Introduction
Obesity is related to several metabolic disorders
such as type 2 diabetes mellitus, coronary heart disease and
hypertension, and is associated with cancer development in
different tissues including colon, prostate and breast (1). It has been clearly demonstrated that
obesity is a risk factor for breast cancer development in
postmenopausal women (2,3). Moreover, an excess of adipose tissue
favors breast cancer recurrence and is associated with higher
mortality (4). Thus, overweight or
obese women with breast carcinoma have a 2.5-fold increased risk of
mortality within five years of diagnosis compared with lean women
(5). Numerous factors have been
proposed to explain the relationship between obesity and breast
cancer (6–8), however, none has been completely
conclusive.
Emerging data suggest that adipose tissue, which is
an endocrine organ producing a large range of factors, may
interfere with cancer development. These factors, mainly secreted
by the adipose tissue, known as adipokines, include angiogenic
factors, paracrine mitogens and anti-mitogens, growth factors and
pro-inflammatory cytokines (IL-1, TNF-α and IL-6) involved in the
mediation or the coordination of inflammatory diseases and obesity
(9,10). Adipokines are produced by different
fat depots, including subcutaneous, visceral and mammary adipose
tissue. Of note, adipokines may act on breast tissue in an
endocrine manner (via external adipose sources), in a paracrine
pathway (via mammary adipose tissue secretion and non adipose
sources including stromal cells and inflammatory cells) and in an
autocrine manner (via the mammary tumor by itself). The structure
of the mammary gland favors a close interaction between mammary
adipose tissue and breast tissue, and suggests that adipokines
produced by mammary adipose tissue and the tumor microenvironment
may be the major link between obesity and disease progression and
metastasis (11–14). We previously investigated
simultaneously the in vitro and in vivo molecular
mechanisms by which leptin induces and, conversely, adiponectin
suppresses, tumor proliferation in breast cancer cells (15,16).
We suggested that these two adipokines have antagonistic properties
in breast cancer development by modulating differentially both
proliferative and apoptotic signaling pathways (17).
Zinc-α2-glycoprotein (ZAG) is a new adipokine whose
gene expression is reduced in subcutaneous fat of obese patients
(18,19). This expression is positively
correlated with adiponectin expression and negatively correlated
with leptin expression, suggesting a protective role for ZAG in
breast cancer (20). ZAG is also a
sound immunohistochemical marker of breast cell differentiation
since ZAG tissue levels are associated with histological grades of
tumors and vary from 4.6 μg/mg in well-differentiated tumors to 0.9
μg/mg in poorly differentiated tumors (21,22).
However, other studies found that circulating levels of ZAG are
significantly higher in cancer patients (51 mg/l) as compared with
levels in healthy women (44 mg/l), particularly in patients with
advanced and node-positive breast cancer (23). Moreover, Bing et al(18) showed that ZAG expression is
upregulated in mice with cancer cachexia. Overexpression in white
adipose tissue of tumor-bearing mice suggests that ZAG plays a role
in the substantial reduction of adiposity in cancer cachexia. ZAG
is also considered a prognostic marker in breast cancer (24). We recently reported ZAG expression
in breast tumor or healthy breast tissue and detected this
expression at high levels in ductal carcinoma and in normal
epithelial adjacent tissue, but not in normal tissue of healthy
women (25). We also found ZAG
expression was positively correlated to leptin receptor and
negatively correlated to adiponectin receptor in cancer tissues.
Our previous data suggested both a potential prognostic role for
ZAG in breast cancer and a close interaction between ZAG and other
major adipokine pathways.
The aim of the present study was to characterize the
involvement of ZAG in breast cancer proliferation. Thus, we
explored the in vitro potential effect of human recombinant
ZAG on i) proliferative/apoptotic response, and ii) the
modifications of gene expression in different breast epithelial
cell lines.
