donated by Dr
Y. Shimada. EC0156 was cultured in Dulbecco’s modified Eagle’s
medium (HyClone, UT, USA) supplemented with 10% fetal calf serum,
100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, NY, USA).
The other cell lines were cultured in RPMI-1640 medium supplemented
with 10% heat-inactivated fetal bovine serum. All the cells were
incubated at 37°C in a humidified atmosphere of 5%
CO2.
Tissue specimens
From January 1999 to 2002, 8 pairs of specimens for
two dimensional electrophoresis were obtained from surgically
resected ESCC tissues in Cancer Hospital of Chinese Academy of
Medical Sciences (CAMS). Another 50 tissues specimes for
immnohistochemistry were also obtained from surgically resected
esophageal carcinoma in Cancer Hospital of CAMS from January 1999
to 2009. Tissue specimens (n=93) for immunohistochemistry were
purchased as microarray (Outdo Biotech Co., Shanghai, China). All
specimens were frozen immediately, stored at liquid nitrogen. The
median age of the patients of the 143 ESCC tissues for
immunochemistry was 60 years (range, 29–84 years).
Protein extraction and
quantification
Approximately 1×107 cells were grown to
80% confluence and washed six times in 1X phosphate-buffered saline
(PBS). Soluble proteins were extracted with lysis (50 mM Tris-HCl
pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS) with protease
inhibitor cocktail (2.5 μM AEBSF, 0.04 μg/ml aprotinin, 0.04 μg/ml
leupeptin, 1 mM EDTA ) by super-sonication and followed by
centrifugation at 12,000 g for 15 min. Protein concentration was
measured by the Bradford method.
Two dimensional electrophoresis
The soluble proteins from individual tissue specimen
and the pooled tissue samples were separated by two dimensional
electrophoresis (2-DE). Commercial IPG strips (pH 3–10NL, 18 cm;
Amersham Biosciences, Uppsala, Sweden), were rehydrated overnight
with 450 μl solution containing 8 M urea, 2% w/v CHAPS, 20 mM DTT,
0.5% v/v IPG buffer, 0.002% bromophenol blue and 1000 μg protein.
Electrofocusing was carried out for 60 kVh at 20°C following the
manufacturer’s instruction. Prior to the second dimension, the IPG
strips were equilibrated for 30 min with 50 mM Tris-HCl pH 8.8, 6 M
urea, 30% v/v glycerol, 2% w/v SDS, and a trace of bromophenol blue
followed by reduction with 1% of DTT and alkylation with 2.5% of
iodoacetamide. The IPG strips were placed into 12%
SDS-polyacrylamide gels (26×20 cm) and were further electrophoresed
by an EttanII-DE system (Amersham Biosciences) with a programmable
power control, 0.5 h at 0.5 W per gel, then at 15 W per gel until
the dye front reached the gel bottom. The separated proteins were
visualized by Coomassie Brilliant Blue staining.
Protein identification
Briefly, images of the stained gels were acquired
with an Image Scanner (Amersham Biosciences) using transmissive
light. The gel images were first analyzed by eyes and subsequently
were analyzed by ImageMaster 2D Elite 4.01 (Amersham Biosciences).
Protein spots with signals differential intensity reaching 2.0 in
2D gels were excised, then were digested in modified trypsin
solution with a final substrate-to-trypsin ratio of 40:1 (W:W) (in
25 mM ammonium bicarbonate). The digested peptides from 2-DE gel
spots were analyzed by MALDI-TOF-MS using an Ettan-MALDI-TOF system
(Amersham Biosciences). Monoisotopic peptide masses obtained from
MALDI-TOF were used to search the NCBInr protein database using
Mascot algorithm.
Immunohistochemistry
For immunohistochemical staining multiple tissue
arrays (MTA) of formalin-fixed and ESCC and their matched adjacent
normal tissues were incubated with stathmin mAb (3352, Cell
Signaling Technology, UK) or control IgG. After washing with 1X
PBS, slides were reacted with the biotin-labeled second antibody
and then visualized using an ultrasensitive
streptavidin-peroxidases system (Maxim Biotech, Fuzhou, China).
Semi-quantitative criteria of the stathmin immunoreaction were
modified according to previous publications (17,18).
