Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common type of cancer worldwide and only 40–50% of
patients survive 5 years after initial diagnosis (1). Among HNSCC, incidence of high-risk
(HR)-human papillomavirus (HPV)-positive oropharyngeal squamous
cell carcinoma (OSCC) has increased over the past 20 years while
the incidence of HR-HPV−HNSCC has steadily decreased
with reduced alcohol and tobacco consumption (1–4). The
high mortality of this disease is due to the development of distant
metastases and the emergence of eventually inoperable local and
regional recurrences that have low responsiveness to radiation
therapy or chemotherapy (1).
However, if HNSCC is divided into subgroups with regard to their
etiology, HR-HPV+ tumors appear to be more responsive to
treatment and patients show better survival rates (5,6).
Identification and characterization of cancer stem
cell-like cells (CSCs) in HNSCC, including OSCC, yields new
insights into the possible causes of the poor prognosis. CSCs are a
small subpopulation of cells within the tumor that exhibit
self-renewing ability and are responsible for tumor maintenance,
growth, metastasis and also for resistance towards chemotherapy and
radiation therapy (7–9). Therefore, tumor cells can be divided
into two subpopulations, a bulk population of non-CSCs and a
smaller population of CSCs (10).
CSCs have been identified by appropriate marker expressions such as
CD44 (11). High aldehyde
dehydrogenase 1 (ALDH1) activity was recently shown to identify
CSC-like cells in HNSCC (12).
ALDH1-positivity also correlates with the number of cells
undergoing epithelial-mesenchymal transition (EMT), a process that
is considered a key prerogative for the formation of metastases
(13,14).
Since it is hypothesized that CSCs play a
significant role in tumor progression, their frequency in primary
tumors may also correlate with the extent of invasion and
metastasis. Tumor cells undergoing EMT and its reverse process,
mesenchymal-to-epithelial transition, are closely related to cells
with CSC phenotype (13). We
previously reported that ALDH1+ putative CSCs expanded
from HNSCC cell lines exhibited traits including self-renewal,
quiescence, and increased expression of the stemness related genes
Oct3/4, SOX2 and NANOG. These cells also possess a higher invading
capacity and upregulated EMT-marker expression, such as Snail1 and
Twist, as well as a significantly increased expression of
mesenchymal markers such as α-smooth muscle actin and vimentin
(14). Yang et al(15) found as an essential mechanism for
EMT that Rac1 activation mediated Twist1-induced cancer cell
migration. Twist1-induced activated tumor cells had a motile
stem-like cancer cell phenotype that correlated with tumor
invasiveness and unfavorable outcome in head and neck cancer
patients. Although there is accumulating experimental evidence for
the biological role of the CSC model, clinical confirmation of the
role of CSCs in malignant progression and metastasis is
limited.
One of the main etiologies of HNSCC is a persisting
infection with oncogenic HR-HPV defining a distinct subgroup of the
disease. HPV association has been detected in 20–30% of tumors
located in all head and neck anatomic subsites and in approximately
50% of OSCCs, which were therefore chosen for this study (1). Currently, 15 confirmed HR-HPV-types
are confirmed carcinogens. As in cervical cancer, HPV type 16 seems
to play the major role in the etiology of HR-HPV-associated HNSCC.
In general, patients affected by HPV+ or HPV−
HNSCC differ in incidence, age, genetic background and prognosis
(5,16,17).
Therefore, it is of interest to investigate comparatively the
biological and clinical differences of CSCs of HR-HPV+
and HR-HPV− OSCC as well as differences between primary
tumors and their metastases.
We designed this study in paired samples of primary
OSCC and their respective lymph node metastases with the aim to
evaluate the relevance of CSC content in various stages of
HPV-related and unrelated OSCC. Understanding the associations and
relevance between HPV status and CSC content, in the progression of
OSCC could support a rationale for the development of specific
therapies for the distinct subgroups of HNSCC and also for
therapies targeting CSC directly.
Materials and methods
Patient characteristics
This study was approved by the Internal Review Board
of the University of Cologne, Germany. Study participants were
between 38 and 79 years of age (median 57.8 years; male to female
ratio 2.6:1) and only patients with no prior history of
malignancies were included. We evaluated 40 paired samples of
primary OSCC and corresponding lymph node metastases. Data on the
histological stage of tumor, differentiation and the TNM
classification were retrieved from the pathology database and
patient charts.
HPV detection and typing
HPV DNA was amplified using a highly sensitive,
group-specific nested PCR with degenerated primers A5/A10 and A6/A8
as previously described (18,19).
Briefly, direct sequence analysis of purified PCR products
(QIAquick PCR Purification kit, Qiagen, Hilden, Germany) was
carried out with an ABI Prism 377 DNA sequencer using the Taq FS
BigDye Terminator cycle sequencing method (PE Applied Biosystems,
Weiterstadt, Germany). Additionally, A6/A8 PCR products (270 base
pairs) were cloned into the vector pCR-Blunt II-Topo using the Zero
Blunt Topo PCR Cloning kit (Invitrogen, Leek, The Netherlands).
Plasmid DNA harboring an EcoRI insert of the expected size
was sequenced as mentioned above. For HPV typing, obtained sequence
information was compared with an HPV database (20).