Materials and methods
Cell culture
The human breast cancer cell lines MCF-7 and
MDA-MB-231, and the human fibrocystic breast cell line MCF-10a were
obtained from the American Type Culture Collection (ATCC). MCF-7
cells were routinely cultured in RPMI-1640 medium supplemented with
10% heat-inactivated fetal calf serum (FCS), L-glutamine (2 mM) and
gentamicin (50 μg/ml) at 37°C under a 5% CO2 atmosphere.
MDA-MB-231 cells were cultured in Leibovitz’s L-15 medium with 15%
heat-inactivated FCS, L-glutamine (2 mM) and gentamicin (50 μg/ml)
at 37°C in humidified conditions. MCF-10a cells were cultured in
DMEM HAM’s F12 medium supplemented with 10% heat-inactivated horse
serum (HS), EGF (0.02 μg/ml), cholera toxin (0.1 μg/ml),
hydrocortisone (0.5 μg/ml), insulin (0.25 UI/ml) and L-glutamine (2
mM) at 37°C under a 5% CO2 atmosphere (16).
Recombinant human ZAG (rh-ZAG)
Recombinant human (rh)-ZAG was kindly provided by
Mracek et al(20) (Obesity
Biology Research Unit, School of Clinical Sciences, University of
Liverpool) and its production was recently described.
Proliferation assay
The human mammary cell lines (MCF-7, MDA-MB-231 and
MCF-10a) were seeded at the density of 5×103 cells in
96-well plates in a complete medium. After 24 h of incubation,
cells were washed with PBS and exposed to fresh medium (control) or
to fresh medium containing different concentrations of rh-ZAG: 1.2,
2.5, 5, 10 and 20 μg/ml. After 96 h, cells were washed with PBS and
incubated with 200 μl of a 25 μg/ml solution of resazurin in
RPMI-1640 medium for 2 h at 37°C. Fluorescence was then measured on
an automated 96-well plate reader (Fluoroskan Ascent FL; Thermo
Fisher Scientific, Wilmington, DE, USA) using an excitation
wavelength of 530 nm and an emission wavelength of 590 nm. Under
these conditions, fluorescence was proportional to the number of
living cells in the well (16). The
cell proliferation assay was performed three times in triplicates
for each concentration tested.
Analysis of gene expression
Total RNA was extracted with Trizol according to the
manufacturer’s recommendations (Invitrogen). The quantity and
quality of RNA was assessed by 260/280 ratio using a NanoDrop 8000
Spectrophotometer (Thermo Fisher Scientific). cDNAs were obtained
with HighCap cDNA RT kit RNAse inhibitor (Applied Biosystems).
Real-time PCR assays of BAG1, BAG3,
MX1, TP53 and 18S mRNA expression levels were
performed using the StepOne instrument (Applied Biosystems) with
Power SYBR-Green (Applied Biosystems) following the manufacturer’s
instructions. The cDNAs were amplified using the primers summarized
in Table I. The PCRs were set up in
48-well plates in a total volume of 20 μl and 20 ng of cDNA. The
PCR conditions were as follows: 2 min at 50°C, 10 min at 95°C, 40
cycles of 15 sec at 95°C and 30 sec at 62°C.
 | Table IPrimers used in qRT-PCR assays. |
Table I
Primers used in qRT-PCR assays.
| BAG1 | F:
5′-CACAGCAATGAGAAGCACG-3′ |
| BAG1 | R:
5′-GTGTTTCCATTTCCTTCAGAG-3 |
| BAG3 | F:
5′-ATGACCCATCGAGAAACTGC-3′ |
| BAG3 | R:
5′-AATTGGGATGTGTCCAGGAG-3′ |
| MX1 | F:
5′-AGCTCGGCAACAGACTCTTC-3′ |
| MX1 | R:
5′-GGATGATCAAAGGGATGTGG-3′ |
| TP53 | F:
5′-GCGCACAGAGGAAGAGAATC-3′ |
| TP53 | R:
5′-AGAGGAGCTGGTGTTGTTGG-3′ |
| 18S | F:
5′-GTCTGTGATGCCCTTAGATG-3′ |
| 18S | R:
5′-AGCTTATGACCCGCACTTAC-3′ |
The comparative cycle threshold (CT) method
(2−ΔΔCT) was used to calculate the relative gene
expression of ZAG-treated cells normalized within the sample to an
endogenous reference gene (18S), and relative to the expression of
the same gene in untreated cells: 2−ΔΔCT method with
ΔΔCT = [ΔCT (ZAG-treated cells) − ΔCT (untreated cells)] and ΔCT =
[CT (target gene) − CT (reference gene)].
