Introduction
Curcumin is a phenolic compound that is extracted
from Zingiberaceae turmeric and has strong anti-inflammatory,
antioxidant and antimutation properties. Recent studies have shown
that curcumin can inhibit the growth, invasion and metastasis of a
variety of tumor cells, it can induce apoptosis through a variety
of mechanisms, and can improve the sensitivity of tumor cells to
chemotherapeutic drugs and radiotherapy (1,2).
Transforming growth factor-β1 (TGF-β1) can induce
tumor cell epithelial-mesenchymal transition (EMT) (3,4). EMT
is involved in tumorigenesis and development and plays an important
role in the promotion of tumor invasion and metastasis and it is
also closely related to drug resistance of tumor cells (5,6). In
some particular physiological or pathological conditions, the
epithelial cells lose their polarity, convert into interstitial
cells and are able to move freely in the cell matrix. The most
important characteristic of EMT cells is the reduction or loss of
the epithelial marker E-cadherin (7,8) and
the induction of the mesenchymal markers such as fibronectin
protein, vimentin.
Studies have shown that the abnormal activation of
Hedgehog (Hh) signaling plays a significant role in tumorigenesis
and metastasis (9,10). The Hh signaling pathway is a highly
conserved signaling pathway, composed of the signal molecule Hh,
two membrane receptors patched (Ptch) and smoothened (Smo), and the
downstream nuclear transcription factor Gli. GIi proteins play a
key role in the Hh signaling pathway. If having no ligands, Ptch
binds with Smo and inhibits the function of Smo. When Hh presented,
Hh ligand binds to its receptor Ptch, Smo is released and activated
and, ultimately, the entire length of Gli transported to the
nucleus and activated the transcription of target genes. Gli target
genes are involved in tumor cell growth, apoptosis, metastasis, EMT
and neovascularization (11).
In the present study, we stimulated the pancreatic
cancer PANC-1 cells with TGF-β1, we treated the cells with
different concentrations of curcumin and then detected the
proliferation and apoptosis of each group of cells. We analyzed the
expression levels of Shh, GLI1, E-cadherin and vimentin and
evaluated the invasion and migration ability of each group of cells
in vitro. The aim of the present study was to investigate
whether curcumin can reverse the TGF-β1-induced EMT of pancreatic
cancer PANC-1 cells and to explore its possible mechanism.
Materials and methods
Cell lines and reagents
Pancreatic cancer PANC-1 cells were purchased from
the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Antibodies of rabbit anti-human Shh, GLI1,
vimentin and E-cadherin were purchased from Cell Signaling
Technology (Beverly, MA, USA). Mouse anti-rabbit secondary antibody
was obtained from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd (Beijing, China). Annexin V/PI apoptosis detection kit was
purchased from Multisciences Biotech Co., Ltd. (Hangzhou, China).
DMEM and fetal bovine serum were purchased from Gibco (Grand
Island, NY, USA). Curcumin and MTT were purchased from
Sigma-Aldrich (Irvine, CA, USA). TGF-β1 was purchased from R&D
Systems China Co., Ltd. (Shanghai, China). BD Matrigel™ Matrix
basement membrane was purchased from BD Biosciences (Franklin
Lakes, NJ, USA). Transwell insert (8 μm) was purchased from Corning
Incorporated (Corning, NY, USA).
Cell treatment
The pancreatic cancer cell line PANC-1 was grown in
complete Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum (FBS), 100 U/ml penicillin and
streptomycin in 5% CO2 at 37°. Cells were changed for
fresh medium every 3 days. Cells were cocultured with TGF-β1 (5
ng/ml) for 7 days. Then, the TGF-β1-stimulated pancreatic cancer
cells were treated with different concentrations of curcumin (10,
20 and 30 μmol/ml) for 48 h.