Immunohistochemistry
Tissue specimens were fixed in 4% buffered
formaldehyde and embedded in paraffin. Sections (4 μm thick) were
mounted on Superfrost Plus glass slides (Microm, Walldorf,
Germany), deparaffinized and rehydrated in a series of graded
ethanol. Immunohistochemical staining was performed using the
two-step IHC detection reagent following the manufacturer’s
instructions (EnVision System-HRP Mouse, Dako, Hamburg, Germany).
Briefly, after microwave treatment (twice for 7 min at 600 W in 10
mM citrate buffer, pH 6.0) for antigen retrieval, endogenous
peroxidase activity was blocked by immersing slides in ChemMate
Peroxidase-Blocking Solution (Dako) 10 min at room temperature.
Slides were incubated with mouse monoclonal antibody specific for
p16 (1:100 dilution, clone DCS-50; Neomarkers, Fremont, CA, USA) or
mouse monoclonal antibody specific for ALDH1A1 (1:100 dilution,
clone 44; BD Biosciences, San Jose, CA, USA) for 2 h, followed by
addition of HRP-labeled rabbit anti-mouse secondary antibody.
Immunoreactive proteins were visualized with 3,3-diaminobenzidine
and counterstained with Mayer’s haematoxylin. Then, the sections
were dehydrated and mounted. Positive and negative controls were
included in each run for quality control of the immunoreactivity.
Normal tonsils served as positive controls and a mouse isotype
control (Dako) was used to replace the primary antibody as negative
controls.
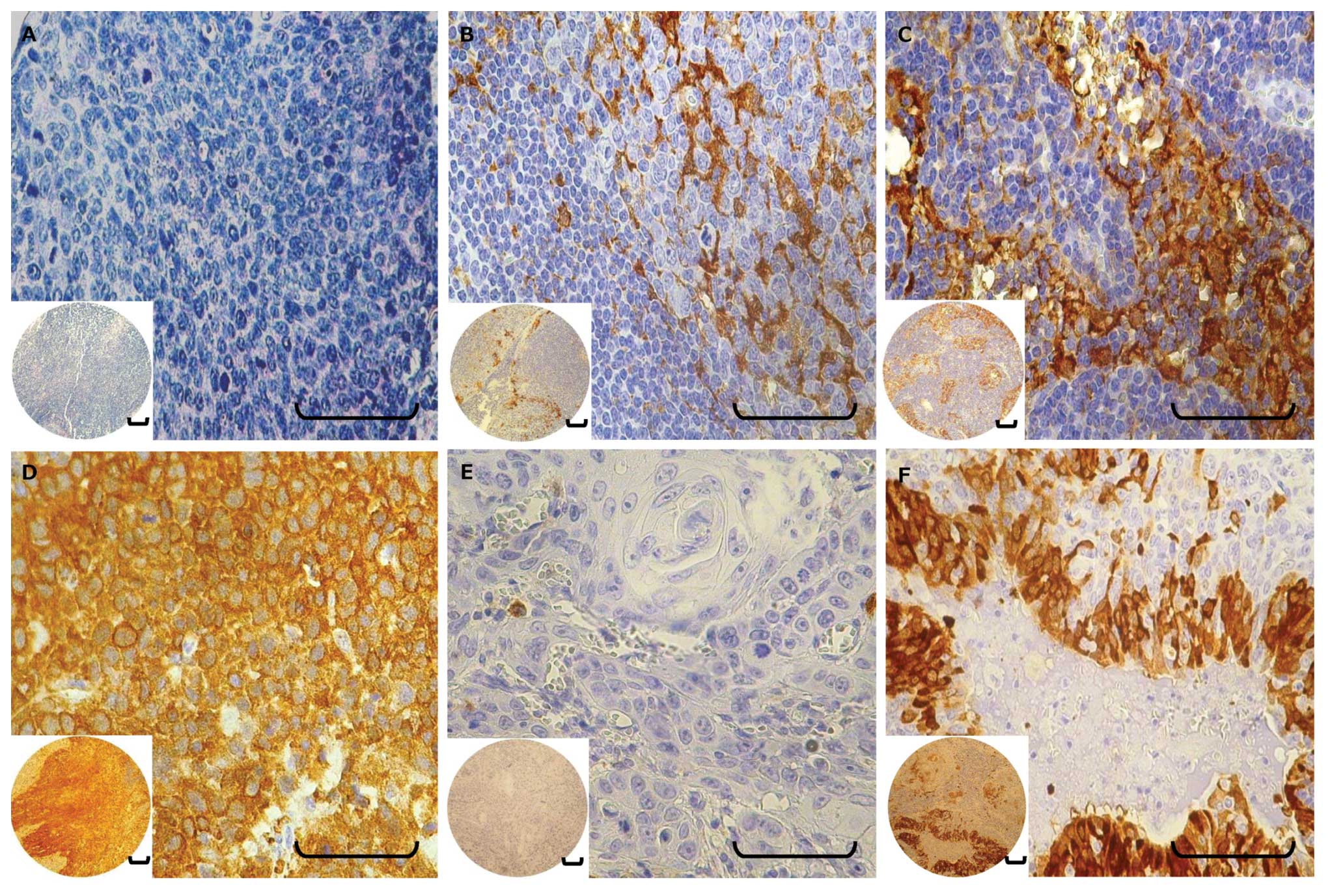
Evaluation of staining
Three independent experienced observers, who were
blinded to the patient clinical information, performed
semiquantitative evaluation of the slides. Discrepancies were
resolved by a consensus meeting using a multiheaded microscope.