Statistical analysis was performed using the paired
Student’s t-test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Western blotting
Cells lysates (containing 10 μg of total proteins)
were separated on 4–12% SDS-PAGE gels (Invitrogen), transferred to
nitrocellulose and blotted with various antibodies. We used primary
antibodies (Santa Cruz Biotechnology, Inc.,) against the following
proteins: p53 (DO-1), p21, Bax, c-Myc and cyclin D1. Secondary
horseradish peroxidase (HRP)-conjugated antibodies were obtained
from Santa Cruz Biotechnology, Inc., (anti-rabbit) or Dako
(anti-mouse). Immunoreactive bands were visualized by incubation
with DURA Western blotting detection system from Thermo Fisher
Scientific. Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH)
monoclonal antibody was used as a loading control. Developed films
were scanned as JPEG images, and the pixel intensities within a
band were measured with ImageJ software. The intensity of each gene
was then reported to GAPDH intensity.
Results
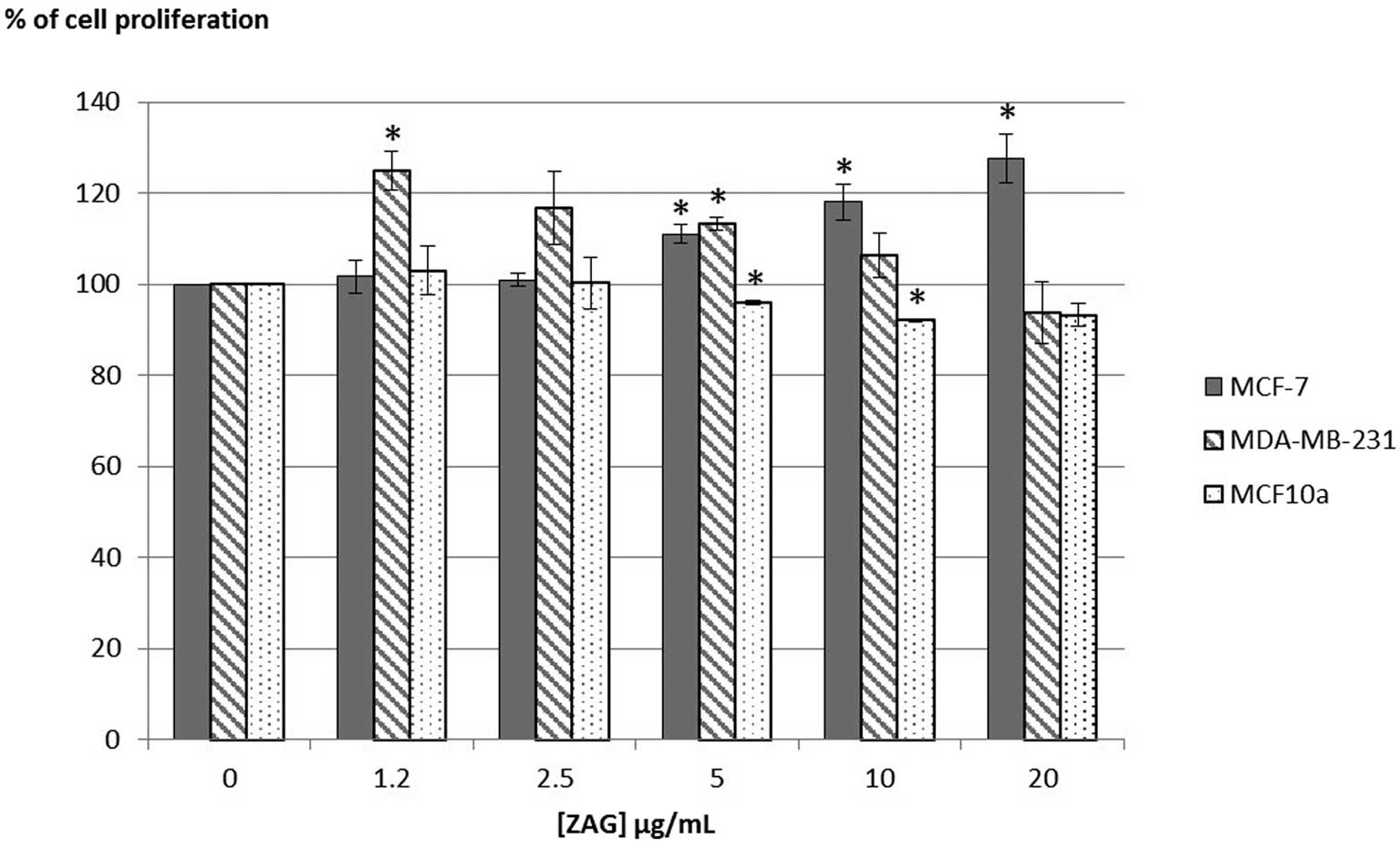
Mammary cell proliferation
The proliferation of estrogen receptor positive
(ER+) mammary cancer cells MCF-7 was increased at the highest
