Introduction
Whole-cell vaccines are a method for providing
target antigens. In this approach, the whole-tumor cell is the
source of immunogens with which to induce an antitumor immune
response. The advantage of using whole-tumor cell vaccine is that a
broad array of tumor-associated antigens (TAAs) is represented,
thereby minimizing immune escape. As whole proteins are present,
there are no HLA restrictions on who can receive the vaccine.
However, it is not ideal to use live pathogens as vaccines due to
safety concerns. Killed but maintained immunogenicity via
γ-irradiation has been reported to have the benefit of vaccines to
induce an appropriate immune response without the issue of pathogen
replication in the host (1–5). Meanwhile, the weak immunogenicity of
many tumors also represents a barrier to the effective induction of
antitumor immunity. Reportedly, cancer cells or other bystander
cells included in the vaccine are transfected with vectors
containing genes that express potent immunostimulating proteins or
cytokines, including B7.1 (CD80), GM-CSF and CCL21 (6–11).
Vascular endothelial growth factor receptor-2
(VEGFR2)is an important receptor responsible for the angiogenic
activity of VEGF (12,13). Overexpression of VEGFR2 is found on
activated endothelial cells of newly formed vessels and is strongly
associated with invasion and metastasis in many types of cancer
(14–16). In addition, it has been reported
that the inhibition of tumor growth and metastasis in many animal
models has been achieved by various techniques that disrupt or
neutralize the functions of either VEGF or VEGFR-2 (17–19).
In our previous research, a xenogeneic homologous VEGFR2 protein
vaccine (qVEGFR) effectively inhibited the tumor growth in LL/2
Lewis lung carcinoma, CT26 colon carcinoma, and Meth A fibrosarcoma
models (20). It is, thus, clear
that the breaking of the immune tolerance against VEGFR2 of
autologous angiogenic endothelial cells is an effective pathway for
cancer therapy with active immunity.
In the present study, we explored the therapeutic
efficacy of an irradiated AdVEGFR2-infected autologous whole-cell
tumor vaccination in the weakly immunogenic and highly metastatic
4T1 murine mammary cancer model. Moreover, we also further
discussed its possible mechanism.
Materials and methods
Cell lines and mice
The 293A (human embryonic kidney) and 4T1 cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA). Cells were cultured in DMEM supplemented with 10% fetal
bovine serum (FBS) and 10 μg/ml gentamicin sulfate, maintained in a
37°C incubator with a humidified 5% CO2 atmosphere. Six-
to 8-week-old female BALB/c mice were obtained from the Laboratory
Animal Center of Sichuan University and maintained in pathogen-free
conditions. All procedures were approved by the institute's Animal
Care and Use Committee.
Construction of recombinant adenoviral
vectors
A recombinant adenovirus carrying the VEGFR2 gene
(AdVEGFR2) was constructed by use of the AdEasy system from
Qbiogene, Inc., according to the procedure provided by the
manufacturer. The recombinant adenovirus without a foreign gene
(Adnull) served as a control. All virus particles were amplified in
HEK 293A cells and titrated as PFU/ml and stored at −80°C.
Preparation of whole-cell vaccines
To vaccinate mice, 4T1 cells were infected by
AdVEGFR2 or Adnull (control) at a MOI of 100 in serum-free
RPMI-1640. Cells were incubated at 37°C for 48 h. Infected cells
and uninfected 4T1 cells were irradiated with 100 Gy and
subsequently injected s.c. into the flanks of the mice.
Breast tumor model and immunotherapy
4T1 breast cancer models were established in BALB/c
mice. Six mice in each group were challenged with 1×106
4T1 cells s.c. in the right flank. For vaccination, 4T1 cells were
infected with AdVEGFR2 or Adnull or were uninfected, and then
irradiated with 100 Gy of X-rays. Irradiated cells were washed
extensively with PBS and vaccination was carried out by s.c.
injection of 1×106 cells, 3 times, on Days 7, 21 and 28
in the left flank of mice after tumor cell inoculation. Additional
control animals were injected with 0.9% NaCl solution. Thus, mice
were divided into 4 groups: the irradiated AdVEGFR2-infected 4T1
cell-treated group (4T1-AdVEGFR2 group), the irradiated
Adnull-infected 4T1 cell-treated group (4T1-Adnull group), the
irradiated 4T1 cell-treated group (4T1 group) and the
saline-treated group (NS group). Tumor dimensions were measured
with calipers every 3 days, and the tumor volume (V) was calculated
according to the following formula: V = 0.52 × length ×
width2.