Areas of carcinoma tissue within the samples and the p16 and ALDH1
expression pattern were evaluated by comparing the intensity and
cellular localization of immunoreactivity with positive and
negative controls. In general, >1,000 cells in five randomly
selected fields of tumor tissue were analyzed for each section at a
magnification of ×400 to determine percentage labeling indices. The
immunoreactivity of positively stained cells was graded into four
categories to enable statistical analysis: grade 0, <5% positive
cells; grade 1, 5–25%; grade 2, 26–50%; and grade 3, >50%. In
tumors showing heterogeneous expression, the grade was judged
according to the predominant pattern.
Data analysis
Statistical analysis was performed using the Stata
9.0-software (StataCorp LP, College Station, TX, USA). Categorical
variables were described by percentages and frequencies, and
numerical variables were represented as means ± SD. Qualitative
data were compared using the Chi-square or the Fisher’s exact test,
as appropriate. For continuous data, between-group comparisons were
performed by either the Mann-Whitney or the Student’s t-test,
depending on the normality of each variable. All statistical
comparisons were 2-sided. A P-value of <0.05 was considered to
indicate statistically significant differences.
Results
In 17.5% of the cases, the OSCC was located in the
tongue, whereas in 82.5% it was located in the tonsil. The
pathological tumor stage according to the TNM classification was as
follows: pT1, 11 (27.5%); pT2, 21 (52.5%); pT3, 8 (20%); pN1, 15
(37.5%); pN2+pN3, 25 (62.5%). As tonsil tumors tend to be
non-keratinizing or basaloid, grading is sometimes ambiguous.
Therefore, 6 cases (22%) were classified as grade 1–2 or 2–3
(Table I).
 | Table IHPV-DNA detection and expression of
HPV, p16 and ALDH1 in primary tumors and metastases. |
Table I
HPV-DNA detection and expression of
HPV, p16 and ALDH1 in primary tumors and metastases.
| HPV-DNA
detection | p16 expression | ALDH1 expression |
|---|
|
|
|
|
|---|
| −, n (%) | +, n (%) | P-value | −, n (%) | +, n (%) | P-value | −, n (%) | +, n (%) | P-value |
|---|
| Total (N=80) | N=48 | N=32 | | N=50 | N=30 | | N=9 | N=71 | |
| Origin |
| Primary
(n=40) | 20 (50) | 20 (50) | 0.068 | 23 (58) | 17 (42) | 0.356 | 5 (13) | 35 (87) | 0.723 |
| Metastasis
(n=40) | 28 (70) | 12 (30) | | 27 (68) | 13 (32) | | 4 (10) | 36 (90) | |
| HPV-DNA
detection |
| HPV+
(n=32) | | | | 5 (16) | 27 (84) | <0.001 | 3 (9) | 29 (91) | 0.665 |
| HPV−
(n=48) | | | | 45 (94) | 3 (6) | | 6 (12) | 42 (88) | |
| p16 expression |
| p16+
(n=30) | | | | | | | 2 (7) | 28 (93) | 0.315 |
| p16−
(n=50) | | | | | | | 7 (14) | 43 (86) | |
| Primary (N=40) | N=20 | N=20 | | N=23 | N=17 | | N=5 | N=35 | |
| Age (years) | 55.7±9.49 | 59.95±10.46 | 0.1863 | 56.48±9.05 | 59.65±11.37 | 0.333 | 58.4±5.08 | 57.74±10.66 | 0.894 |
| Gender |
| Female (n=11) | 6 (55) | 5 (45) | 1.000 | 7 (64) | 4 (36) | 0.726 | 2 (18) | 9 (82) | 0.603 |
| Male (n=29) | 14 (48) | 15 (52) | | 16 (53) | 13 (47) | | 3 (10) | 26 (90) | |
| Primary site |
| Tongue (n=7) | 5 (71) | 2 (29) | 0.407 | 4 (57) | 3 (43) | 1.000 | 0 | 7 (100) | 0.565 |
| Tonsil (n=33) | 15 (45) | 18 (55) | | 19 (58) | 14 (42) | | 5 (15) | 28 (85) | |
