Introduction
Lung cancer is the most common cause of
cancer-related death in the world at present (1). Currently, the survival of lung cancer
patients is poor with only 15% of patients surviving 5 years
follosing diagnosis. Although new chemotherapy agents and
radiotherapy have improved the survival and quality of life of
patients, the overall effect in the last decade has been mainly on
palliation rather than reduction in mortality. Two important
hypotheses have been postulated to explain the genesis, formation,
growth and metastasis of epithelial cancer: the role of cancer stem
cells (CSCs) or tumor-initiating cells (TICs) and the involvement
of ‘so called’ epithelial-mesenchymal transition (EMT). The CSC
hypothesis asserts that only a small subset of cells within a tumor
has the ability of both tumor initiation and sustaining tumor
growth (2,3). These cells, that express stemness
markers, are capable of forming floating spheres in serum-free
medium, a property associated with stem cells and are able to
differentiate into an aberrant cell phenotype constituting tumor
heterogeneity (4). There are two
basic concepts that support the hypothesis that CSCs originate from
normal tissue stem cells. First of all, CSCs have normal stem cell
features such as self-renewal, differentiation, drug resistance and
migration capacity. Moreover, the longevity of stem cells makes
them susceptible to accumulating genetic and epigenetic damages so
as to make them a suitable candidate for the emergence of
neoplastic transformation. CSCs are the only cells that are capable
of generating tumors similar to the original patient specimens when
transplanted into immunocompromised mice such as NOD/SCID mice.
Finally, CSCs have been identified in a variety of solid tumors
including glioblastomas (5), breast
(6) and lung cancer (7,8).
During tumorigenesis, subsets of tumor cells
localized within the primary tumor may acquire features of
invasiveness and motility and enter blood or lymph vessels.
Mechanisms involved in this process are still under investigation;
however, they have been reported to be linked to variable
interactions between tumor cells and their surrounding stroma,
including response to hypoxia and metalloproteinase-dependent
invasion into surrounding tissue, (neo-)vascularization of the
tumor (9), as well as gain of a
phenotype revealing signatures of EMT observed in at least a
subpopulation of tumor cells with certain ‘stemness’ properties
(10). EMT is a key program in
embryonic development, often activated during cancer invasion and
metastasis and by which cells undergo a morphological switch from
the epithelial polarized phenotype to the mesenchymal fibroblastoid
phenotype. As a result of EMT, epithelial cells lose their defined
cell-cell/cell-substratum contacts and their structural/functional
polarity, and they become spindle-shaped and morphologically
similar to activated fibroblasts (11) by downregulation of epithelial
differentiation markers including cytokeratins and E-cadherin and
transcriptional induction of mesenchymal markers such as vimentin,
fibronectin and N-cadherin. CTCs can infiltrate and survive in and
colonize to distant organs (12).
Recent advances in this field are supportive for the early
dissemination model of metastasis, through the observation that
disseminated tumor cells (DTCs) isolated from bone marrow or lymph
nodes display disparate changes on all levels of genomic resolution
as compared to primary tumor cells (13). Cancer cell dissemination may be
followed by a dormancy period before relapse in one or more organs
(14). Current research on DTCs and
CTCs presents a challenge, as these cells are well-defined targets
for understanding tumor biology and tumor cell dissemination in
cancer patients (15), and may open
new avenues for the early detection of metastatic spread and its
successful treatment.
The aim of the present study was to correlate EMT
markers, CTCs and CSCs with clinicopathological parameters and
follow-up of patients with NSCLC to verify whether these markers
contribute to accurate stratification of patients at risk for
recurrent and metastatic disease.
Materials and methods
Patients and follow-up
Forty-five consecutive patients undergoing resection
for primary NSCLC were enrolled. As previously described (16), in all cases, preoperative staging
was performed in order to exclude patients with advanced disease.