Adoptive transfer in vivo
Ten mice in each group were immunized with
1×106 irradiated AdVEGFR2-infected 4T1 cells,
Adnull-infected 4T1 cells, 4T1 cells or NS s.c. in the right flank
3 times on Days 1, 14 and 28. Sera derived from the mice on Day 7
after the third immunization were adoptively and intraperitoneally
transferred 1 day (100 μl serum/mouse) before mice were challenged
with 1×106 4T1 cells s.c. in the right flank and then
were treated once per day for 10 days. In addition, isolated spleen
lymphocytes from the immunized mice were adoptively and
intravenously transferred (1×106 cells/100 μl/mouse) and
were then treated twice per week for 2 weeks. Tumor dimensions were
measured with calipers every 4 days for 29 days, and tumor volume
(V) was calculated according to the following formula: V = 0.52 ×
length × width2.
Histological analysis
Tumors from each group were embedded in paraffin,
and sections (3–5 μm) were immunohistochemically stained to
determine the infiltration of lymphocytes and quantify the
microvessel density in the tumor tissue using rat anti-mouse CD4
antibody, rat anti-mouse CD8 antibody and rabbit anti-mouse CD31
antibody (Abcam, Inc.). Vascular density was quantified by counting
the number of microvessels per high power field. Images were
acquired using an Olympus BX60 microscope.
Quantitative assessment of apoptosis
Tumor species were prepared as previously described
(21). The presence of apoptotic
cells within the tumor sections was determined using the In Situ
Cell Death Detection kit (DeadEnd™ Fluorometric TUNEL System,
Promega, Madison, WI, USA), according to the manufacturer's
protocol. In tissue sections, five high power fields were randomly
chosen and analyzed. The apoptotic index (AI) was defined as
follows: AI (%) = 100 × apoptotic cells/total tumor cells. Images
were acquired using a LEICA DM2500 microscope.
Western blot analysis
The 293A cells infected with AdVEGFR2 or Adnull for
48 h were lysed to analysis the expression of VEGFR2 using rabbit
anti-mouse VEGFR2 antibody (Abcam). 4T1 cells infected with
AdVEGFR2 or Adnull for 48 h and uninfected 4T1 cells were
irratiated with 100 Gy, and their lysates were subjected to western
blot analysis with rabbit anti-mouse HMGB1 and HSP70
antibodies.
FCAS
For FACS analysis, we prepared single-cell
suspensions of tumors from 4T1-AdVEGFR2-treated or
4T1-Adnull-treated mice. Briefly, tumors were minced using a razor
blade and digested with collagenase I for 30 min at 37°C. For
extracellular staining of immune markers, 5×105 of
freshly prepared cells were stained with PE CD4 and FITC CD8.
Fluorescence data were collected on FACScalibur and analyzed using
cell quest software (BD Biosciences).
ELISA
For ELISA, 96-well plates were coated with 4T1 cells
(1×104 cells/well) in 10% RPMI-1640 overnight at 4°C.
Plates were washed with PBST (0.05% Tween 20 in PBS) and were fixed
in 10% formalin for 15 min at room temperature. Then, plates were
washed with ddH2O and blocked for 1 h at 37°C with 200
μl/well 1% bovine serum albumin (BSA) in PBST. Mouse sera from
treated mice diluted serially in PBS were added for 2 h at 37°C,
followed by a dilution of anti-mouse immunoglobulin G (IgG)
subclass or anti-IgM or anti-IgA antibody conjugated to alkaline
phosphatase. Enzyme activity was measured using an enzyme-linked
immunosorbent assay (ELISA) reader (Multiskan MK3).
Statistical analysis
SPSS 16.0 was used for statistical analysis. Data
are expressed as means ± SD. The statistical analysis in all the
experiments was performed using one-way analysis of variance
(ANOVA). P-value <0.05 was considered to indicate a
statistically significant result.