| Tumor grade |
| G1 (n=1) | 0 | 1 (100) | 0.591 | 0 | 1 (100) | 0.438 | 1 (100) | 0 | 0.009 |
| G1–2, G2
(n=19) | 10 (53) | 9 (47) | | 12 (63) | 7 (37) | | 2 (11) | 17 (89) | |
| G2–3, G3
(n=20) | 10 (50) | 10 (50) | | 11 (55) | 9 (45) | | 2 (7) | 28 (93) | |
| Tumor stage |
| T1 (n=11) | 4 (36) | 7 (64) | 0.505 | 5 (45) | 6 (55) | 0.637 | 2 (18) | 9 (82) | 0.270 |
| T2 (n=21) | 11 (52) | 10 (48) | | 13 (62) | 8 (38) | | 1 (5) | 20 (95) | |
| T3 (n=8) | 5 (63) | 3 (37) | | 5 (63) | 3 (37) | | 2 (25) | 6 (75) | |
| HPV-DNA
detection |
| HPV+
(n=20) | | | | 4 (20) | 16 (80) | <0.001 | 3 (15) | 17 (85) | 1.000 |
| HPV−
(n=20) | | | | 19 (95) | 1 (5) | | 2 (10) | 18 (90) | |
| p16 expression |
| p16+
(n=17) | | | | | | 2 (12) | 15 (88) | 1.000 | |
| p16−
(n=23) | | | | | | 3 (13) | 20 (87) | | |
| Metastasis
(N=40) | N=28 | N=12 | | N=27 | N=13 | | N=4 | N=36 | |
| N-Stage |
| N1 (n=15) | 12 (80) | 3 (20) | 0.477 | 11 (73) | 4 (27) | 0.730 | 4 (27) | 11 (73) | 0.015 |
| N2, N3 (n=25) | 16 (64) | 9 (36) | | 16 (64) | 9 (36) | | 0 | 25 (100) | |
| HPV-DNA
detection |
| HPV+
(n=12) | | | | 1 (8) | 11 (92) | <0.001 | 0 | 12 (100) | 0.297 |
| HPV−
(n=28) | | | | 26 (93) | 2 (7) | | 4 (14) | 24 (86) | |
| p16 expression |
| p16+
(n=13) | | | | | | 0 | 13 (100) | 0.284 | |
| p16−
(n=27) | | | | | | 4 (15) | 23 (85) | | |
The correlation between positive HR-HPV status and
p16 expression in total specimens (P<0.001), primary tumors
(P<0.001) and metastases was highly significant (P<0.001)
(Table I). A total of 80% of
HR-HPV-DNA positive primary tumors co-expressed p16 and 95% of
HPV-DNA negative tumors were p16-negative. Furthermore, 92% of
HR-HPV-positive metastases were p16-positive and 93% of HPV-DNA
negative metastases were p16-negative. Positive p16-status has a
specificity of 90% for HR-HPV-DNA positivity in this study. All 20
HPV-DNA negative primary tumors had negative HPV-specific PCR
findings for their metastases, and all 23 p16-negative primary
tumors had p16-negative metastases. Eight of 20 HR-HPV-DNA positive
primary tumors had metastases where no HPV-DNA was detectable. In 4
out of 17 p16-positive primary tumors, the corresponding metastasis
was p16-negative. No significant association was observed between
HPV-DNA status or p16 expression and clinicopathological
parameters.
Expression of ALDH1 was detected in 87.5% of primary
tumors and in 90% of the metastases. ALDH1 positivity was
significantly correlated with lower tumor differentiation (P=0.009)
and higher N classification (P=0.015) (Table I). Subsequently, samples were
divided into groups with different ALDH1 expression grades (0–3).
Higher expression grades of ALDH1 were also more frequent in
primary tumors with lower tumor differentiation (P=0.022) and
higher N classification, (P=0.025) (Table II). Next, we analyzed the
HPV-status, p16, and ALDH1 positivity in metastases, however, no
significant correlation was found. By contrast, ALDH1 expression
grades were significantly elevated in metastases vs. primary tumors
(P=0.012) (Table III).
 | Table IICorrelation between ALDH1 grade and
clinicopathological characteristics. |
Table II
Correlation between ALDH1 grade and
clinicopathological characteristics.