Cell proliferation assay (MTT)
MTT was used to investigate the cellular growth
inhibition rate. Following digestion with 0.25% trypsin,
1×104 TGF-β1-stimulated pancreatic cancer cells were
seeded in a 96-well plate and allowed to adhere to the plate for 24
h at 37°C. The cells were then divided into the control group and
the curcumin-treated group (10, 20 and 30 μmol/ml) and were treated
for 48 h. Thereafter, the cells in 96-well plates were centrifuged
at 500 × g for 3 min, with 100 μl supernatant in each well removed.
Then, 10 μl (5 mg/ml) MTT in PBS was added to each well and the
microtiter plates were incubated for an additional 4 h at 37°C.
DMSO (100 μl) was then added to each well and OD570 value was
detected with enzyme-labeled immunoassay instrument after shaking
for 10 min. The growth inhibition rate was calculated as follows:
Inhibition rate = (1 − Atreatment group/Acontrol
group) × 100%.
Cell apoptosis assay by flow
cytometry
Cell apoptosis was investigated with the Annexin
V-FITC apoptosis detection kit according to the corresponding
protocols: 1×105 TGF-β1-stimulated pancreatic cancer
cells were seeded in 6-well plates for 24 h at 37°C. Then, the
cells were divided into the control and the curcumin-treated group
(10, 20 and 30 μmol/ml). Forty-eight hours later, cells in 6-well
plates were collected and centrifuged at 800 × g for 5 min. After
adding buffer solution (50 μl), V-FITC (5 μl) and PI (10 μl) for 5
min, cells were transferred for flow cytometry assay.
Western blot analysis of Shh, GLI1,
vimentin and E-cadherin
After PANC-1 cells were cocultured with TGF-β1 (5
ng/ml) for 7 days, the expression levels of Shh, GLI1, vimentin and
E-cadherin were detected by western blot analysis.
For western blot analysis, we prepared separation
and spacer gels. Cells in the above groups were collected
separately and total proteins in each group were extracted with the
one-shot method. After mixing with 4X loading buffer, boiling for 5
min, and centrifuging at 12,000 rpm for 3 min, 20 μl of the
resulting sample was used for SDS-PAGE analysis at 100 V. When the
separation step was completed, the gel was washed once with
Millipore H2O, electroblotted onto transfer membranes at
100 V for 2 h, and then blocked with blocking reagent for 2 h. The
blots were incubated with corresponding primary antibodies for 24
h. After washing four times (10 min each time) with Tris-buffered
saline with Tween-20 (TBST), the membranes were incubated 1 h with
secondary antibodies conjugated with horseradish peroxidase. The
membranes were again washed four times (10 min each time) with TBST
and incubated with ECL. Band Leader software was used for
densitometry analysis of protein bands with GAPDH as internal
control.
Subsequently, the TGF-β1-stimulated pancreatic
cancer PANC-1 cells were treated with curcumin (30 μmol/ml) for 48
h. The expression levels of Shh, GLI1, vimentin and E-cadherin were
also detected by western blot analysis, as described above.
Transwell cell invasion assay
Transwell chambers were placed in a 24-well plate.
Matrigel was mixed with serum-free DMEM (1:3). Each small chamber
was supplemented with 30 μl of the Matrigel mixture, and then
placed at 37°C for 30 min. Then, 30 μl serum-free DMEM containing
0.1% BSA was added to each chamber. Cells were then divided into
the TGF-β1-stimulated PANC-1 control group and the curcumin-treated
group (30 μmol/ml). Cells of each group (3×104) were
resuspended in 300 μl serum-free DMEM with 0.1% BSA and seeded in
the transwell upper chamber. DMEM (600 μl) containing 10% FBS was
added to the lower chamber of the transwell. The cells were then
incubated at 37°C, 5% CO2 for 48 h, the culture medium
was absorbed and the cells of the microporous membranes were gently
wiped off with a cotton swab; the invaded cells of the lower
surface of the microporous membrane were retained in ice methanol
for 1 min and H&E stained. The invading cells were counted in
five randomly selected high magnification fields (x200). The
experiment was repeated 5 times.
Wound healing assay
TGF-β1-stimulated pancreatic cancer cells
(1×106) were seeded in 6-well plates. Cells were divided
into the TGF-β1-stimulated PANC-1 control group and the
curcumin-treated group (30 μmol/ml). Upon 80% confluence, cells
were scraped across the cell monolayer using a plastic 200 μl tip.