Results
Construction of the recombinant
VEGFR2-expressing adenovirus
Positive clones were confirmed by restriction enzyme
analysis and DNA sequencing. The PacI-digested pAdVEGFR2
plasmid was transfected into 293 cells. At the early stage, cells
producing the adenovirus first appear as patches of rounding, dying
cells. As the infection proceeded, cells containing the viral
particles lysed and infected neighboring cells. A plaque began to
form. On Days 8–10 post-transfection, the infected neighboring
cells lysed, forming a plaque that was clearly visible (Fig. 1A). The expression of VEGFR2 in the
AdVEGFR2-infected 293 cells was detected using western blotting
(Fig. 1B).
Induction of therapeutic antitumor
immunity
We tested the therapeutic efficacy of lethally
irradiated AdVEGFR2-infected 4T1 cells used as vaccines in
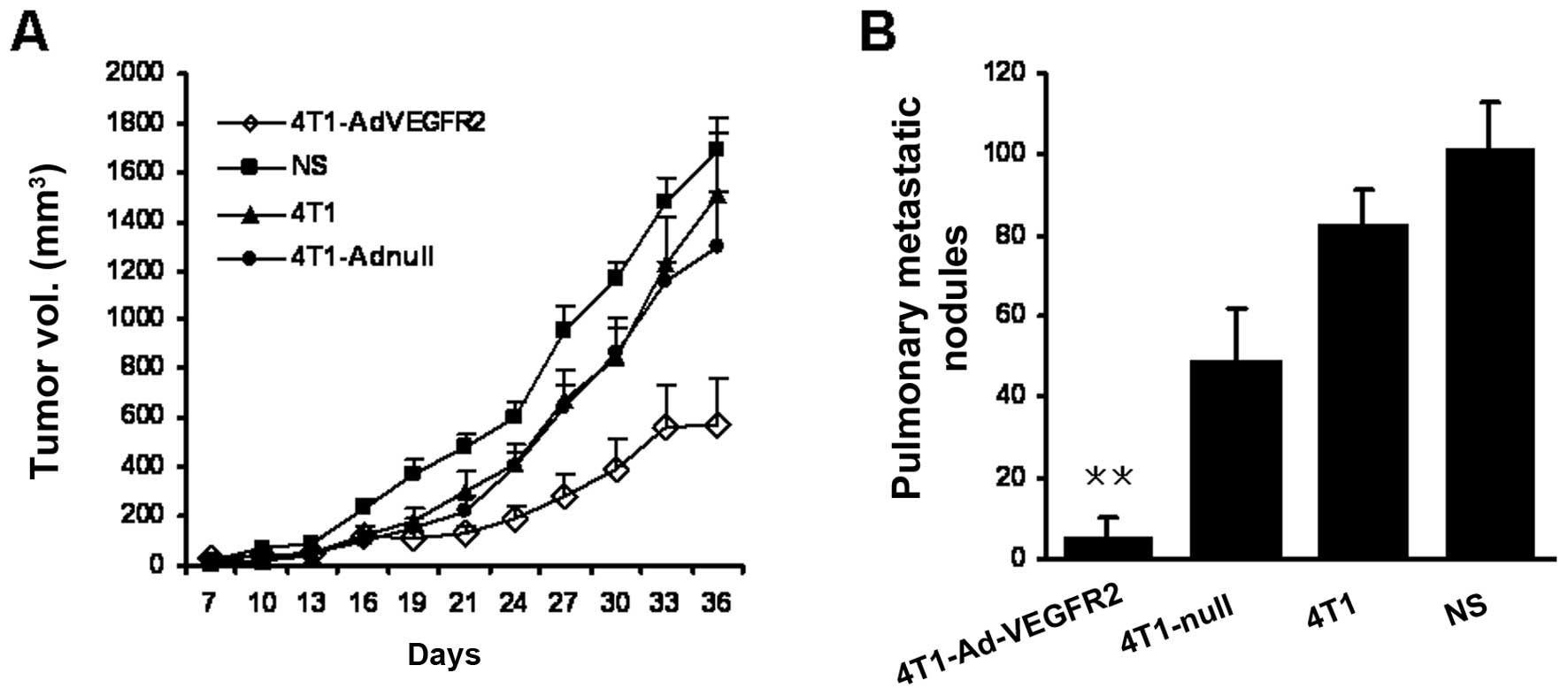
established tumors. We treated the mice on Day 7 after 4T1 cell
inoculation, when the tumors were visible and palpable. Following
treatment with the vaccine 3 times on Days 7, 21 and 28, the size
of the tumor nodes in the 4T1-AdVEGFR2-treated group was
significantly smaller in comparison with those in the control
groups starting on Day 24 (P<0.05) (Fig. 2A). Furthermore, lung metastatic
nodules of mice sacrificed at the termination of the experiment
were counted under a dissecting microscope. Lung metastatic nodules
in the 4T1-AdVEGFR2-treated group were nearly absent compared with
the control groups (P<0.05) (Fig.