concentrations tested [11 to 27% with (ZAG) = 5 to 20 μg/ml]
(Fig. 1). In ER- mammary cancer
cells MDA-MB-231, rh-ZAG had a proliferative effect at the lowest
concentrations tested [+24% with (ZAG) = 1.2 μg/ml; +13% with (ZAG)
= 5 μg/ml]. By contrast, rh-ZAG had an anti-proliferative effect on
fibrocystic breast cells MCF-10a when used at the concentrations of
5 and 10 μg/ml (−5 and −8% in proliferation decrease).
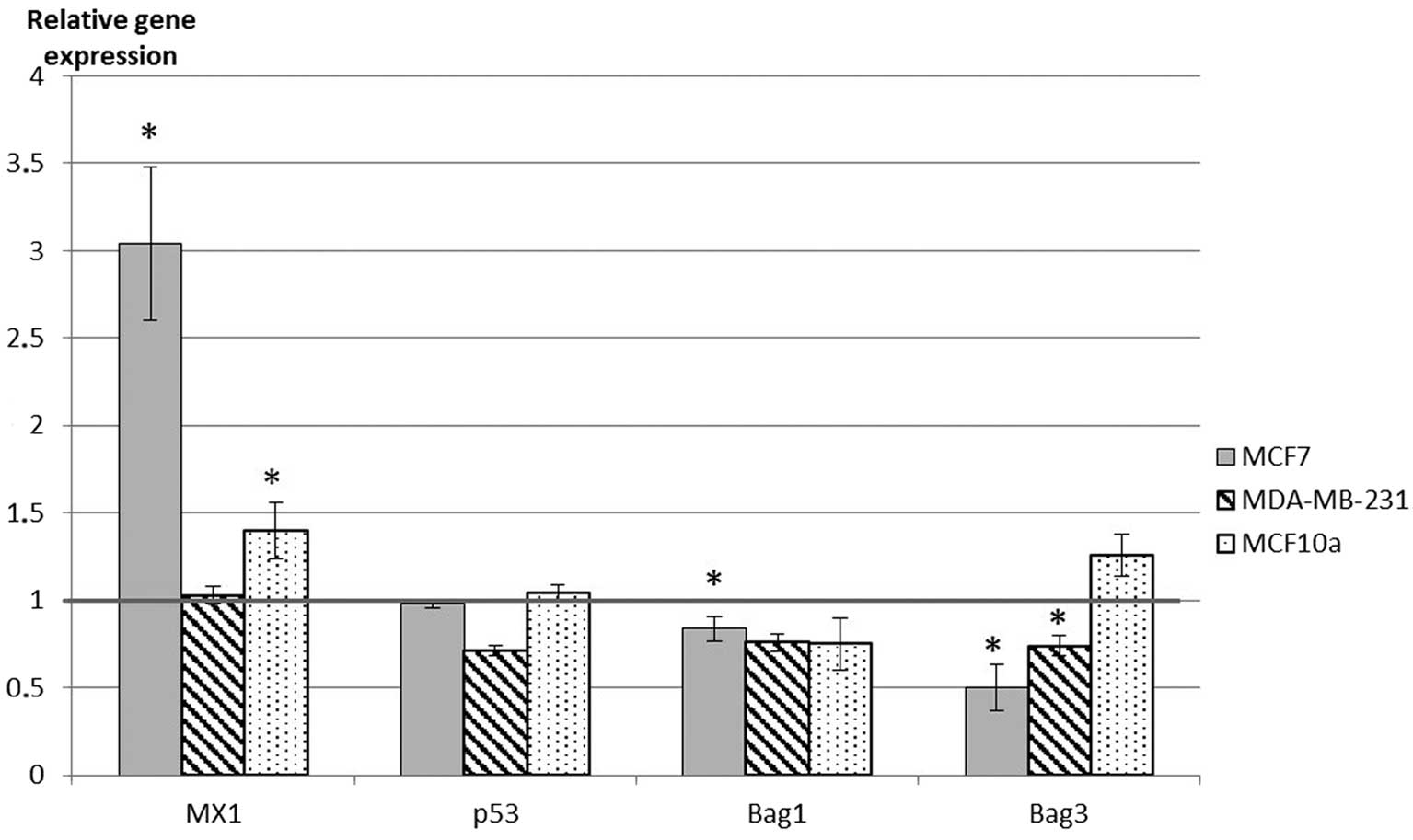
Gene expression
qRT-PCR was performed to study the effects of a 72-h
rh-ZAG treatment on the expression of two pro-apoptotic genes
(p53, mx1) and two anti-apoptotic genes (Bag1
and Bag3) in the three studied breast cell lines. We used
rh-ZAG at the respective concentration points leading to an optimal
effect on cell growth: 20 μg/ml for MCF-7 cells, 1 μg/ml for
MDA-MB-231 cells and 10 μg/ml for MCF-10 cells.
Bag1 and Bag3 expressions were
downregulated by rh-ZAG in MCF-7 and MDA-MB-231 cells, but remained
unaltered in MCF-10a cells (Fig.
2). Concomitantly, Mx1 gene expression was upregulated
by rh-ZAG in MCF-7 and MCF-10a and more strongly in MCF-7
cells.
Protein expression
Fig. 3 shows the
results obtained in cells treated for 24, 48 or 72 h with rh-ZAG