Photomicrographs were captured at 24 h. The measured ratio of the
remaining wound area relative to the initial wound area was
quantified and reported. The data are described as closed
width/scratch width (%, mean ± SD). The experiment was repeated 5
times, independently.
Statistical analysis
SPSS 13.0 software was used for statistical analysis
and data are shown as the means ± SD. Statistical differences were
determined by t-test analysis and P<0.05 was considered to
indicate a statistically significant result.
Results
Expression level of Shh, GLI1, E-cadherin
and vimentin in TGFβ1-stimulated PANC-1 cells
The Hh signaling pathway is abnormally activated in
a variety of tumors and is associated with development and
invasion, and plays an important role in the transfer process. Hh
signaling target genes are related to tumor cell growth, apoptosis,
metastasis, EMT. To explore whether TGF-β1 induces the EMT of
PANC-1 cells through activation of the Hh signaling, the expression
levels of Shh, GLI1, E-cadherin and vimentin in TGF-β1-stimulated
PANC-1 cells were detected by western blot analysis.
Taking GAPDH as internal control, the results were
obtained by semi-quantitative analysis of the gray scales of bands
using Band Leader software. The results demonstrated that the
expression levels of Shh and GLI1 in the TGF-β1-stimulated group
were significantly increased, compared with those in the control
group (t=7.30, P<0.01; t=9.12, P<0.01, respectively). The
expression level of E-cadherin in the TGF-β1-stimulated group was
significantly decreased, compared with that in the control group
(t=7.89, P<0.01), and the expression level of vimentin in the
TGF-β1-stimulated group was also significantly elevated, compared
with that in the control group (t=8.15, P<0.01). These results
suggest that the TGF-β1-stimulated PANC-1 cells are able to induce
activation of the Hh signaling pathway and the EMT of PANC-1 cells,
as shown in Fig. 1.
Curcumin inhibits proliferation and
induces apoptosis of TGF-β1-stimulated PANC-1 cells
TGF-β1 can induce pancreatic cancer PANC-1 cell EMT.
Our experimental results also suggest that following stimulation
with TGF-β1, the proliferation, invasiveness and migration ability
of PANC-1 cells increased and the cell morphology changed,
suggesting the occurrence of EMT.
Curcumin can inhibit the growth of various tumor
cells through a variety of mechanisms and it can induce apoptosis.
In order to verify the role of curcumin in TGF-β1-stimulated PANC-1
cells, we treated the cells with different concentrations of
curcumin (10, 20 and 30 μmol/ml) for 48 h.
The results indicated that as the curcumin
concentration increased, its proliferation inhibitory effect on
TGF-β1-stimulated PANC-1 cells also increased. Using t-test
analysis, the proliferation inhibiting effect of the 10 μmol/ml
curcumin-treated group was significantly higher than that of the
control group (P<0.01). The proliferation inhibiting effect of
the 20 μmol/ml curcumin-treated group was significantly higher than
that of the 10 μmol/ml curcumin-treated and the control group
(P<0.01, respectively), and the proliferation inhibiting effect
of the 30 μmol/ml curcumin-treated group was also significantly
higher than that of the 10 and 20 μmol/ml curcumin-treated and the
control group (P<0.01, respectively) as shown in Table I.
 | Table IGrowth inhibition rates of
TGF-β1-stimulated PANC-1 cells treated with curcumin detected by
MTT (%, mean ± SD). |
Table I
Growth inhibition rates of
TGF-β1-stimulated PANC-1 cells treated with curcumin detected by
MTT (%, mean ± SD).