2B). We monitored the mice treated with the vaccines every 3
days throughout the entire experiment. No severe toxic effects were
observed in terms of gross measures, such as weight loss, ruffling
of fur and feeding. Thus, the therapy with the irradiated
AdVEGFR2-infected cell vaccine not only inhibited the growth of the
implanted tumors, but also restrained tumor metastasis.
Induction of tumor apoptosis and
inhibition of tumor angiogenesis
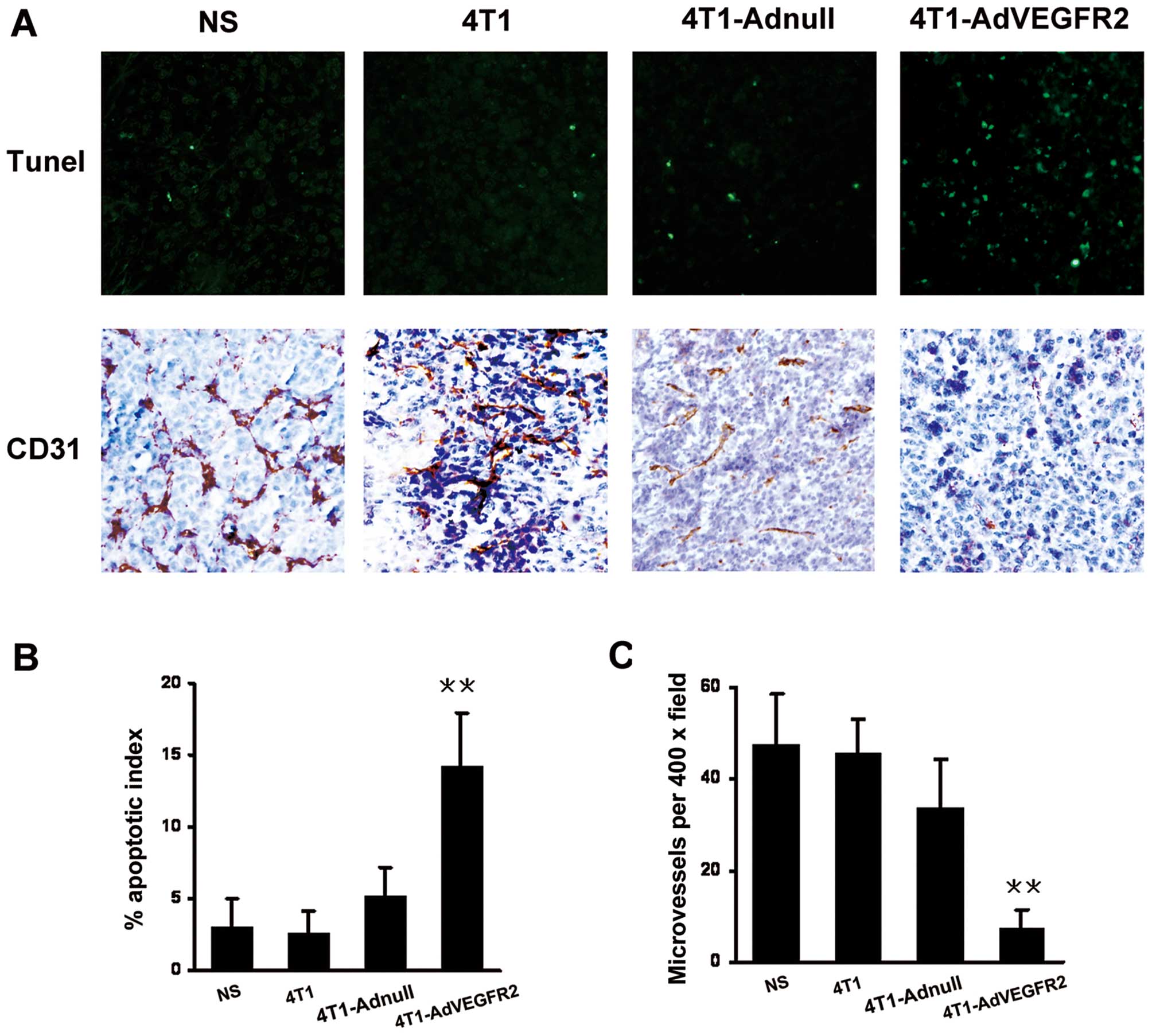
To explore the role of the irradiated
AdVEGFR2-infected 4T1 cell vaccine on the apoptosis of tumor cells,
TUNEL assay of tumor sections was performed. As shown in Fig. 3A, within a similar high-power field,
more apoptotic cells were noted in the tumor tissues from the
4T1-AdVEGFR2-treated mice, and the differences were significant
compared with those of the control groups (P<0.001) (Fig. 3B). As VEGFR2 is closely related to
tumor angiogenic blood vessels, we hypothesized that the therapy
with the irradiated AdVEGFR2-infected 4T1 cell vaccine would act
partly via an antiangiogenic mechanism to promote tumor regression.
Thus, we investigated the microvessel density in the tumor sections
by immunohistochemistry using an antibody specific for CD31.
Results showed that the 4T1 tumor regression after irradiated
AdVEGFR2-infected 4T1 cell vaccine treatment was accompanied by a
corresponding decrease in microvessel density compared with the
controls (P<0.001) (Fig.
3C).
Cellular and humoral immune response in
irradiated AdVEGFR2-infected 4T1 cell vaccine-induced antitumor
activity
To explore the possible mechanism through which the
antitumor activity was induced by the irradiated AdVEGFR2-infected