(0, 1, 10 ng/ml). In MCF-7 cells treated with rh-ZAG, an increase
in p53 protein expression at 24 h [+100% with (ZAG) = 1 ng/ml;
+300% with (ZAG) = 10 ng/ml] but a decrease at 72 h (-32% with
(ZAG) = 10 ng/ml) were observed. The expression of c-Myc was
increased only at 24 h (+160%) whereas the expression of p21 and
Bax slightly increased during the experiments (p21: +21, +39 and
28% at 24, 48 and 72 h, respectively; Bax: +58, +37 and +64% at 24,
48 and 72 h, respectively). In MDA-MB-231 cells, there was no
evidence of ZAG-modulated variations in the expression of the
different proteins tested. In MCF-10a cells, rh-ZAG enhanced c-Myc
expression at 72 h [+80% with (ZAG) = 10 ng/ml] while
downregulating both p53 (−95%) and Bax (−90%) protein expression at
the same incubation time.
Discussion
ZAG, a well-known lipid mobilizing factor, which is
downregulated in obesity, is now considered as an adipokine. We
previously reported in vivo ZAG expression in breast
malignant cells and in normal epithelial adjacent tissue (25). The present study showed ZAG had a
proliferative activity on the two human breast tumor cell lines
(MCF-7 and MDA-MB-231), but an anti-proliferative effect on MCF-10a
cells derived from a non-cancerous fibrocystic tissue.