Immunostaining was scored as follows: 0, negative; 1, weak; 2,
moderate; and 3, strong staining. The percentage of stathmin
staining area was graded as 0, no positive staining; 1, <10%; 2,
10–50%; or 3, 50–100%. The staining index was calculated as the
multiples of staining intensity and staining area, as described
(18,19) The staining index <1 was
considered as negative, while 1–4 as weak and >4 as strong.
Western blot analysis
Western blotting was performed as previously
described (20). In briefly,
protein extracted from cell lines and tissues specimens were
separated using 12% SDS-PAGE, then transferred to polyvinylidene
fluoride (PVDF) membranes (Millipore, Bedford, MA). Membranes were
blocked by 10% skim milk in 1X PBS. The membranes were incubated
with the primary antibodies against stathmin (ab52630, Abcam, UK)
or β-actin (Cat. No. A-5316, Sigma, MO) in suitable dilutions.
Secondary antibodies were anti-rabbit IgG and anti-mouse IgG,
respectively. Signals were detected by chemiluminescence using the
ECL kit.
siRNA transfection
The double-strand small interfering RNAs (siRNAs)
were synthesized in duplex and purified forms using Genechem Co.
(Shanghai, China). siRNAs targeting stathmin
[5′-GAAACGAGAGCACGAGAAAtt-3′ (forward) and
5′-UUUCUCGUGCUCUCGUUUCtt-3′ (reverse)] and non-specific scramble
siRNA oligonucletide sequences [5′-UUC UCCGAACGUGUCACGUtt-3′
(forward) and 5′-ACGUGAC ACGUUCGGAGAAtt-3′ (reverse)] were
transfected separately into KYSE30 and KYSE410 cells using
Lipofectamine 2000 (Invitrogen, CA, USA) according to
manufacturer′s protocol. After 72 h, proteins were extracted.
In vitro wound-healing assay
KYSE30, KYSE410 or EC0156 cells were incubated
overnight yielding confluent monolayer for wounding. Wounds were
scratched using a pipette tip, photographs were taken immediately
(time 0 h) and 24 h after wounding. The distance migrated by the
cell monolayer to close the wounded area during this time period
was measured. Photos were taken by Leica DMCI microscope (Leica,
German) at ×100 magnification.
Statistical analysis
Data were analyzed by SPSS 16.0 (SPSS Inc., Chicago,
IL, USA). χ2 test was used to analyze the relationship
between stathmin expression with clinicopathologic characteristics.
P-values <0.05 were considered as statistically significant.
Results
The identification of stathmin in ESCC
tissues
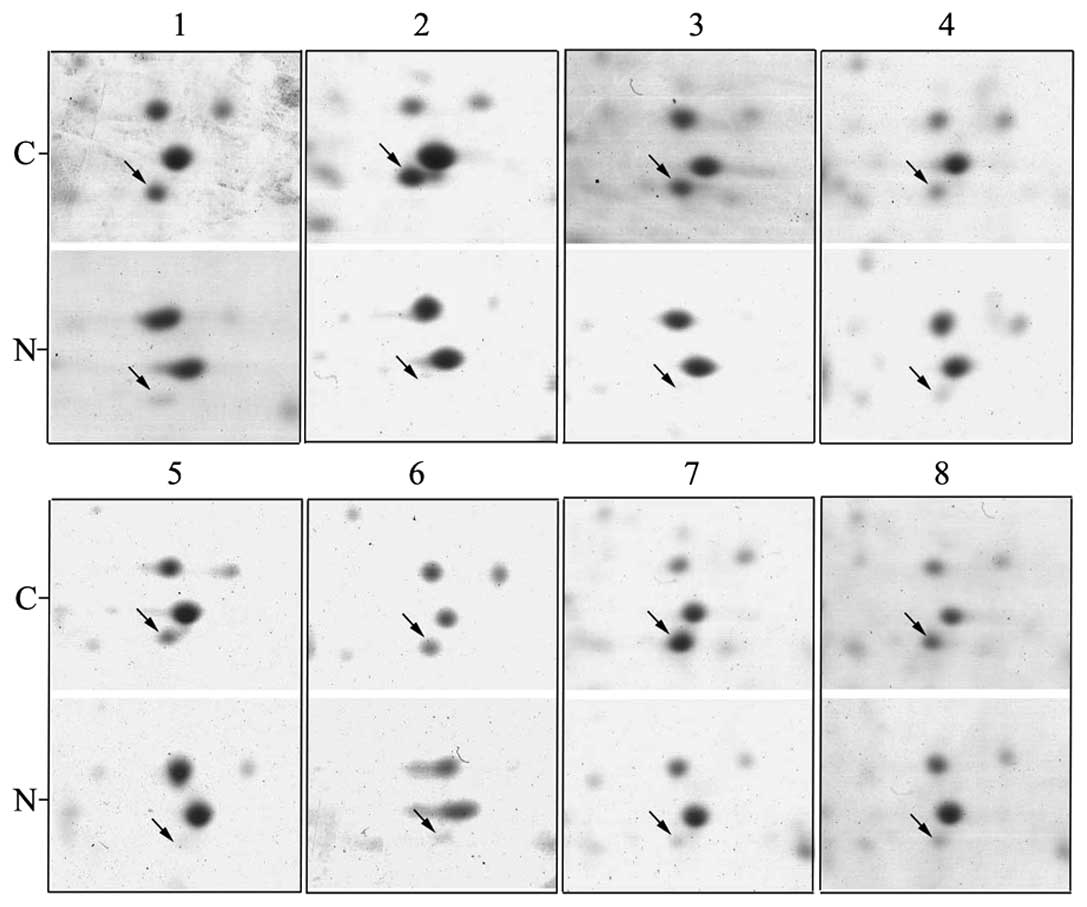
Firstly, we analyzed differientially expressed
proteins using proteomic method between tumor and corresponding
normal tissues in ESCC. Results showed stathmin was detected in all
2-DE gels of the 8 pairs of ESCC tissues (Fig. 1), as an obvious differentially