Generally, lung resection consisted of lobectomy or pneumonectomy
with systematic mediastinal lymphadenectomy. Immediately after
surgical resection, tumor-draining pulmonary vein stumps were
punctured to obtain blood samples. Follow-up studies included
physical examination, chest X-ray, and blood tests at a 3-month
interval and an additional thoracic chest and abdomen (CT) scan,
along with flexible bronchoscopy at a 6-month interval. Thus,
relapses and deaths were recorded in our files. Ethics committee
approval was provided in all instances (IRB approval no. 556).
Demographics
Patient characteristics are documented in Table I. There were 14 female patients. The
median age at the time of surgery was 74.2 years (range, 48–81).
The median follow-up duration was 16.3 months (range, 1–26).
Pathological stage I was recorded in 32 patients, stage II in 8
patients and stage III in 5 patients. Relapses were recorded in 11
cases, with a mean time of 14 months. At the end of the follow-up,
death was observed in 6 cases, 32 patients were alive without
disease and 7 patients were alive with disease.
 | Table ICharacteristics of patients enrolled
in this study and distribution of CTCs and CD133. |
Table I
Characteristics of patients enrolled
in this study and distribution of CTCs and CD133.
| Patients
(%)(n=45) | Relation to CD133
positivity P-value |
|---|
| Age (years) |
| <60 | 26.7 | 0.656279 |
| ≥60 | 73.3 | |
| Gender |
| Male | 68.9 | 1 |
| Female | 31.1 | |
| Stage |
| I | 71.1 | 0.56015 |
| II | 17.8 | |
| III | 11.1 | |
| Histological
type |
| Squamous cell | 55.6 | 0.543478 |
|
Adenocarcinoma | 33.3 | |
| Large-cell | 11.1 | |
| Relapse |
| No | 68.9 | 0.136 |
| Yes | 31.1 | |
| Status |
| Alive | 71.1 | 0.26018 |
| Alive with
disease | 15.6 | |
| Death due to
tumor | 13.3 | |
| Circulating
cells |
| Absence | 75.6 | 0.588 |
| Presence | 24.4 | |
| CD117 |
| Absence | 73.3 | 0.044 |
| Presence | 26.7 | |
Histology of primary tumors
All cases were reviewed according to WHO
classification. In particular, pathological stage, histological
type and when applicable cancer grade were determined.
Isolation of cancer cells from NSCLC
biopsies and flow cytometry
Fresh NSCLC biopsies were minced with scissors,
filtered through 40-mm nylon meshes and subsequently analyzed for
EMT and CSC markers using flow cytometry. At least 200,000 cells
were incubated with 1 μg/μl of fluorescent-labelled monoclonal
antibodies or respective isotype controls at 4°C for 30 min in the
dark. After incubation, the samples were washed and analyzed by
flow cytometry using a FACSAria II (Becton-Dickinson, USA). The
antibodies used were: CD133/2 PE (Miltenyi Biotec S.r.l., Calderara
di Reno, Bologna, Italy), CSC marker CD326 PE (EpCAM, Miltenyi
Biotec), CD90 FITC (BD Pharmingen, Buccinasco, Milan, Italy),
mesenchymal marker CD45 CY (BD Pharmingen) and leukocyte marker.
All data were analyzed using DIVA 6.6 software.
Isolation of mononuclear cells from
pulmonary venous blood and CTC identification
The tumor-draining pulmonary vein was punctured
subsequent to thoracotomy and prior to intrathoracal preparation
for lung resection. Pulmonary venous blood (10 ml) was drawn into a
sterile syringe. The obtained pulmonary venous blood, yielding
between 5×106 and 6×107 (mean,
2.5×107) mononuclear cells, was centrifuged through 20
ml Ficoll-Hypaque for 30 min at 1,200 rpm. The interface layer,
which contains mononuclear cells, was collected and brought to a
final concentration of 106 cells/ml. The obtained cells
were fixed with Cytolit™ solution and then processed according to
the ThinPrep2000™ method (Cytyc Corp., Marlborough, MA).