| Group | Growth inhibition
rate |
|---|
| Control group | 0 |
| Curcumin (10
μmol/ml)a | 13.60±3.39 |
| Curcumin (20
μmol/ml)a,b | 17.43±3.70 |
| Curcumin (30
μmol/ml)a,b,c | 62.18±6.87 |
The experimental results of the flow cytometry also
showed that when the curcumin concentration increased, the
apoptosis of TGF-β1-stimulated PANC-1 cells also increased. With
t-test analysis, the apoptosis of the 10 μmol/ml curcumin-treated
group was significantly different from that of the control group
(P<0.01). The apoptosis of the 20 μmol/ml curcumin-treated group
was significantly different from that of the 10 μmol/ml
curcumin-treated and the control group (P<0.01, respectively),
and the apoptosis of the 30 mol/ml curcumin-treated group was also
significantly different from that of the 10 and 20 μmol/ml
curcumin-treated and the control group (P<0.01, respectively) as
shown in Table II, Fig. 2.
 | Table IIApoptosis rate of TGF-β1-stimulated
PANC-1 cells treated with curcumin detected by flow cytometery (%,
mean ± SD). |
Table II
Apoptosis rate of TGF-β1-stimulated
PANC-1 cells treated with curcumin detected by flow cytometery (%,
mean ± SD).
| Group | Apoptosis rate |
|---|
| Control group | 7.62±0.32 |
| Curcumin (10
μmol/ml)a | 9.52±0.56 |
| Curcumin (20
μmol/ml)a,b | 12.92±0.67 |
| Curcumin (30
μmol/ml)a,b,c | 35.57±1.26 |
Effects of curcumin on the expression
levels of Shh, GLI1, E-cadherin and vimentin in TGFβ1-stimulated
PANC-1 cells
The antitumor mechanism of curcumin is highly
complicated and involves several signaling pathways, such as EGFR,
HER-2, Wnt/β-catenin and its downstream signaling molecules ERK,
AKT, NF-κB, STATs. In order to investigate whether curcumin is able
to reverse the EMT of TGF-β1-stimulated PANC-1 cells by inhibiting
the Hh signaling pathway, the TGF-β1-stimulated PANC-1 cells were
treated with 30 μmol/ml curcumin for 48 h, and the expression
levels of Shh, GLI1, vimentin and E-cadherin were then detected by
western blot analysis.
Taking GAPDH as internal control, the results were
obtained by semi-quantitative analysis of the gray scales of bands
using Band Leader software. The results revealed that the