4T1 cell vaccine, anti-CD4 and anti-CD8 monoclonal antibodies were
used in immunohistochemical staining and FACS. As shown in Fig. 4A, the infiltration of
CD4+ and CD8+ T lymphocytes was apparently
increased in the 4T1-AdVEGFR2-treated group. Results from FACS
indicated the number of CD8+ lymphocytes was increased
by 63.8% and the number of CD4+ lymphocyte cells was
increased 53.4% in the 4T1-AdVEGFR2-treated group compared with the
4T1-Adnull-treated group (Fig. 4B).
These results indicate that both CD4+ and
CD8+ lymphocytes are important for the therapeutic
activity of the 4T1-AdVEGFR2 vaccine against 4T1 breast tumors. To
identify the autoantibodies against 4T1 cells within sera from
treated mice, we investigated the sera by ELISA. The autoantibodies
were increased in the 4T1-AdVEGFR2-treated group (8566.667±351.1885
pg/ml) when compared with the control groups (P<0.001) (Fig. 4C).
Serum and lymphocyte adoptive transfer in
vivo
Given that the autoantibodies and T lymphocytes were
increased in the 4T1-AdVEGFR2-treated mouse blood and tumor
sections, we sought to investigate the protection from tumor growth
of serum and lymphocyte adoptive transfer. As expected, treatment
with lymphocytes from the spleens of the mice immunized with the
irradiated AdVEGFR2-infected 4T1 cell vaccine resulted in apparent
inhibition of tumor growth, compared with those from mice immunized
with 4T1-Adnull, 4T1 or NS (Fig.
5A). Yet, the adoptive transfer of sera from mice immunized
with 4T1-AdVEGFR2 did not effectively inhibit tumor growth
(Fig. 5B). These results indicated
that the immune responses to the irradiated AdVEGFR2-infected cell
vaccine were mainly cellular immune responses.