expressed spot.
Stathmin is overexpressed in ESCC
tissues
Subsequently, we employed immunohistochemistry to
analyze the expression of stathmin in 143 ESCC tissue microarray
using the anti-stathmin antibody. Strong staining was seen in ESCC
tissues, however, the normal tissues were weaker or negatively
stained (Fig. 2). The strong
staining of stathmin in cancer was 70.63% (101/143), while normal
in 27.97% (40/143). In addition, the weak staining of stathmin in
cancer was 20.28% (29/143), while normal in 15.38% (22/143). There
was a significantly different staining mode between cancer and
normal (P<0.05). Statistical analysis showed that overexpression
of stathmin was significantly correlated with histological grade
(P<0.05) (Table I). However, no
correlation was found between stathmin expression and age, gender
and lymph node metastasis (P>0.05) (Table I).
 | Table IThe correlation between stathmin
expression and clinicopathological characteristics in ESCC
specimens. |
Table I
The correlation between stathmin
expression and clinicopathological characteristics in ESCC
specimens.
| | Stathmin | |
|---|
| |
| |
|---|
|
Characteristics | All cases (n)
(%) | Negative (%) | Weak (%) | Strong (%) | P-value |
|---|
| Age | | | | | >0.05 |
| ≥60 | 80 (55.94) | 7 (8.75) | 14 (17.50) | 59 (73.75) | |
| <60 | 63 (44.06) | 6 (9.52) | 15 (23.81) | 42 (66.67) | |
| Gender | | | | | >0.05 |
| Male | 108 (75.52) | 11 (10.19) | 24 (22.22) | 73 (67.59) | |
| Female | 35 (24.48) | 2 (5.71) | 5 (14.29) | 28 (80.00) | |
| Tumor grade | | | | | 0.019 |
| Well
differentiated | 36 (25.17) | 8 (22.22) | 8 (22.22) | 20 (55.56) | |
| Moderately
differentiated | 80 (55.94) | 5 (6.25) | 16 (20.00) | 59 (73.75) | |
| Poor
differentiated | 27 (18.89) | 0 (0.00) | 5 (18.52) | 22 (81.48) | |
| Lymph node
metastasisa | | | | | >0.05 |
| Present | 38 (40.43) | 3 (7.90) | 3 (7.90) | 32 (84.20) | |
| Not present | 56 (59.57) | 2 (3.57) | 7 (12.50) | 47 (83.93) | |
siRNA-mediated reduction in stathmin
expression resulted in impaired cell migration
We chose two ESCC cell lines (KYSE30 and KYSE410) as
a model. SiRNA was employed to knockdown stathmin, and
western blotting to detect the effect of siRNA. The results showed
that the expression of stathmin was reduced after transfection of
siRNA oligonucleotide for 72 h in KYSE30 and KYSE410 (Fig. 3A). Subsequently, wound-healing assay
showed that the speed of wound recovery of siRNA stathmin
was much slower than the scramble in both KYSE30 and KYSE410
(Fig. 3B). The results revealed
that cell migration was impaired when deficient of stathmin.