Tumor growth should be viewed as the result of a
balance between cell proliferation and cell death. Our aim was to
assess whether ZAG can regulate mammary cancer proliferation not
only through an inhibitory pathway but also by triggering a
programmed cell death. Markedly, ZAG had a proliferative effect in
MCF-7 cells while upregulating pro-apoptotic Mx1 and
downregulating the gene expression of anti-apoptotic genes
Bag1 and Bag3. Such a modulation on apoptotic markers
should result in a slowing process of cell proliferation.
Our data also suggest that ZAG-induced cell-growth
inhibition in MCF-10a breast cancer cells could be mediated through
the c-Myc pathway. Indeed, we found ZAG upregulates c-Myc protein
expression in these cells. Despite its role in promoting
tumorigenesis, there is increasing evidence that c-Myc also induces
apoptosis in cancer cells (26,27).
Epithelial cells have also been shown to be susceptible to
apoptosis by c-Myc (28). In
addition, it was reported that MCF-7 breast carcinoma cells
deprived of glucose exhibit both c-Myc elevation and significant
cell death, which can be blocked by the addition of antisense c-Myc
oligonucleotides (29).
We previously reported that endogenous ZAG was not
associated with Bax and Bcl2 apoptosis biomarkers in breast cancer
tissues (25). In the present
study, we found exogenous ZAG did not consistently affect Bax
expression in the two human breast tumor cell lines (MCF-7 and
MDA-MB-231). By contrast, ZAG downregulated the expression of both
pro-apoptotic proteins p53 and Bax in MCF-10a cells. We also
observed an anti-proliferative effect for ZAG in these cells,
therefore, apoptosis may not be the only mechanism to explain the
resulting inhibitory effect on cell proliferation. In squamous
carcinoma, ZAG inhibits cell proliferation by downregulating cdc2
expression and affecting cell cycle (30). In this study, ZAG did not affect the
expression of the genes involved in apoptosis or differentiation
(PCNA, p53, c-Myc and Bcl-2). Of note, if adipokines generally
modulate the apoptotic response in vitro, they may exert
their activities through different pathways from one cell type to
another since breast cancer cell lines display distinct patterns of
apoptosis-regulatory genes (31).
For example, MCF-7 cells are caspase-3 negative, ZR-75 cells are
Bcl-2 negative and the p53 status is either wild or mutant,
depending on the cell line.
Thus, our in vitro approach does not seem to
reflect the complex regulation that may occur in vivo.
Metabolic dysregulations associated with obesity (such as
hypoadiponectinemia and hyperleptinemia) are likely to promote
cancer cell growth via both systemic and local mechanisms.
Furthermore, when mammary cells are engaged in the process of
carcinogenesis, they produce adipokines (mainly leptin) able to act
on surrounding cancer cells in a paracrine and/or autocrine manner.
Adipokines act via their receptors on mammary tumor cells to i)
influence tumor cell proliferation, migration and invasion in
breast cancer; ii) regulate the production of epithelial-derived
proteins, angiogenic proteins and growth factors; iii) stimulate
other cells in the tumor microenvironment to invade and
proliferate. For example, a study reported a proliferative effect
of adipocyte-secreted factors on the MCF-7 breast cancer cell line,
through the regulation of genes involved in cell motility,
migration, survival, apoptosis and angiogenesis (32). In the same way, Celis et
al(33) identified in mammary
adipose tissue 359 protein components and excreted factors that may
provide insight into the close interplay between mammary
epithelium, stroma and fat tissue. Among these proteins, they
identified several cell cycle regulators, including p53 and p21.
Perera et al(34) used a
proteomic approach and described for the first time the secretion
of epithelial-derived proteins in MCF-7 cells in response to
leptin. The secretion of such proteins in breast cancer cells in
response to ZAG has yet to be reported.