Expression of HMGB1 and HSP70 in tumor
cells infected with AdVEGFR2
Reportedly, HSP70 and the alarmin
high-mobility-group box 1 protein (HMGB1) are involved in the
activation of tumor antigen-specific T-cell immunity (22–25).
Our findings showed that the therapeutic antitumor immunity of the
vaccine was mainly cellular immunity. Thus, we investigated the
expression of HMGB1 and HSP70 by western blot analysis in
whole-cell lysates of irradiated 4T1 tumors infected with AdVEGFR2,
Adnull or uninfected. As shown in Fig.
6, the surface expression of HMGB1 and HSP70 in irradiated
AdVEGFR2-transfected 4T1 cell tumors was obviously increased, and
HSP70 was scarcely expressed in the groups treated with 4T1
cells.
Discussion
In the present study, we demonstrated that the
immunotherapy based on the irradiated AdVEGFR2-infected 4T1 cell
vaccine had an increased antitumor effect when compared with the
irradiated Adnull-infected 4T1 cell or irradiated 4T1 cell
vaccines. In vivo, irradiated AdVEGFR2-infected 4T1 cell
vaccine significantly prevented local tumor growth and pulmonary
metastasis. The autoantibodies against 4T1 cells were increased in
the vaccine-treated mouse sera, yet the antitumor activity was not
caused by the adoptive transfer of sera. Instead, the adoptive
transfer of spleen lymphocytes caused an apparent antitumor
activity. The number of CD4+ and CD8+ T
lymphocytes was increased in the tumors treated with the irradiated
AdVEGFR2-infected cell vaccine, and angiogenesis was markedly
inhibited. The surface exposures of HMGB1 and HSP70 in the 4T1
cells were apparently increased in vitro. The antitumor
mechanisms of the vaccine may be due to induction of celluar
immunity by targeted inhibition of tumor cells and tumor
vessels.
Whole-cell vaccines are characterized by their broad
array of tumor-associated antigens (26,27).
Vaccination with irradiated tumor cells has been studied in various
animal models as early as the 1970s. Yet, tumor cells are not very
immunogenic, thus many proteins or cytokines were infected into
tumor cells to stimulate immunogenicity. These immunostimulating
proteins include B7.1 (CD80), CCL21 and GM-CSF (8–11).
Reportedly, vaccination with irradiated tumor cells engineered to
secrete murine GM-CSF stimulated potent, specific, and long-lasting
antitumor immunity (28).
Angiogenesis is important not only for normal
embryonic development but also for the development of pathologic
conditions such as cancer, retinopathies and rheumatoid arthritis
(29–32). There is accumulating evidence that
the growth and persistence of solid tumors and their metastasis are
angiogenesis-dependent (14,33,34).
VEGFR-2 is the main receptor responsible for the angiogenic
activity of VEGF. Antiangiogenic therapy targeting VEGFR2
represents a good alternative for the treatment of tumors (35,36).
Our previous studies demonstrated that a quail homologous VEGFR2
protein vaccine effectively induced protective and therapeutic
antitumor immunity in several solid and hematopoietic tumor models
in mice (20), which suggested that
VEGFR2 gene therapy warrants further research.
In the present study, 4T1 cells were infected with
the VEGFR2 gene, which synchronously stimulating the immune
response to tumor cells and tumor vessels. The increase in
CD4+ and CD8+ T lymphocytes in tumors after
treatment with the AdVEGFR2-infected cell vaccine showed that
cellular immunity was involved in the antitumor immune response,
which was further confirmed by the significant inhibition of tumor
growth by spleen lymphocyte adoptive transfer. Reportedly, the
activation of tumor antigen-specific T-cell immunity involves
secretion or surface exposure of the high-mobility-group box 1
(HMGB1) alarmin protein and HSP70 by dying tumor cells (22–25).
Our results demonstrated that HMGB1 and HSP70 were upregulated in
the irradiated AdVEGFR2-infected 4T1 cells. Thus, cell immunity
played an important role in the irradiated infected VEGFR2
whole-cell vaccine treatment.