| ALDH1 expression
grade |
|---|
|
|
|---|
| | 0 | 1 | 2 | 3 | |
|---|
|
|
|---|
|
Characteristics | n | n (%) | n (%) | n (%) | n (%) | P-value |
|---|
| Primary site |
| Tongue | 7 | 2 (29) | 2 (29) | 2 (29) | 1 (14) | 0.725 |
| Tonsil | 33 | 12 (36) | 5 (15) | 3 (9) | 13 (39) | |
| Origin |
| Primary | 40 | 14 (35) | 7 (17) | 4 (10) | 15 (38) | 0.012 |
| Metastasis | 40 | 4 (19) | 3 (8) | 8 (20) | 25 (62) | |
| Tumor
differentiation grade |
| G1 | 1 | 1 (100) | 0 | 0 | 0 | 0.022 |
| G1–2, G2 | 19 | 8 (42) | 6 (32) | 2 (10) | 3 (16) | |
| G2–3, G3 | 20 | 5 (25) | 1 (5) | 2 (10) | 12 (60) | |
| Tumor (T)
stage |
| T1 | 11 | 6 (55) | 3 (27) | 0 | 2 (18) | 0.206 |
| T2 | 21 | 5 (24) | 4 (19) | 2 (10) | 10 (48) | |
| T3 | 8 | 3 (38) | 0 | 2 (25) | 3 (38) | |
| Nodal (N)
stage |
| N1 | 15 | 4 (27) | 1 (7) | 1 (7) | 9 (60) | 0.025 |
| N2, N3 | 25 | 0 | 2 (8) | 7 (28) | 16 (64) | |
| HPV-DNA in total
specimens |
|
HPV+ | 32 | 11 (34) | 5 (16) | 4 (13) | 12 (38) | 0.130 |
|
HPV− | 48 | 7 (15) | 5 (10) | 8 (17) | 28 (58) | |
| HPV-DNA in
primaries |
|
HPV+ | 20 | 11 (55) | 5 (25) | 1 (5) | 3 (15) | 0.004 |
|
HPV− | 20 | 3 (15) | 2 (10) | 3 (15) | 12 (60) | |
| HPV-DNA in
metastasis |
|
HPV+ | 12 | 0 | 0 | 3 (25) | 9 (75) | 0.429 |
|
HPV− | 28 | 4 (14) | 3 (11) | 5 (18) | 16 (57) | |
| p16 expression in
total specimens |
|
p16+ | 30 | 7 (23) | 5 (17) | 4 (13) | 14 (47) | 0.831 |
|
p16− | 50 | 11 (22) | 5 (10) | 8 (16) | 26 (52) | |
| p16 expression in
primaries |
|
p16+ | 17 | 7 (41) | 5 (29) | 1 (6) | 4 (24) | 0.200 |
|
p16− | 23 | 7 (30) | 2 (9) | 3 (13) | 11 (48) | |
| p16 expression in
metastasis |
|
p16+ | 13 | 0 | 0 | 3 (23) | 10 (77) | 0.362 |
|
p16− | 27 | 4 (15) | 3 (11) | 5 (19) | 15 (56) | |
 | Table IIICorrelation between HPV-DNA detection
with p16 and ALDH1 expression. |
Table III
Correlation between HPV-DNA detection
with p16 and ALDH1 expression.
| | | | | ALDH1 expression
grade |
|---|
| | | | |
|
|---|
| | | | | 0 | 1 | 2 | 3 | |
|---|
| | | | |
|
|---|
|
Characteristics | n |
ALDH1+ |
ALDH1− | P-value | n (%) | n (%) | n (%) | n (%) | P-value |
|---|
| Primary tumor |
|
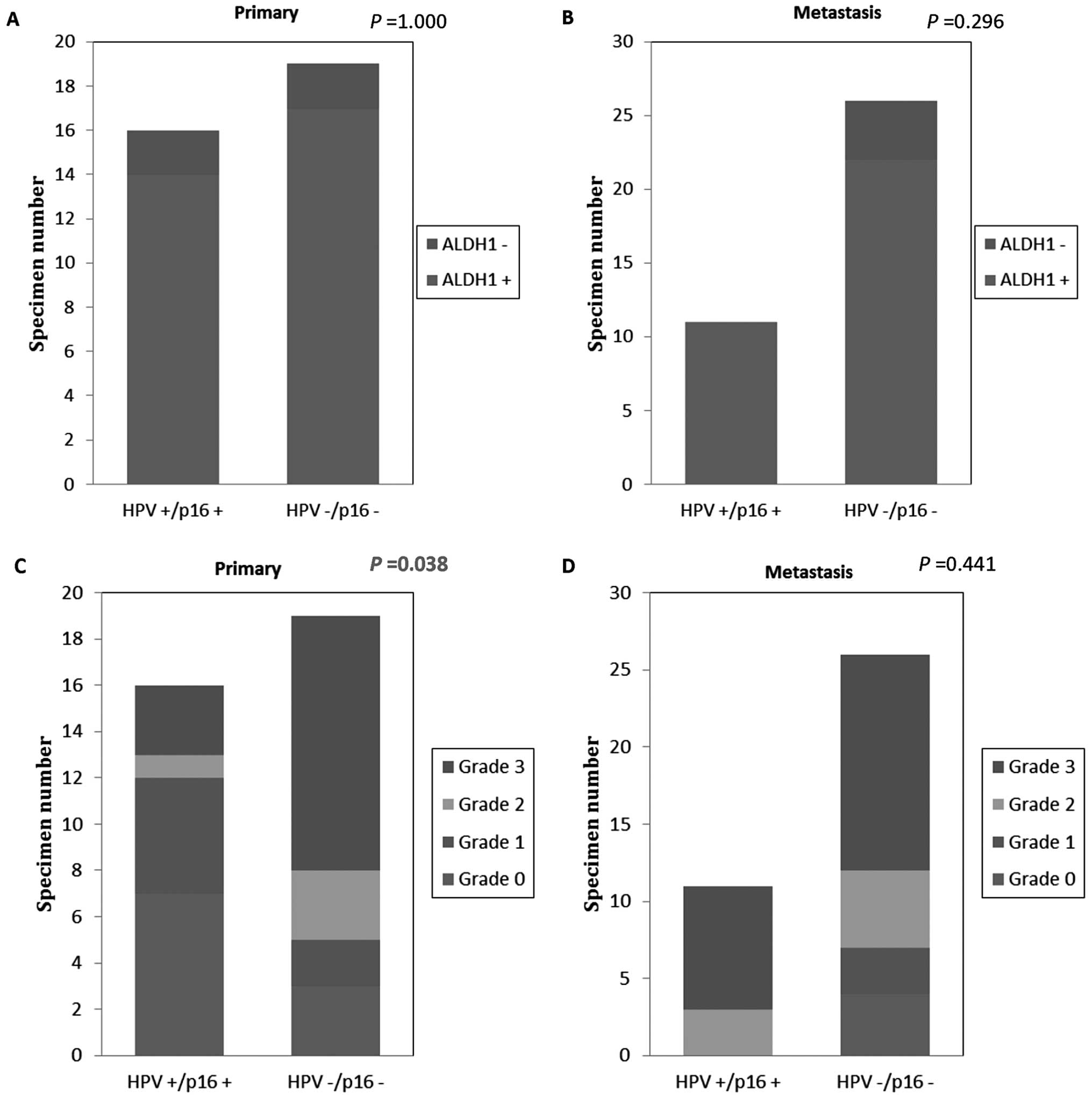
HR-HPV+/p16+ | 16 | 14 (88) | 2 (12) | 1.000 | 7 (44) | 5 (31) | 1 (6) | 3 (19) | 0.038 |
|
HPV−/p16− | 19 | 17 (89) | 2 (11) | | 6 (32) | 2 (11) | 3 (16) | 11 (58) | |
| Metastasis |
|
HR-HPV+/p16+ | 11 | 11 (100) | 0 | 0.296 | 0 | 0 | 3 (27) | 8 (73) | 0.441 |
|
HPV−/p16− | 26 | 22 (85) | 4 (15) | | 4 (15) | 3 (11) | 5 (19) | 14 (54) | |
Comparing HPV-negative and -positive tumors, there
was no correlation between ALDH1 expression and HPV status in total
specimens (P=0.665), primary tumors (P=1) and metastases (P=0.297)
(Table I). Significant
correlations, however, were found between higher ALDH1 expression
grades and negative HPV status for primary tumors (P=0.004), but
not for metastases (P=0.429) (Table
II). There was no correlation between ALDH1 expression and p16
expression for primary tumors (P=1) and metastases (P=0.284)
(Table I). There was no significant
correlation between the ALDH1 expression grade and p16 expression
between the primary tumors (P=0.2) and metastases (P=0.362)
(Table II).
Subgroups of HR-HPV-DNA+/p16+
and HPV-DNA−/p16− tumors were correlated with
ALDH1 expression. There were no correlations between these two
groups concerning ALDH1-positive and -negative expression in