Occult tumor cells of pulmonary venous blood were
detected by immunohistochemical staining using pan-cytokeratins.
Antigen retrieval was performed on all slides obtained by the
ThinPrep2000 method. The first step of antigen retrieval lasted for
30 min at ~100°C with a citrate-based buffer (Leica Bond Epitope
retrieval solution, pH 5.9–6.1). CK 8/18/19 (pan-CK) mouse
monoclonal antibody (clone A45-B/B3, 1:100 dilution; Miltenyi
Biotec GmbH, Gladbach, Germany) was applied and incubation was
carried out for 60 min, followed by a washing step to remove any
excess antibody. The antibody was visualized using the peroxidase
detection system (RE7120-K; Novocastra, Newcastle, UK) and
diaminobenzidine (DAB) as contrasting chromogen.
TMA construction
As previously described, a tissue microarray (TMA)
was constructed using the most representative areas from each
single case (16) using a
semiautomated tissue arrayer (Galileo TMA CK 3500).
Immunohistochemistry
Primary antibodies included anti-human CD133 (AC133,
dilution 1.150; Miltenyi Biotec), c-kit (polyclonal, diluition
1:500; Dako) and CD90 (EPR3132, dilution 1:150; Lifespan). Antigen
retrieval was performed by microwave pretreatment in 0.01 M citrate
buffer for 10 min. Sections were incubated with mouse anti-rabbit
or goat anti-mouse secondary immunoglobulin (IgG) biotiny-lated
secondary antibody for 30 min. Immunoreactivity was visualized by
means of avidin-biotin-peroxidase complex kit reagents (Novocastra)
as the chromogenic substrate. Finally, sections were weakly
counterstained with hematoxylin and mounted. Appropriate inner
cells were considered as controls. For each antibody, cytoplasmic
and membranous staining was recorded. Tissues were scored
semi-quantitatively by evaluating the proportion of positive tumor
cells over the total number of tumor cells (percentage of positive
tumor cells per tissue microarray punch). Negative, low expression
and high expression cases were recorded when neoplastic cells
expressing chemokine receptor were between 0 and 10%, <30% and
>30%, respectively.
CD133 qRT-PCR
Total RNA was isolated from frozen biopsies from our
Institutional Bio-Bank, using RNeasy Mini kit (Qiagen GmbH, Hilden,
Germany) following the manufacturer's instructions. Samples were
treated with RNase-free DNase (Qiagen GmbH) to prevent
amplification of genomic DNA. A total of 1 μg RNA was subjected to
cDNA synthesis for 1 h at 37°C using the Ready-To-Go You-Primer
First-Strand Beads kit (code 27-9264-01; Amersham Biosciences
Europe GmbH, Freiburg, Germany) in a reaction mixture containing
0.5 μg random hexamers (GeneAmp RNA PCR random hexamers set
N808-0127; Applied Biosystems, Foster City, CA).
Quantitative RT-PCR was performed using a
LightCycler system (Roche Molecular Biochemicals, Mannheim,
Germany) using TaqMan® analysis. In this system, all
reactions were run in glass capillaries with LightCycler TaqMan
Master Mix (code 04735536001; Roche Molecular Biochemicals), 10 μl
in a volume of 20 μl containing 2 μl of cDNA and 1 μl of specific
TaqMan Gene Expression Assays for human CD133 (Real-Time Designer
Assay code 05583055001; Roche Molecular Biochemicals), according to
the manufacturer's instructions. All reactions were performed in
triplicate. The thermal cycling conditions included a step of 20
sec at 95°C followed by 40 cycles of 95°C for 1 sec and 60°C for 20
sec. The comparative Ct method was employed to determine
the human CD133 gene variation, using as reference gene TaqMan
Endogenous Controls Human ACTB (β-actin) (Real-Time Designer Assay
code 05532957001; Roche Molecular Biochemicals). We identified a
calibrator cell line that represents the unitary amount of the
target of interest. Consequently, the samples express n-fold mRNA
relative to the calibrator. Final amounts of target were determined
as follows: Target amount = 2 − Ct, where Ct
= [Ct (CD133) − Ct (ACTB)]sample −
[Ct (CD133) − Ct
(ACTB)]calibrator.