Collectively, our data in the present study suggest
that immunotherapy with AdVEGFR2 whole-cell vaccine was effective
for therapeutic antitumor immunity in a breast tumor model. This
antitumor effect may result from eliciting the host CTL response
against 4T1 cells and tumor vessels. These findings may be of
importance in further exploration of the potential application of
this vaccine in the treatment of breast cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30901773).
References
|
1
|
Perez CA, Fu A, Onishko H, Hallahan DE and
Geng L: Radiation induces an antitumour immune response to mouse
melanoma. Int J Radiat Biol. 85:1126–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chakravarty PK, Guha C, Alfieri A, et al:
Flt3L therapy following localized tumor irradiation generates
long-term protective immune response in metastatic lung cancer: its
implication in designing a vaccination strategy. Oncology.
70:245–254. 2006. View Article : Google Scholar
|
|
3
|
Nakajima K, Yanagawa T, Watanabe H and
Takagishi K: Hyperthermia reduces migration of osteosarcoma by
suppression of autocrine motility factor. Oncol Rep. 28:1953–1958.
2012.PubMed/NCBI
|
|
4
|
Weiss EM, Frey B, Rodel F, et al: Ex vivo-
and in vivo-induced dead tumor cells as modulators of antitumor
responses. Ann NY Acad Sci. 1209:109–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Das A and Ali N: Vaccine prospects of
killed but metabolically active Leishmania against visceral
leishmaniasis. Expert Rev Vaccines. 11:783–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dols A, Smith JW II, Meijer SL, et al:
Vaccination of women with metastatic breast cancer, using a
costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic,
breast cancer cell line: clinical and immunological results. Hum
Gene Ther. 14:1117–1123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riedl K, Baratelli F, Batra RK, et al:
Overexpression of CCL-21/secondary lymphoid tissue chemokine in
human dendritic cells augments chemotactic activities for
lymphocytes and antigen presenting cells. Mol Cancer. 2:352003.
View Article : Google Scholar
|
|
8
|
Li B, VanRoey M, Wang C, Chen TH, Korman A
and Jooss K: Anti-programmed death-1 synergizes with granulocyte
macrophage colony-stimulating factor-secreting tumor cell
immunotherapy providing therapeutic benefit to mice with
established tumors. Clin Cancer Res. 15:1623–1634. 2009. View Article : Google Scholar
|
|
9
|
van Elsas A, Hurwitz AA and Allison JP:
Combination immunotherapy of B16 melanoma using anti-cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage
colony-stimulating factor (GM-CSF)-producing vaccines induces
rejection of subcutaneous and metastatic tumors accompanied by
autoimmune depigmentation. J Exp Med. 190:355–366. 1999.
|
|
10
|
van den Eertwegh AJ, Versluis J, van den
Berg HP, et al: Combined immunotherapy with granulocyte-macrophage
colony-stimulating factor-transduced allogeneic prostate cancer
cells and ipilimumab in patients with metastatic
castration-resistant prostate cancer: a phase 1 dose-escalation
trial. Lancet Oncol. 13:509–517. 2012.
|
|
11
|
Guckel B, Stumm S, Rentzsch C, Marme A,
Mannhardt G and Wallwiener D: A CD80-transfected human breast
cancer cell variant induces HER-2/neu-specific T cells in
HLA-A*02-matched situations in vitro as well as in vivo. Cancer
Immunol Immunother. 54:129–140. 2005.PubMed/NCBI
|
|
12
|
Gille H, Kowalski J, Li B, et al: Analysis
of biological effects and signaling properties of Flt-1 (VEGFR-1)
and KDR (VEGFR-2). A reassessment using novel receptor-specific
vascular endothelial growth factor mutants. J Biol Chem.
276:3222–3230. 2001. View Article : Google Scholar
|
|
13
|
Zeng H, Dvorak HF and Mukhopadhyay D:
Vascular permeability factor (VPF)/vascular endothelial growth
factor (VEGF) peceptor-1 down-modulates VPF/VEGF
receptor-2-mediated endothelial cell proliferation, but not
migration, through phosphatidylinositol 3-kinase-dependent
pathways. J Biol Chem. 276:26969–26979. 2001. View Article : Google Scholar
|
|
14
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
15
|
Vajkoczy P, Farhadi M, Gaumann A, et al:
Microtumor growth initiates angiogenic sprouting with simultaneous
expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin
Invest. 109:777–785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Breier G, Blum S, Peli J, et al:
Transforming growth factor-beta and Ras regulate the
VEGF/VEGF-receptor system during tumor angiogenesis. Int J Cancer.