primary tumors or metastases. However, when comparing these
subgroups concerning ALDH1 expression grades, we found that
HPV-DNA+/p16+ primary tumors exhibited
significantly lower ALDH1 expression grades, whereas
HPV-DNA−/p16− primary tumors presented with
higher ALDH1 grades (P=0.038) (Fig.
1). There were no similar findings for metastases (P=0.441).
Representative examples are shown in Fig. 2.
Discussion
In elucidating HNSCC tumorigenesis, CSC research is
currently a promising field that could lead to a better
understanding of the formation of recurrence, metastasis, and
resistance to radiotherapy and chemotherapy. Prince et
al(11) were among the first to
demonstrate that the CD44+ cell population in HNSCC
possesses properties of cancer stem cells. Later, Chen et
al(12) showed that
ALDH1+ cells derived from HNSCC were tumorigenic and
displayed resistance towards radiotherapy. In another study, the
authors found that silencing of Bmi-1, a transcriptional repressor
essential for maintaining the self-renewal abilities of adult stem
cells and CSCs, significantly increased the sensitivity of
ALDH1+ HNSCC cells to chemo-radiation and the degree of
chemo-radiation-mediated apoptosis (21). Therefore, measuring ALDH1 expression
is promising as a marker for therapeutic success and prognosis.
In the current study, we specifically focused on the
clinical and histopathological characteristics of OSCC in relation
to ALDH1 expression. We investigated the presence of
ALDH1+ CSCs in primary OSCC as a subgroup of HNSCC that
is in approximately half of the cases HR-HPV-positive, and in
corresponding lymph node metastases from clinical paraffin-embedded
specimens. We demonstrated that higher ALDH1 grades are
significantly associated with a lower grade of tumor
differentiation and a higher nodal classification of the OSCC. This
indicates that the frequency of CSCs is related to poorly
differentiated OSCC and the occurrence of nodal metastasis and that
the frequency of CSCs is related to the progression of OSCC and
possibly plays a role in this process. In support of these
findings, numerous recent studies of carcinomas of different origin
also presented traits of high-grade malignancy that could be
specifically traced to the presence of CSCs, indicating that CSCs
may be the critical drivers of tumor progression (22,23).
Moreover, CSCs have been defined by their key trait,
the ability to seed new tumors and metastases (13). In our previous study in HNSCC cell
lines, the invasiveness of tumor cells was explored and correlated
with the CSC phenotype (14). CSCs
reside in close proximity to blood vessels and can give rise to
tumor endothelium (24). It was
reported that endothelial-derived factors inhibit anoikis of
ALDH+CD44+ head and neck cancer stem cells
(25). EMT is regarded a necessary
process that empowers CSCs to disseminate from primary tumors and
to seed metastases. In order to understand the role of
ALDH1+ CSCs in the progression of OSCC, we analyzed
their frequency and distribution in primary tumors and their
metastases. Only few previous studies have thus far comparatively
studied CSCs in primary tumors with their associated metastases. It
was reported that increased ALDH1 expression in patients with
breast cancer lymph node metastases serves as a prognostic factor
of poor clinical outcome (26).