The phosphorylation of stathmin reduced
the motility of EC0156
Considering phosphorylation had been closely
associtated with the function of stathmin, we wondered whether
stathmin phosphorylation has influence on ESCC cell lines. We
selected EC0156 for the next study.
EC0156 was treated with paclitaxel, a compound
extracted from taxus plants, at gradient dosage from 0 to 1.6
μg/ml. The results revealed that the 19 kDa band of stathmin was
decreased after paclitaxel treatment, whereas a new band at about
21 kDa gradiently increased in a dose-dependent manner. We
confirmed the new band was phosphorylated stathmin at Ser-16. The
tendency increased dramatically between 0.1 and 1.6 μg/ml, reaching
a peak at a dosage of 0.8 μg/ml. Conversely, KYSE30 and EC0156
without treatment had no changes (Fig.
4A). Besides, rounded cells were observed in most of the EC0156
through microscopic analysis (Fig.
4B). The number of cells appered slightly reduced.
Wound-healing assay showed that the speed of wound recovery of
EC0156 cells treated with pacitaxel was much slower than the
control, suggesting that cell migration was impaired after
stabilized phophorylation of stathmin (Fig. 4C).
Discussion
Previously, we analyzed differientially expressed
proteins using proteomic methods between tumor and nomal tissues in
ESCC. We found an obviously overexpressed spot in all tumor 2-DE
gels, which was identified as stathmin. Stathmin is ubiquitous,
highly conserved 19 kDa cytosolic phosphoprotein that regulates
microtubule dynamics (21).
We employed immunohistochemistry to detect the
expression of stathmin in ESCC tissue microarray. Here we
demonstrated strong expression in 77.14% (108/140) of ESCC tissues.
In addition, overexpression of stathmin was significantly
correlated with histological grade (P<0.05). However, no
correlation was found with age, gender and lymph node metastasis
(P>0.05). Wang et al revealed that the positive rate of
stathmin in 75 ESCC samples was 81.3% and the relative contents of
stathmin were significantly correlated with the differentiation
degree, lymph node metastasis, invasive depth and TNM stage of ESCC
(13). In addition, the basic
tendency of stronger stathmin staining in ESCC was consistent with
our previous study, which only used 13 ESCC samples (22). So far, many studies have
demonstrated stathmin to be overexpressed in many human cancers,
including mesothelioma tumor (11),
malignant pheochromocytomas (23,24),
cervical carcinoma (25), primary
nasopharyngeal carcinoma (26),
gastric cancer (27),
hepatocellular carcinoma (28,29),
medulloblastoma (30), endometrial
cancer (18) and urothelial
carcinoma (31). Above all,
overexpression of stathmin was significantly correlated with
clinical stage, tumor grade and lymph node metastasis in cancers,
including cervical carcinoma, nasopharyngeal carcinoma, gastric
cancer, hepatocellular carcinoma and endometrial cancer (18,25–30).
Moreover, stathmin may be regarded as a survival prognosis factor
in ovarian cancer (32),
endometrial cancer (18) and
urothelial carcinoma (31). Our
previous work, although the ESCC cases were only 13 for
immunohistochemistry to analyze the expression of stathmin, we
still found the expression of stathmin was overexpressed in ESCC
tissue, but there was no correlation with tumor grades (22). In the present study, we expanded the
ESCC case numbers for immunohistochemitry. Finally, we found
overexpression of stathmin was significantly correlated with
histological grade. However, the relationship between stathmin
expression and lymph node metastasis did not replicate. There may
be two reasons. The limited cases with lymph node metastasis in
tissue microarray may lead to information being lost in our study.
Another factor worth considered is the tiny difference of the
pathologic criteria. In addition, the case number of the present
study was much greater than the previous reports in ESCC.
We demonstrated that the speed of wound recovery in
stathmin knockdown cells was much slower than the scramble in both
KYSE30 and KYSE410 cells, suggesting cell migration was impaired
when deficient of stathmin. Similar report exists in gastic cancer,
in which cell invasion was significantly reduced by stathmin siRNA
in the Matrigel invasion assay (27). Stathmin depletion with siRNA caused
significant inhibition of lamellipodia formation, which directed by
Pak1-WAVE2-kinesin complex (33).