expression levels of Shh and GLI1 in the curcumin-treated group
were significantly decreased compared with those in the control
group (t=14.66, P<0.01; t=7.46, P<0.01, respectively), the
expression level of E-cadherin in the curcumin-treated group was
significantly increased compared with that in the control group
(t=4.37, P<0.01), and the expression level of vimentin in the
curcumin-treated group was also significantly decreased compared
with that in the control group (t=5.00, P<0.01). The results
suggest that curcumin inhibits the activation of the Hh signaling
pathway and reverses the EMT of TGF-β1-stimulated PANC-1 cells, as
shown in Fig. 3.
Curcumin inhibits cell invasion and
migration of TGF-β1-stimulated PANC-1 cells
EMT is defined as particular physiological or
pathological conditions in which the epithelial cells with polarity
lose their polarity and convert into interstitial cells with the
ability to move freely in the cell matrix. The tumor cells that
acquire EMT phenotype have increased invasion ability and are easy
to transfer. To verify whether curcumin can inhibit the metastatic
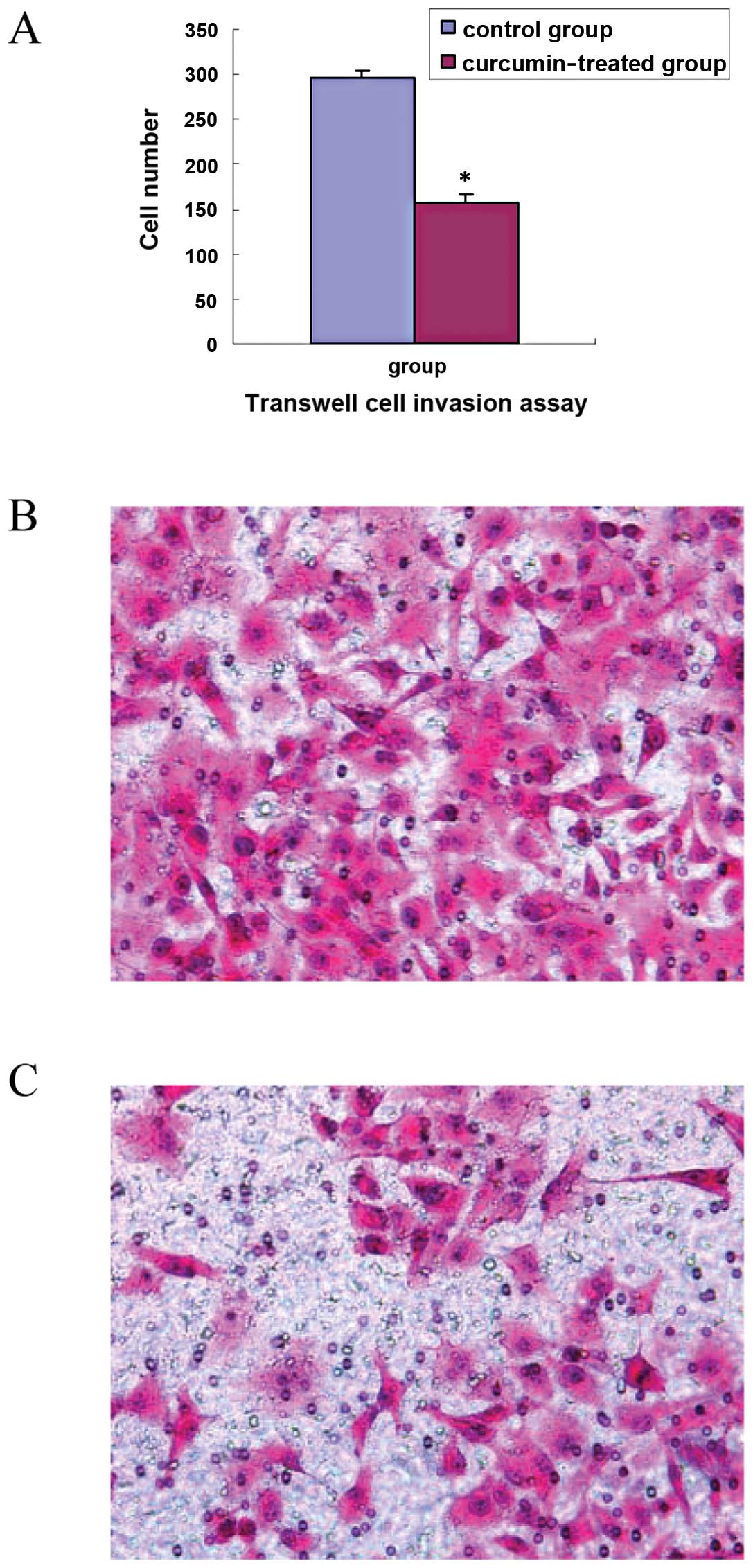
ability of TGF-β1-stimulated PANC-1 cells, we performed Transwell
cell invasion assay and wound healing assay. The results showed
that cell invasion in the curcumin-treated group (30 μmol/ml) was
significantly decreased compared with that in the control group
(t=7.68, P<0.01), as shown in Fig.
4A. The scratch wound in the curcumin-treated group healed
slower than that in the control group (t=12.90, P<0.01), as
shown in Fig. 5A. Representative
images of invasion and wound healing are shown in Figs. 4B and C, and 5B and C. The results suggest that curcumin
inhibits cell invasion and migration of TGF-β1-stimulated PANC-1
cells.
Discussion
Abnormal and sustained activations of several
signaling pathways are the main mechanisms of malignant tumor
development, invasion, metastasis, recurrence and resistance.
Curcumin has chemopreventive and therapeutic roles in a variety of
tumor cells, including breast, gastric, colorectal, lung,
pancreatic and prostate cancer (12–14).