In summary, these preliminary data show that
recombinant ZAG has a pro-carcinogenic effect on breast cancer
cells and conversely an anti-carcinogenic effect on non malignant
breast cells. ZAG clearly modulates signaling pathways involved in
proliferation and apoptosis but this modulation cannot fully
explain the effects we previously observed with ZAG in breast
cancer tissues.
References
|
1
|
Klein S, Wadden T and Sugerman HJ: AGA
technical review on obesity. Gastroenterology. 123:882–932. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lorincz AM and Sukumar S: Molecular links
between obesity and breast cancer. Endocr Relat Cancer. 13:279–292.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolk A, Gridley G, Svensson M, Nyren O,
McLaughlin JK, Fraumeni JF and Adam HO: A prospective study of
obesity and cancer risk (Sweden). Cancer Causes Control. 12:13–21.
2001. View Article : Google Scholar
|
|
4
|
Chlebowski RT, Aiello E and McTiernan A:
Weight loss in breast cancer patient management. J Clin Oncol.
20:1128–1143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daling JR, Malone KE, Doody DR, Johnson
LG, Gralow JR and Porter PL: Relation of body mass index to tumor
markers and survival among young women with invasive ductal breast
carcinoma. Cancer. 92:720–729. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Belfiore A and Frasca F: IGF and insulin
receptor signaling in breast cancer. J Mammary Gland Biol
Neoplasia. 13:381–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cold S, Hansen S, Overvad K and Rose C: A
woman’s build and the risk of breast cancer. Eur J Cancer.
34:1163–1174. 1998.
|
|
8
|
Pischon T, Nothlings U and Boeing H:
Obesity and cancer. Proc Nutr Soc. 67:128–145. 2008. View Article : Google Scholar
|
|
9
|
Fantuzzi G: Adipose tissue, adipokines,
and inflammation. J Allergy Clin Immunol. 115:911–919. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tilg H and Moschen AR: Adipocytokines:
mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–783. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perrier S, Caldefie-Chezet F and Vasson
MP: IL-1 family in breast cancer: potential interplay with leptin
and other adipocytokines. FEBS Lett. 583:259–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rose DP, Komninou D and Stephenson GD:
Obesity, adipocytokines, and insulin resistance in breast cancer.
Obes Rev. 5:153–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaffler A, Scholmerich J and Buechler C:
Mechanisms of disease: adipokines and breast cancer - endocrine and
paracrine mechanisms that connect adiposity and breast cancer. Nat
Clin Pract Endocrinol Metab. 3:345–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vona-Davis L, Howard-McNatt M and Rose DP:
Adiposity, type 2 diabetes and the metabolic syndrome in breast
cancer. Obes Rev. 8:395–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jarde T, Caldefie-Chezet F, Damez M,
Mishellany F, Perrone D, Penault-Llorca F, Guillot J and Vasson MP:
Adiponectin and leptin expression in primary ductal breast cancer
and in adjacent healthy epithelial and myoepithelial tissue.
Histopathology. 53:484–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jarde T, Caldefie-Chezet F,
Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F and
Vasson MP: Involvement of adiponectin and leptin in breast cancer:
clinical and in vitro studies. Endocr Relat Cancer. 16:1197–1210.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jarde T, Perrier S, Vasson MP and
Caldefie-Chezet F: Molecular mechanisms of leptin and adiponectin
in breast cancer. Eur J Cancer. 47:33–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bing C, Bao Y, Jenkins J, Sanders P,
Manieri M, Cinti S, Tisdale MJ and Trayhurn P:
Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed
in adipocytes and is up-regulated in mice with cancer cachexia.
Proc Natl Acad Sci USA. 101:2500–2505. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marrades MP, Martinez JA and Moreno-Aliaga
MJ: ZAG, a lipid mobilizing adipokine, is downregulated in human
obesity. J Physiol Biochem. 64:61–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mracek T, Ding Q, Tzanavari T, Kos K,
Pinkney J, Wilding J, Trayhurn P and Bing C: The adipokine
zinc-alpha2-glycoprotein (ZAG) is downregulated with fat mass
expansion in obesity. Clin Endocrinol. 72:334–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bundred NJ, Miller WR and Walker RA: An
immunohistochemical study of the tissue distribution of the breast
cyst fluid protein, zinc alpha 2 glycoprotein. Histopathology.