Statistical analysis
The primary outcome variables were overall survival
(OS) and disease-free survival (DFS). Survival curves were
estimated using the Kaplan-Meier method, and differences among
groups were analyzed using the log-rank test. OS was calculated as
the time from the date of surgical resection to death of any cause
or until the date of the last follow-up at which point data were
censored. DFS was defined as the time between surgical resection
until signs of disease progression (PD), death (DCD) from any cause
and last radiological assessment. Data on survivors were censored
at the last follow-up. We used non-parametric tests to compare
independent groups of numerical data (Mann-Whitney test) and
categorical data (χ2-test and Fisher's exact test). The
correlations among numerical variables were assessed by Spearman's
rank correlation analysis. Multivariate analyses, with backward
variable selection, were conducted using the Cox's
proportional-hazard regression model. Variables at the 0.10 level
in univariate analysis were included in the multivariate model. The
level of significance was defined as P<0.05. All the statistical
analyses were carried out using the Statistical Package for the
Social Science 8.0 software (SPSS Inc., Chicago, IL, USA).
Results
Cytokeratin expression in CTCs in
tumor-draining venous blood and TMA analyses
Cells positive for cytokeratins were detectable in
11 (23.9%) cases from tumor-draining pulmonary venous blood and
were distributed as showed in Table
I. The number of cells positive for cytokeratins ranged from 2
to 10 (Fig. 1).
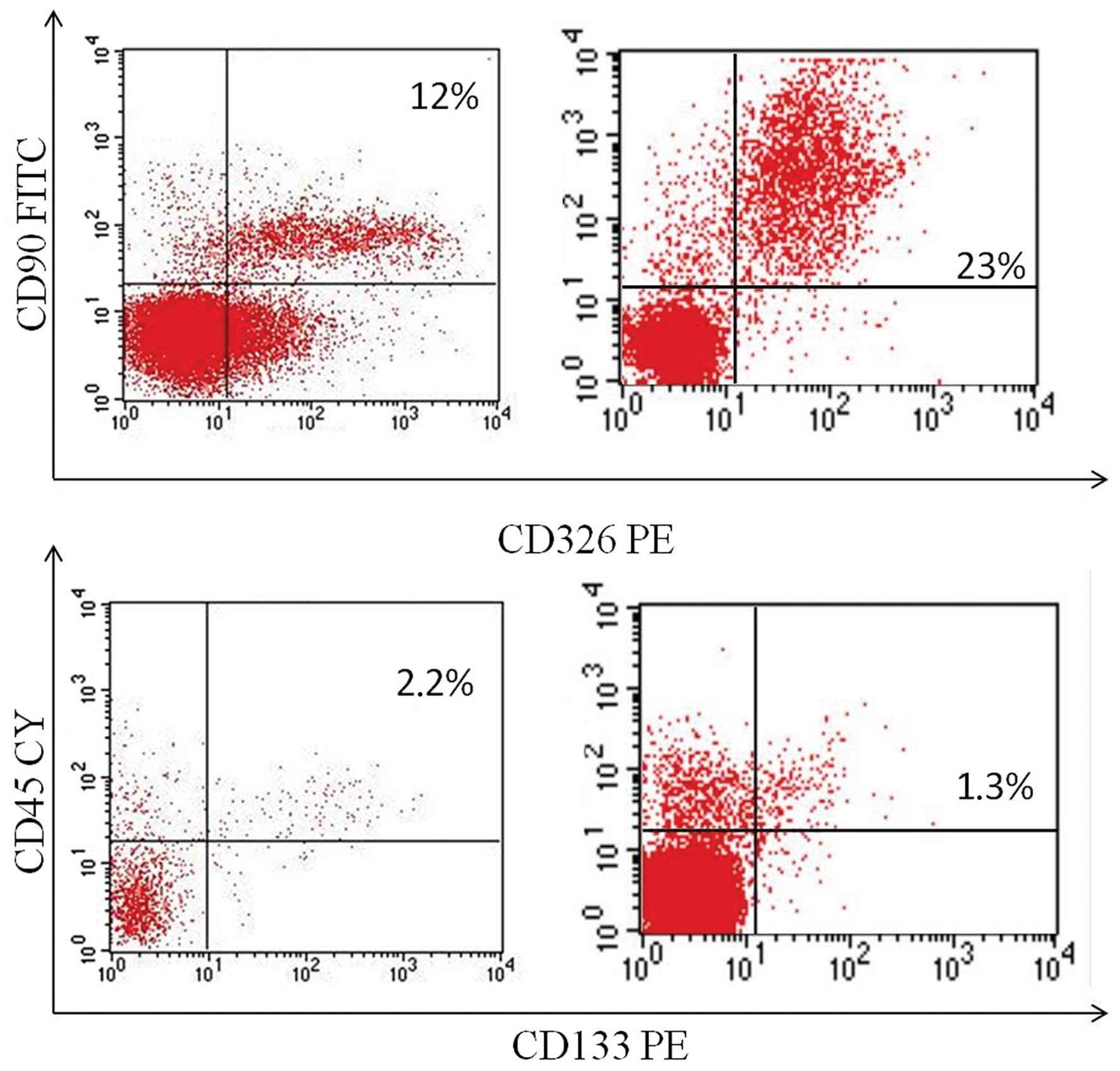
Flow cytometric analysis and
prognosis
Fresh surgical biopsies of NSCLC obtained from 45
patients undergoing tumor resection were mechanically
disaggregated, and the resulting single-cell suspensions were
analyzed by flow cytometry to identify both the cell population
expressing the stemness marker CD133, and the cell population