We have demonstrated the expression of ALDH1 in
pairs of primary OSCC and corresponding lymph node metastases. In
the total case collection, metastasis did not display a
significantly increased number of ALDH1+ cells when
compared to the primary tumor. However, if the percentage of
ALDH1+ cells in the materials were classified into
grades 0–3, significant differences between primary tumor and
corresponding lymph node metastases became apparent. Lower ALDH1
grades in the primary were seen in HPV+ tumors and
higher grades in HPV− primaries. By contrast, this
difference was not observed in metastases. In general a higher
grade of ALDH1 expression was more frequently found in metastases
and also correlated with a higher nodal metastasis status of the
patient. The observation that the number of CSCs was increased in
metastases may reflect the fact that successful seeding of
metastases requires a more motile cellular phenotype that is
supported by the ability of CSCs to undergo EMT. It remains to be
explored if the increase of CSC numbers manifests transiently e.g.
in the initiation period during the metastatic colonization and
decreases later or displays a stable state. In accordance with our
findings, Malanchi et al(27) found that in a breast cancer model,
the relative size of the CSC population increased during the early
period of metastatic colonization in the secondary target organ,
i.e. the lung. In our study, metastases presented higher grades of
ALDH1 expression than the corresponding primary tumor. This
evidence could also be indicative of the active function of CSCs in
the process of metastasis formation.
In the present study, we showed, for the first time,
a significant association between ALDH1+ OSCC and their
nodal metastases. Taken together, the results of our study of EMT
capacity and ALDH association of HNSCC cell lines and the current
data from clinical paraffin-embedded specimens, ALDH1+
CSCs are highly invasive and have metastatic ability. Recently, Xu
et al(28) investigated the
ALDH1+ CSC content of primary metastatic or
non-metastatic HNSCC. ALDH1 expression in primary tumors correlated
with lymph node metastasis. Patients with low ALDH1 expression
levels in primary tumors had a better 5-year survival rate than
those with high ALDH1 expression levels.
With regard to the etiology of OSCC, the HPV status
was also explored in the present study. In the past, much attention
has focused on the possible role of HPV infection in the
pathogenesis of OSCC. HPV and protein p16INKa (p16) are
among the most commonly reported OSCC markers. In agreement with
previous studies, our findings demonstrate that HPV and p16 are
highly correlated in both primary OSCC and metastases (5,29).
Numerous studies reported that HR-HPV+ OSCC patients
showed better survival than those with HPV− OSCC,
although HPV+ tumors are less differentiated than
HPV− tumors and present more frequently with nodal
metastases (30,31). These counterintuitive findings
suggest HR-HPV associated tumors have distinct biological features.
We observed that there was a significant correlation between a
higher ALDH1 expression grade in the tissue and negative HPV status
for primary tumors, but not for metastases. By subgroup analyses,
we found HR-HPV-DNA+/p16+ primary tumors
exhibited lower ALDH1 expression grades representing a lower number
of CSCs, while HPV-DNA−/p16− primary tumors
had higher ALDH1 grades representing a higher CSC frequency. Our
data support the existence of distinct tumor cell characteristics
defined by the HPV status. This difference is reflected by
different ALDH1 grading in the primary tumors.
Since ALDH1+ CSCs might play an important
role in the progression of malignancies, our findings may help to
explain the better survival of HPV+ OSCC patients. It is
evident that only enumerating the frequency of CSC populations in
HR-HPV-related OSCC (low in primary and high in metastases) does
not yield a satisfying answer. The function of HPV in CSCs as well
as the genetic and epigenetic changes in OSCC remain to be
elucidated in future studies.
In conclusion, in this initial study we
characterized the patterns of ALDH1, p16 expression and HPV-DNA
status in primary OSCC and their lymph node metastases. Our data
suggest that enumeration alone does not suffice, and that studying
phenotypical and functional states of CSCs is critical in
understanding their specific abilities that determine differences
in the prognosis of HPV-related and unrelated OSCC. These insights
may support therapeutic design and decisions in the future.
Acknowledgements
The authors thank Ms. Erika Berg for her technical
assistance. Dr Xu Qian received a stipend from ZAST-organization
dedicated to support scientific exchange between China and
Germany.
References
|
1
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar
|
|
2
|
Chaturvedi AK, Engels EA, Pfeiffer RM, et
al: Human papillomavirus and rising oropharyngeal cancer incidence
in the United States. J Clin Oncol. 29:4294–4301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Licitra L, Zigon G, Gatta G, Sanchez MJ
and Berrino F; EUROCARE Working Group. Human papillomavirus in
HNSCC: a European epidemiologic perspective. Hematol Oncol Clin
North Am. 22:1143–1153. vii–viii. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nasman A, Attner P, Hammarstedt L, et al:
Incidence of human papillomavirus (HPV) positive tonsillar
carcinoma in Stockholm, Sweden: an epidemic of viral-induced
carcinoma? Int J Cancer. 125:362–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ang KK, Harris J, Wheeler R, et al: Human
papillomavirus and survival of patients with oropharyngeal cancer.