The lamellipodia formation may promote cell migration and invasion.
Thus, stathmin depletion might inactivate lamellipodia formation
leading to reduction of cell migration.
We demonstrated that the band at 19 kDa of stathmin
was decreased when EC0156 was treated by paclitaxel. Meantime, a
new band at about 21 kDa appeared in a dose-dependent manner.
Subsequently, the new band was confirmed as phosphorylated stathmin
at Ser-16. The basic function of stathmin was closely associated
with its phosphorylated state. The stathmin-tubulin interaction was
dependent on phosphorylation of stathmin (34). Rapidly switching phosphorylation of
stathmin regulated microtubule assembly (21). Phosphorylation at Ser-16 and Ser-63
strongly reduced stathmin-tubulin complex formation (35). The effects of stathmin on dynamic
instability were strongly attenuated by phosphorylation at
Ser-16-and Ser-63 (6). Many protein
kinases, including CDC2 (36), MAP
(37), Auro B (38) and BGLF4 (39), can phosphorylate stathmin. Stathmin
is known to undergo phosphorylation at Ser16 upon cell stimulation,
such as paclitaxel at low concentration (40). Paclitaxel is an anticancer drug
which interferes with microtubules (41). In the present study, we found
paclitaxel may induce stathmin phosphorylation at Ser-16 and
influenced the function of stathmin.
Through microscopic analysis, rounded cells were
observed in most of EC0156 after the treatment with paclitaxel. The
total number EC0156 appeared slightly reduced. Besides, the wound
recovery speed of EC0156 treated with paclitaxel was diminished
compared to the control, suggesting that cell migration may be
impaired after stathmin phophorylation. It is possibly that
stabilized phosphorylation of stathmin may disrupt microtubule
dynamics and finally impaired the motility of EC0156, which were
supported by other reports. Migration can be viewed as a repeated
sequences of events that include formation of pseudopodia
protrusions, attachment and translocation of the cell body in the
direction of the new adhesion sites. The microtubule dynamics
instability is important to generate an asymmetrical microtubules
array (42). Stronger or long time
stabilized phosphorylation of stathmin may impair the microtuble
dynamics. Cernuda-Morollon et al found that T-cell receptor
(TCR)-induced stathmin phosphorylation prevented T cell
polarization on ICAM-1 by increasing Rac1 activity and reduced the
migration of T cell and changed microtubule dynamics leading to
loss of migratory polarity (43).
Di Paolo et al revealed that phosphorylation at three sites
(Ser-16, Ser-25, Ser-63) completely inhibited the tubulin binding
capacity of stathmin (35). This
phenomenon was also observated in cancer cells. Belletti et
al revealed that Ser-16 phosphorylation of stathmin enhanced
sarcoma cell adhesion and inhibited sarcoma cell motility by mutant
methods (44). The present study
revealed that paclitaxel may act as a stimulus factor to induce the
phosphorylaiton of stathmin leading to impairment of microtubule
dynamics. Our observations provide new evidence for understanding
the interaction between stathmin phosphorylation and
microtubules.
Our observation at cell level was not in accordance
with immunohistochemistry analysis. Except for information lost or
cancer diversity, different microenvironment between cultured cells
and tissues may also contribute. In summary, stathmin was
overexpressed in ESCC tissues and overexpression of stathmin was
significantly correlated with histological grade. In addition,
deficiency or stabilized phosphorylation of stathmin both impaired
the migration of ESCC cells. The present study might highlight the
potential of stathmin in the therapy and diagnosis of ESCC.
Acknowledgements
We thank Dr Rong Wang in Mount Sinai School of
Medicine and Dr Si-qi Liu in Beijing Genomics Institute for
critical suggestion. This study was supported by National High-Tech
R&D Program (No. 2012AA020206, 2012AA02A503), SKPBR (No.