expressing EMT markers CD90 (mesenchymal marker) and CD326
(epithelial marker). The co-expression of CD90 and CD326 identifies
the EMT subpopulation. The co-expression of CD90/CD326 markers was
detectable in 42 of the 45 (93.3%) samples with a mean percentage
of 10.41% (range, 0–55.2%) (Fig.
2). The expression of the CD133 marker was detectable in 40 of
the 45 (88.8%) samples with a mean percentage of 1.6% (range,
0–9.22%).
TMA evaluation and RT-PCR
Prognostic TMA analyses showed a heterogeneous
expression of c-kit as detected by immunohistochemistry. In
particular, 12 of the 45 cases were positive. CD133 was observed as
positive focal membrane expression in neoplastic cells (Fig. 3), and CD133 mRNA was overexpression
as detected by qRT-PCR, in all cases documented as positive in
cytofluorimetry.
Statistical analysis
Using cytometry, the CD133 antigen showed a high
statistically significant correlation with deceased patients when
compared with CD90/CD326 co-expression (32.5 vs. 9.5%, P=0.01).
Additionally, CD133 expression exhibited a strong correlation,
although not statistically significant, with patients with
progression disease when compared to CD90/CD326 co-expression (15
vs. 7.1%, P=0.21) (Table II). A
statistically significantly inverse correlation (P=0.04) was also
noted between CD133 expression and that of c-kit. Moreover, no
statistically significant relationship existed between the presence
of CTCs and CD133 expression.
 | Table IICorrelation among CSCs positive for
CD133, EMT markers and patient prognosis. |
Table II
Correlation among CSCs positive for
CD133, EMT markers and patient prognosis.
| DCD (%) | P-value | PD (%) | P-value |
|---|
| Patients expressing
CD133 | 32.5 | 0.01 | 15 | 0.21 |
| Patients
co-expressing CD90/CD326 | 9.5 | | 7.1 | |
CD133 expression was significantly related to
shorter DFS (P=0.001) (Fig. 4). A
non-significant relationship was noted between OS and all of the
analyzed markers.