97:142–148. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prewett M, Huber J, Li Y, et al:
Antivascular endothelial growth factor receptor (fetal liver kinase
1) monoclonal antibody inhibits tumor angiogenesis and growth of
several mouse and human tumors. Cancer Res. 59:5209–5218. 1999.
|
|
18
|
Wood JM, Bold G, Buchdunger E, et al:
PTK787/ZK 222584, a novel and potent inhibitor of vascular
endothelial growth factor receptor tyrosine kinases, impairs
vascular endothelial growth factor-induced responses and tumor
growth after oral administration. Cancer Res. 60:2178–2189.
2000.
|
|
19
|
Thomas AL, Morgan B, Drevs J, et al:
Vascular endothelial growth factor receptor tyrosine kinase
inhibitors: PTK787/ZK 222584. Semin Oncol. 30:32–38. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JY, Wei YQ, Yang L, et al:
Immunotherapy of tumors with vaccine based on quail homologous
vascular endothelial growth factor receptor-2. Blood.
102:1815–1823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li G, Tian L, Hou JM, et al: Improved
therapeutic effectiveness by combining recombinant CXC chemokine
ligand 10 with cisplatin in solid tumors. Clin Cancer Res.
11:4217–4224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seong SY and Matzinger P: Hydrophobicity:
an ancient damage-associated molecular pattern that initiates
innate immune responses. Nat Rev Immunol. 4:469–478. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang D, Liang J, Fan J, et al: Regulation
of lung injury and repair by Toll-like receptors and hyaluronan.
Nat Med. 11:1173–1179. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsung A, Sahai R, Tanaka H, et al: The
nuclear factor HMGB1 mediates hepatic injury after murine liver
ischemia-reperfusion. J Exp Med. 201:1135–1143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Apetoh L, Ghiringhelli F, Tesniere A, et
al: Toll-like receptor 4-dependent contribution of the immune
system to anticancer chemotherapy and radiotherapy. Nat Med.
13:1050–1059. 2007. View
Article : Google Scholar
|
|
26
|
Soliman H: Developing an effective breast
cancer vaccine. Cancer Control. 17:183–190. 2010.PubMed/NCBI
|
|
27
|
Solbrig CM, Saucier-Sawyer JK, Cody V,
Saltzman WM and Hanlon DJ: Polymer nanoparticles for immunotherapy
from encapsulated tumor-associated antigens and whole tumor cells.
Mol Pharm. 4:47–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Wang H, Yang Z, et al: Enhancement
of cancer radiation therapy by use of adenovirus-mediated
secretable glucose-regulated protein 94/gp96 expression. Cancer
Res. 65:9126–9131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shalaby F, Rossant J, Yamaguchi TP, et al:
Failure of blood-island formation and vasculogenesis in
Flk-1-deficient mice. Nature. 376:62–66. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei YQ, Wang QR, Zhao X, et al:
Immunotherapy of tumors with xenogeneic endothelial cells as a
vaccine. Nat Med. 6:1160–1166. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pe'er J, Shweiki D, Itin A, Hemo I,
Gnessin H and Keshet E: Hypoxia-induced expression of vascular
endothelial growth factor by retinal cells is a common factor in
neovascularizing ocular diseases. Lab Invest. 72:638–645.
1995.PubMed/NCBI
|
|
32
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fine BA, Valente PT, Feinstein GI and Dey
T: VEGF, flt-1, and KDR/flk-1 as prognostic indicators in
endometrial carcinoma. Gynecol Oncol. 76:33–39. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yancopoulos GD, Klagsbrun M and Folkman J:
Vasculogenesis, angiogenesis, and growth factors: ephrins enter the
fray at the border. Cell. 93:661–664. 1998. View Article : Google Scholar : PubMed/NCBI
|