2011CB910703) and NSFC (No. 91029725, 81071789, 81071811) of
China.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Lin DC, Du XL and Wang MR: Protein
alterations in ESCC and clinical implications: a review. Dis
Esophagus. 22:9–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinmetz MO: Structure and thermodynamics
of the tubulin-stathmin interaction. J Struct Biol. 158:137–147.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Belmont LD and Mitchison TJ:
Identification of a protein that interacts with tubulin dimers and
increases the catastrophe rate of microtubules. Cell. 84:623–631.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cassimeris L: The oncoprotein 18/stathmin
family of microtubule destabilizers. Curr Opin Cell Biol. 14:18–24.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manna T, Thrower DA, Honnappa S, Steinmetz
MO and Wilson L: Regulation of microtubule dynamic instability in
vitro by differentially phosphorylated stathmin. J Biol Chem.
284:15640–15649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marklund U, Larsson N, Gradin HM,
Brattsand G and Gullberg M: Oncoprotein 18 is a
phosphorylation-responsive regulator of microtubule dynamics. EMBO
J. 15:5290–5298. 1996.PubMed/NCBI
|
|
8
|
Amayed P, Pantaloni D and Carlier MF: The
effect of stathmin phosphorylation on microtubule assembly depends
on tubulin critical concentration. J Biol Chem. 277:22718–22724.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niethammer P, Bastiaens P and Karsenti E:
Stathmin-tubulin interaction gradients in motile and mitotic cells.
Science. 303:1862–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Lu H, Shi H, et al: PUMA
overexpression induces reactive oxygen species generation and
proteasome-mediated stathmin degradation in colorectal cancer
cells. Cancer Res. 65:1647–1654. 2005. View Article : Google Scholar
|
|
11
|
Kim JY, Harvard C, You L, et al: Stathmin
is overexpressed in malignant mesothelioma. Anticancer Res.
27:39–44. 2007.PubMed/NCBI
|
|
12
|
Nakashima D, Uzawa K, Kasamatsu A, et al:
Protein expression profiling identifies maspin and stathmin as
potential biomarkers of adenoid cystic carcinoma of the salivary
glands. Int J Cancer. 118:704–713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Wang LX, He W, Zhu LN, Zhao PR and
Fan QX: Expression of stathmin in esophageal squamous cell
carcinoma and its biological significance. Nan Fang Yi Ke Da Xue
Xue Bao. 30:1552–1557. 2010.(In Chinese).
|
|
14
|
Wang X, Fan QX, Zhao PR and Wang F:
Expression and significance of stathmin in esophageal squamous cell
carcinoma. J Basic Clin Oncol. 120:372–374. 2007.
|
|
15
|
Wang F, Wang LX, Fan QX and Zhao PR:
Inhibitory effects of ASODN of stathmin gene on cultured Eca109
cell lines. J Zhengzhou University (Medical Sciences). 43:414–418.
2008.
|
|
16
|
Wang Q, Xu Y, Zhao X, et al: A facile
one-step in situ functionalization of quantum dots with preserved
photoluminescence for bioconjugation. J Am Chem Soc. 129:6380–6381.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kouzu Y, Uzawa K, Koike H, et al:
Overexpression of stathmin in oral squamous-cell carcinoma:
correlation with tumour progression and poor prognosis. Br J
Cancer. 94:717–723. 2006.PubMed/NCBI
|
|
18
|
Trovik J, Wik E, Stefansson IM, et al:
Stathmin overexpression identifies high risk patients and lymph
node metastasis in endometrial cancer. Clin Cancer Res.
17:3368–3377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salvesen HB, Das S and Akslen LA: Loss of
nuclear p16 protein expression is not associated with promoter
methylation but defines a subgroup of aggressive endometrial
carcinomas with poor prognosis. Clin Cancer Res. 6:153–159.
2000.PubMed/NCBI
|
|
20
|
Sun Y, Mi W, Cai J, et al: Quantitative
proteomic signature of liver cancer cells: tissue transglutaminase
2 could be a novel protein candidate of human hepatocellular
carcinoma. J Proteome Res. 7:3847–3859. 2008. View Article : Google Scholar
|
|
21
|
Rana S, Maples PB, Senzer N and Nemunaitis
J: Stathmin 1: a novel therapeutic target for anticancer activity.