Discussion
Lung cancer is the leading cause of cancer-related
deaths worldwide. Generally, the overall survival of lung cancer
patients is low, due to late presentation, frequent tumor relapse
and lack of effective systemic therapy. Recently, the CSC theory
proposes that cancer maintenance is guaranteed by tumor cells that
possess stem or progenitor cell features. Thus, CSCs are able to
initiate tumor formation and differentiate along multi-potent
pathways. In addition, they are relatively resistant to
conventional chemotherapy (17).
Various stem cell markers of normal tissues have been proposed to
detect CSCs. In particular, the CD133 antigen appears to be the
most frequently demonstrated marker in liver, brain, colon and lung
cancers (5,7,18,19).
Thus, recent studies using NSCLC cell lines and fresh lung tumor
tissues suggest CD133 as a lung CSC marker (7,8,20,21–23).
A biochemical study showed that CD133 plays a functional role in
cell cycle regulation and proliferation but not in tumor initiation
(24). Yet, numerous other markers
have been identified in lung cancer CSCs, with conflicting results.
Thus, the marker profile of lung CSCs remains to be explored.
Studies performed on lung cancer cell lines by flow cytometry
demonstrated no significant CD133 expression by either
semi-quantitative RT-PCR, IHC, immunoblotting or flow cytometry
analysis. For example, only 0.7% of cells with CD133 expression was
noted in H1299 cells, while no expression was found in our study
(20,25). Our data confirmed the relative low
percentage of cells positive for CD133 as identified by flow
cytofluorimetry in fresh lung cancer tissues.
The relationship between CD133 expression and
patient prognosis has been documented in many tumor types. Thus,
CD133 expression is related to poor prognosis in cancer of the
colorectum (26–29), brain (30), liver (31), stomach (32), endometrium (33), ovary (34) and lung (35). The majority of these studies are
small in sample size and are further limited by use of the
immunohistochemistry method, which results generally in high
background noise with the commercial antibody. Several of the
studies utilized CD133 mRNA alone or in combination with other
markers. In our study, we evaluated CD133 expression using three
different methods, with substantial superimposable results. In
fact, cases with CD133 expression by flow cytometry were confirmed
by immunohistochemical analysis on paraffin-embedded tumor tissue
and by real-time PCR. Although a small series was studied, the use
of different methods reinforced the finding that CD133 expression
is a significant predictor of lung cancer prognosis.
Other markers have been proposed as CSC biomarkers
in lung cancer. Among these, c-kit receptors and embryonic markers
have been suggested (36).
In particular, c-kit has been considered a stemness
biomarker in many tumor histotypes, with conflicting results in
term of prognosis in small and non-small cell lung cancer. In our
series we observed no relationship between c-kit expression and
patient prognosis, and a significantly inverse relation was noted
between c-kit-expressing and CD133-expressing cases. The
significance of stemness in cancer varies from one tumor to
another, and research aimed to determine an accurate profile of
CSCs for each tumor type appears to be the next frontier to
elucidate the role of this biological feature in the clinical
setting for each patient. In fact, in our series, CD133 was a
strong predictor and c-kit was not. Moreover, using
immunohistochemistry, these two markers did not identify the same
neoplastic cells, probably due to the fact that c-kit expression in
this context was not related to stemness properties but to other
biological events, such as gene mutations that are described in
other tumors.