11:603–610. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diez-Itza I, Sanchez LM, Allende MT,
Vizoso F, Ruibal A and Lopez-Otin C: Zn-alpha 2-glycoprotein levels
in breast cancer cytosols and correlation with clinical,
histological and biochemical parameters. Eur J Cancer.
29A:1256–1260. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bundred NJ, Scott WN, Davies SJ, Miller WR
and Mansel RE: Zinc alpha-2 glycoprotein levels in serum and breast
fluids: a potential marker of apocrine activity. Eur J Cancer.
27:549–552. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sanchez LM, Vizoso F, Diez-Itza I and
Lopez-Otin C: Identification of the major protein components in
breast secretions from women with benign and malignant breast
diseases. Cancer Res. 52:95–100. 1992.PubMed/NCBI
|
|
25
|
Dubois V, Delort L, Mishellany F, Jarde T,
Billard H, Lequeux C, Damour O, Penault-Llorca F, Vasson MP and
Caldefie-Chezet F: Zinc-alpha2-glycoprotein: a new biomarker of
breast cancer? Anticancer Res. 30:2919–2925. 2010.PubMed/NCBI
|
|
26
|
Meyer N, Kim SS and Penn LZ: The
Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol.
16:275–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prendergast GC: Mechanisms of apoptosis by
c-Myc. Oncogene. 18:2967–2987. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakamuro D, Eviner V, Elliott KJ, Showe L,
White E and Prendergast GC: c-Myc induces apoptosis in epithelial
cells by both p53-dependent and p53-independent mechanisms.
Oncogene. 11:2411–2418. 1995.PubMed/NCBI
|
|
29
|
Lee YJ, Galoforo SS, Berns CM, Tong WP,
Kim HR and Corry PM: Glucose deprivation-induced cytotoxicity in
drug resistant human breast carcinoma MCF-7/ADR cells: role of
c-myc and bcl-2 in apoptotic cell death. J Cell Sci. 110:681–686.
1997.PubMed/NCBI
|
|
30
|
He N, Brysk H, Tyring SK, Ohkubo I and
Brysk MM: Zinc-alpha(2)-glycoprotein hinders cell proliferation and
reduces cdc2 expression. J Cell Biochem Suppl. 36:162–169. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zapata JM, Krajewska M, Krajewski S, Huang
RP, Takayama S, Wang HG, Adamson E and Reed JC: Expression of
multiple apoptosis-regulatory genes in human breast cancer cell
lines and primary tumors. Breast Cancer Res Treat. 47:129–140.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iyengar P, Combs TP, Shah SJ, Gouon-Evans
V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C,
Lisanti MP, Pestell RG and Scherer PE: Adipocyte-secreted factors
synergistically promote mammary tumorigenesis through induction of
anti-apoptotic transcriptional programs and proto-oncogene
stabilization. Oncogene. 22:6408–6423. 2003. View Article : Google Scholar
|
|
33
|
Celis JE, Moreira JM, Cabezon T, Gromov P,
Friis E, Rank F and Gromova I: Identification of extracellular and
intracellular signaling components of the mammary adipose tissue
and its interstitial fluid in high risk breast cancer patients:
toward dissecting the molecular circuitry of epithelial-adipocyte
stromal cell interactions. Mol Cell Proteomics. 4:492–522. 2005.
View Article : Google Scholar
|
|
34
|
Perera CN, Spalding HS, Mohammed SI and
Camarillo IG: Identification of proteins secreted from leptin
stimulated MCF-7 breast cancer cells: a dual proteomic approach.
Exp Biol Med. 233:708–720. 2008. View Article : Google Scholar : PubMed/NCBI
|