Expert Rev Anticancer Ther. 8:1461–1470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Liu F, Sun YL and Zhao XH:
Significance of STMN1 expression in esophageal squamous cell
carcinoma. World Chin J Digestol. 18:1306–1312. 2010.
|
|
23
|
Sadow PM, Rumilla KM, Erickson LA and
Lloyd RV: Stathmin expression in pheochromocytomas, paragangliomas,
and in other endocrine tumors. Endocr Pathol. 19:97–103. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bjorklund P, Cupisti K, Fryknas M, et al:
Stathmin as a marker for malignancy in pheochromocytomas. Exp Clin
Endocrinol Diabetes. 118:27–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xi W, Rui W, Fang L, Ke D, Ping G and
Hui-Zhong Z: Expression of stathmin/op18 as a significant
prognostic factor for cervical carcinoma patients. J Cancer Res
Clin Oncol. 135:837–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng AL, Huang WG, Chen ZC, et al:
Identification of novel nasopharyngeal carcinoma biomarkers by
laser capture microdissection and proteomic analysis. Clin Cancer
Res. 14:435–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeon TY, Han ME, Lee YW, et al:
Overexpression of stathmin1 in the diffuse type of gastric cancer
and its roles in proliferation and migration of gastric cancer
cells. Br J Cancer. 102:710–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan RH, Jeng YM, Chen HL, et al: Stathmin
overexpression cooperates with p53 mutation and osteopontin
overexpression, and is associated with tumour progression, early
recurrence, and poor prognosis in hepatocellular carcinoma. J
Pathol. 209:549–558. 2006. View Article : Google Scholar
|
|
29
|
Gan L, Guo K, Li Y, et al: Up-regulated
expression of stathmin may be associated with hepatocarcinogenesis.
Oncol Rep. 23:1037–1043. 2010.PubMed/NCBI
|
|
30
|
Kuo MF, Wang HS, Kuo QT, et al: High
expression of stathmin protein predicts a fulminant course in
medulloblastoma. J Neurosurg Pediatr. 4:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin WC, Chen SC, Hu FC, et al: Expression
of stathmin in localized upper urinary tract urothelial carcinoma:
correlations with prognosis. Urology. 74:1264–1269. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su D, Smith SM, Preti M, et al: Stathmin
and tubulin expression and survival of ovarian cancer patients
receiving platinum treatment with and without paclitaxel. Cancer.
115:2453–2463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takahashi K and Suzuki K: Membrane
transport of WAVE2 and lamellipodia formation require Pak1 that
mediates phosphorylation and recruitment of stathmin/Op18 to
Pak1-WAVE2-kinesin complex. Cell Signal. 21:695–703. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ravelli RB, Gigant B, Curmi PA, et al:
Insight into tubulin regulation from a complex with colchicine and
a stathmin-like domain. Nature. 428:198–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di Paolo G, Antonsson B, Kassel D,
Riederer BM and Grenningloh G: Phosphorylation regulates the
microtubule-destabilizing activity of stathmin and its interaction
with tubulin. FEBS Lett. 416:149–152. 1997.PubMed/NCBI
|
|
36
|
Moreno FJ and Avila J: Phosphorylation of
stathmin modulates its function as a microtubule depolymerizing
factor. Mol Cell Biochem. 183:201–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Antonsson B, Kassel DB, Ruchti E and
Grenningloh G: Differences in phosphorylation of human and chicken
stathmin by MAP kinase. J Cell Biochem. 80:346–352. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gadea BB and Ruderman JV: Aurora B is
required for mitotic chromatin-induced phosphorylation of
Op18/Stathmin. Proc Natl Acad Sci USA. 103:4493–4498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen PW, Lin SJ, Tsai SC, et al:
Regulation of microtubule dynamics through phosphorylation on
stathmin by Epstein-Barr virus kinase BGLF4. J Biol Chem.
285:10053–10063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andersen SS: Spindle assembly and the art
of regulating microtubule dynamics by MAPs and Stathmin/Op18.
Trends Cell Biol. 10:261–267. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mistry SJ, Bank A and Atweh GF:
Synergistic antiangiogenic effects of stathmin inhibition and taxol
exposure. Mol Cancer Res. 5:773–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Honore S, Pasquier E and Braguer D:
Understanding microtubule dynamics for improved cancer therapy.
Cell Mol Life Sci. 62:3039–3056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cernuda-Morollon E, Millan J, Shipman M,
Marelli-Berg FM and Ridley AJ: Rac activation by the T-cell
receptor inhibits T cell migration. PLoS One. 5:e123932010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Belletti B, Nicoloso MS, Schiappacassi M,
et al: Stathmin activity influences sarcoma cell shape, motility,
and metastatic potential. Mol Biol Cell. 19:2003–2013. 2008.
View Article : Google Scholar : PubMed/NCBI
|