It is well known that DTCs can be found in the
circulation of patients with metastatic cancer, and it has been
hypothesized that these DTCs may represent cancer stem cells or a
cell population with high metastatic potential (37). In recent years, significant effort
has been made to develop technologies that achieve specific and
sensitive detection and capture of CTCs. In a recent study
(16), we identified in the same
set of patients CTCs by immunohistochemistry in pulmonary vein
blood obtained after the surgical intervention. No association was
found between the presence of CTCs and patient prognosis. Herein,
we found that no statistical relationship exists between the
presence of CTCs and CD133 expression. This is probably due to the
fact that all CTCs have stemness properties, responsible for
circulation and colonization to distant organs and recirculation,
i.e. to propagate tumor progression. In fact, the majority of CTCs
will either die in the bloodstream due to mechanical shear forces,
immune surveillance, and/or other regulatory mechanisms; a few
cells will successfully extravasate and form new colonies at
distant sites. Based on this view, only CSCs are able to survive
the difficulties of metastatic development. For this reason,
clinical reports evaluating CTCs have provided contradictory
results with various studies indicating that CTC detection may be
associated with poor prognosis (38,39)
whereas others have failed to show such an association (40).
In this context, it is important to consider the
role of EMT in metastases. In the present study, we evaluated cells
undergoing EMT using CD90 and CD326 markers from cells derived from
fresh biopsy samples. CD90 is a main mesenchymal marker, while
CD326 is an epithelial marker expressed both in normal and
pathological epithelial tissues. Their co-expression indicates
cells expressing both epithelial and mesenchymal markers; hence,
cells undergoing EMT. Our results demonstrated that this cell
subset existed, but in respect to CD133 expression, it was not
correlated with patient prognosis. In conclusion, our data
reinforce the role of CD133 expression in primary tumors in terms
of prognosis, although a complete profile of CSCs in lung cancer is
needed for correct patient stratification. In addition, the lack of
association between the presence of CTCs and CD133 suggests that
numerous factors are responsible, but only stemness favors tumor
growth in distant sites and cancer maintenance.
Acknowledgments
The authors would like to thank Dr Alessandra
Trocino, librarian of the National Cancer Institute, Naples, for
providing excellent bibliographic services and assistance. This
study was financially supported by the Italian Government. This
study was presented at the 48th Annual Meeting of The Society of
Thoracic Surgeons, Fort Lauderdale, FL, February, 2012.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Locke M, Heywood M, Fawell S and Mackenzie
IC: Retention of intrinsic stem cell hierarchies in
carcinoma-derived cell lines. Cancer Res. 65:8944–8950. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
6
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
et al: Isolation and in vitro propagation of tumorigenic breast
cancer cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tirino V, Camerlingo R, Franco R, et al:
The role of CD133 in the identification and characterisation of
tumour-initiating cells in non-small-cell lung cancer. Eur J
Cardiothorac Surg. 36:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonnomet A, Brysse A, Tachsidis A, et al:
Epithelial-to-mesenchymal transitions and circulating tumor cells.
J Mammary Gland Biol Neoplasia. 15:261–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005.
|
|
12
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein CA: Parallel progression of primary
tumours and metastases. Nat Rev Cancer. 9:302–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aguirre-Ghiso JA: Models, mechanisms and
clinical evidence for cancer dormancy. Nat Rev Cancer. 7:834–846.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pantel K, Alix-Panabières C and Riethdorf
S: Cancer micrometastases. Nat Rev Clin Oncol. 6:339–351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franco R, Pirozzi G, Scala S, Cantile M,
Scognamiglio G, Camerlingo R, Botti G and Rocco G: CXCL12-binding
receptors expression in non-small cell lung cancer relates to
tumoral microvascular density and CXCR4 positive circulating
tumoral cells in lung draining venous blood. Eur J Cardiothorac
Surg. 41:368–375. 2012. View Article : Google Scholar
|
|
17
|
Dick JE: Future prospects for animal
models created by transplanting human haematopoietic cells into
immune-deficient mice. Res Immunol. 145:380–384. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma S, Chan KW, Hu L, et al: Identification
and characterization of tumorigenic liver cancer stem/progenitor
cells. Gastroenterology. 132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YC, Hsu HS, Chen YW, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertolini G, Roz L, Perego P, Tortoreto M,
Fontanella E, et al: Highly tumorigenic lung cancer
CD133+ cells display stem-like features and are spared
by cisplatin treatment. Proc Natl Acad Sci USA. 106:16281–16286.