N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillison ML, D’Souza G, Westra W, et al:
Distinct risk factor profiles for human papillomavirus type
16-positive and human papillomavirus type 16-negative head and neck
cancers. J Natl Cancer Inst. 100:407–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koukourakis MI, Giatromanolaki A, Tsakmaki
V, Danielidis V and Sivridis E: Cancer stem cell phenotype relates
to radio-chemotherapy outcome in locally advanced squamous cell
head-neck cancer. Br J Cancer. 106:846–853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: an evolving concept. Nat Rev Cancer. 12:133–143.
2012.PubMed/NCBI
|
|
9
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albers AE, Chen C, Koberle B, et al: Stem
cells in squamous head and neck cancer. Crit Rev Oncol Hematol.
81:224–240. 2012. View Article : Google Scholar
|
|
11
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YC, Chen YW, Hsu HS, et al: Aldehyde
dehydrogenase 1 is a putative marker for cancer stem cells in head
and neck squamous cancer. Biochem Biophys Res Commun. 385:307–313.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Wei Y, Hummel M, et al: Evidence
for epithelial-mesenchymal transition in cancer stem cells of head
and neck squamous cell carcinoma. PLoS One. 6:e164662011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang WH, Lan HY, Huang CH, et al: RAC1
activation mediates Twist1-induced cancer cell migration. Nat Cell
Biol. 14:366–374. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gillison ML, Koch WM, Capone RB, et al:
Evidence for a causal association between human papillomavirus and
a subset of head and neck cancers. J Natl Cancer Inst. 92:709–720.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klussmann JP, Mooren JJ, Lehnen M, et al:
Genetic signatures of HPV-related and unrelated oropharyngeal
carcinoma and their prognostic implications. Clin Cancer Res.
15:1779–1786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klussmann JP, Weissenborn SJ, Wieland U,
et al: Prevalence, distribution, and viral load of human
papillomavirus 16 DNA in tonsillar carcinomas. Cancer.
92:2875–2884. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wieland U, Ritzkowsky A, Stoltidis M, et
al: Papillomavirus DNA in basal cell carcinomas of immunocompetent
patients: an accidental association? J Invest Dermatol.
115:124–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Myers G, Baker C, Münger K, Sverdrup F,
McBride A and Bernard HU: Human Papillomaviruses 1997. Los Alamos
National Laboratory; Los Alamos, NM: 1997
|
|
21
|
Chen YC, Chang CJ, Hsu HS, et al:
Inhibition of tumorigenicity and enhancement of
radiochemosensitivity in head and neck squamous cell cancer-derived
ALDH1-positive cells by knockdown of Bmi-1. Oral Oncol. 46:158–165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charafe-Jauffret E, Ginestier C, Iovino F,
et al: Breast cancer cell lines contain functional cancer stem
cells with metastatic capacity and a distinct molecular signature.
Cancer Res. 69:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pang R, Law WL, Chu AC, et al: A
subpopulation of CD26+ cancer stem cells with metastatic
capacity in human colorectal cancer. Cell Stem Cell. 6:603–615.
2010.
|
|
24
|
Wang R, Chadalavada K, Wilshire J, et al:
Glioblastoma stem-like cells give rise to tumour endothelium.
Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campos MS, Neiva KG, Meyers KA,
Krishnamurthy S and Nor JE: Endothelial derived factors inhibit
anoikis of head and neck cancer stem cells. Oral Oncol. 48:26–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nogami T, Shien T, Tanaka T, et al:
Expression of ALDH1 in axillary lymph node metastases is a
prognostic factor of poor clinical outcome in breast cancer
patients with 1–3 lymph node metastases. Breast Cancer. Mar
10–2012.(Epub ahead of print).
|
|
27
|
Malanchi I, Santamaria-Martinez A, Susanto
E, et al: Interactions between cancer stem cells and their niche
govern metastatic colonization. Nature. 481:85–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu J, Muller S, Nannapaneni S, et al:
Comparison of quantum dot technology with conventional
immunohistochemistry in examining aldehyde dehydrogenase 1A1 as a
potential biomarker for lymph node metastasis of head and neck
cancer. Eur J Cancer. 8:1682–1689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D’Souza G, Kreimer AR, Viscidi R, et al:
Case-control study of human papillomavirus and oropharyngeal
cancer. N Engl J Med. 356:1944–1956. 2007.PubMed/NCBI
|
|
30
|
Begum S and Westra WH: Basaloid squamous
cell carcinoma of the head and neck is a mixed variant that can be
further resolved by HPV status. Am J Surg Pathol. 32:1044–1050.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mendelsohn AH, Lai CK, Shintaku IP, et al:
Histopathologic findings of HPV and p16 positive HNSCC.
Laryngoscope. 120:1788–1794. 2010. View Article : Google Scholar : PubMed/NCBI
|