2009.PubMed/NCBI
|
|
22
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basak SK, Veena MS, Oh S, Huang G,
Srivatsan E, et al: The malignant pleural effusion as a model to
investigate intratumoral heterogeneity in lung cancer. PLoS One.
4:e58842009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y and Wu PY: CD133 as a marker for
cancer stem cells: progresses and concerns. Stem Cells Dev.
18:1127–1134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leung EL, Fiscus RR, Tung JW, et al:
Non-small cell lung cancer cells expressing CD44 are enriched for
stem cell-like properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li CY, Li BX, Liang Y, et al: Higher
percentage of CD133+ cells is associated with poor
prognosis in colon carcinoma patients with stage IIIB. J Transl
Med. 7:562009.PubMed/NCBI
|
|
27
|
Xi HQ and Zhao P: Clinicopathological
significance and prognostic value of EphA3 and CD133 expression in
colorectal carcinoma. J Clin Pathol. 64:498–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Artells R, Moreno I, Diaz T, et al: Tumour
CD133 mRNA expression and clinical outcome in surgically resected
colorectal cancer patients. Eur J Cancer. 46:642–649. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horst D, Kriegl L, Engel J, et al: CD133
expression is an independent prognostic marker for low survival in
colorectal cancer. Br J Cancer. 99:1285–1289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Metellus P, Nanni-Metellus I, Delfino C,
et al: Prognostic impact of CD133 mRNA expression in 48
glioblastoma patients treated with concomitant radiochemotherapy: a
prospective patient cohort at a single institution. Ann Surg Oncol.
18:2937–2945. 2011. View Article : Google Scholar
|
|
31
|
Sasaki A, Kamiyama T, Yokoo H, et al:
Cytoplasmic expression of CD133 is an important risk factor for
overall survival in hepatocellular carcinoma. Oncol Rep.
24:537–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishigami S, Ueno S, Arigami T, et al:
Prognostic impact of CD133 expression in gastric carcinoma.
Anticancer Res. 30:2453–2457. 2010.PubMed/NCBI
|
|
33
|
Nakamura M, Kyo S, Zhang B, et al:
Prognostic impact of CD133 expression as a tumor-initiating cell
marker in endometrial cancer. Hum Pathol. 41:1516–1529. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silva IA, Bai S, McLean K, et al: Aldehyde
dehydrogenase in combination with CD133 defines angiogenic ovarian
cancer stem cells that portend poor patient survival. Cancer Res.
71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Woo T, Okudela K, Mitsui H, et al:
Prognostic value of CD133 expression in stage I lung
adenocarcinomas. Int J Clin Exp Pathol. 4:32–42. 2010.PubMed/NCBI
|
|
36
|
Levina V, Marrangoni A, Wang T, et al:
Elimination of human lung cancer stem cells through targeting of
the stem cell factor-c-kit autocrine signaling loop. Cancer Res.
70:338–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maheswaran S and Haber DA: Circulating
tumor cells: a window into cancer biology and metastasis. Curr Opin
Genet Dev. 20:96–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gudemann CJ, Weitz J, Kienle P, et al:
Detection of hematogenous micrometastasis in patients with
transitional cell carcinoma. J Urol. 164:532–536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naito T, Tanaka F, Ono A, et al:
Prognostic impact of circulating tumor cells in patients with small
cell lung cancer. J Thorac Oncol. 7:512–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guzzo TJ, McNeil BK, Bivalacqua TJ,
Elliott DJ, Sokoll LJ and Schoenberg MP: The presence of
circulating tumor cells does not predict extravesical disease in
bladder cancer patients prior to radical cystectomy. Urol Oncol.
30:44–48. 2012. View Article : Google Scholar : PubMed/NCBI
|