The antitumor mechanism of curcumin is to regulate multiple
signaling molecules, such as downregulation of EGFR, HER-2,
SHH/GLI1 Wnt/β-catenin and its downstream signaling molecules ERK,
AKT, NF-κB, STATs, and upregulation of p21, p27, cell cycle kinase
inhibitors (13,15–17).
Curcumin can inhibit pancreatic cancer cell
proliferation, induce apoptosis, anti-angiogenesis,
chemosensitizing, but its mechanism is not yet fully understood.
Curcumin may inhibit EGFR, STAT-3, NF-κB and their downstream
target genes, multidrug resistance-associated protein 5 expression
(18–21). Curcumin inhibits Panc-28 and L3.6pL
pancreatic cancer cell proliferation through downregulation of
NF-κB-dependent genes and Sp1, Sp2, Sp3 transcription factors
(18). Curcumin can suppress the
transplantation tumor growth of L3.6pL pancreatic cells in nude
mice through intraperitoneal injection (20). Curcumin can improve the
antiproliferative effects of gemcitabine on BxPC-3, Panc-1,
MIAPaCa-2 pancreatic cancer cells and can induce apoptosis
(19,22). In animal models of pancreatic
cancer, the combination of curcumin and gemcitabine is more
effective than gemcitabine alone in the inhibition of tumor growth
and anti-angiogenesis (18,21). The chemotherapy sensitizing effect
of curcumin is partly due to the inhibition of the expression of
STAT-3 and NF-κB-regulated genes such as cyclins, c-Myc, Bcl-2,
Bxl-xl, MMPs and VEGF (18,21).
Studies have found that Hh signaling mediated tumor
development. The cross-talk between the Hh signaling with other
signaling pathways, such as EGFR, Wnt/β-catenin and transforming
growth factor-β (TGF-β)/TGF-β receptors, also play important roles
(23). Morton et al(23) also found that the Hh signaling
pathway has a synergistic effect with activated K-ras signaling by
reducing tumor cell dependence on sustained activation of MAPK and
PI3-K/Akt/mTOR signals, thereby enhancing the K-ras-induced
pancreatic cancer development. In pancreatic ductal adenocarcinoma
the downstream nuclear transcription factor GLI1 of the Hh
signaling pathway is not completely dependent on upstream
Shh-Ptch-Smo signals and is also regulated by TGF-β and KRAS
signals (24).
TGF-β1, a multifunctional polypeptide cytokine, has
an impact on a variety of cell growth, differentiation, apoptosis
and immune regulations. The signaling pathway of TGF-β1 is to
inhibit epithelial cell growth and to induce apoptosis of
epithelial cells. However, it has been found that the majority of
tumor cells can secrete TGF-β1 by autocrine or paracrine modes. The
high level of TGF-β1 in the tumor tissue can not only promote the
growth of tumor cells, and inhibit apoptosis, but it can also
promote the occurrence of EMT. Pancreatic cancer cells secrete
TGF-β1; the tumor-derived TGF-β1 plays an important role in
pancreatic cancer microenvironment. miR-23a regulates TGF-β-induced
EMT by suppression of E-cadherin and contributes to EGFR-TKI
resistance in lung cancer cells (25).
In our experiment, the proliferation, invasion and
migration activities of pancreatic cancer PANC-1 cells increased
significantly by stimulation with TGF-β1 (5 ng/ml) for 7 days. The
expression levels of Shh and GLI1 increased, E-cadherin decreased,
while vimentin expression increased as well. Compared with the
control group, the difference was significant. These results
indicate that TGF-β1 promotes EMT transition of PANC-1 cells.
Subsequently, we treated the TGF-β1-stimulated
PANC-1 cells with different concentrations of curcumin. We found
that with the gradual increase of the concentration of curcumin,
the inhibition of cell proliferation also increased, and 30 μmol/ml
of curcumin significantly inhibited the proliferation of PANC-1
cells. After the PANC-1 cells were treated with curcumin, the rate
of apoptosis was significantly increased. Our experimental results
suggest that curcumin inhibits PANC-1 cell proliferation and
induces cell apoptosis in vitro.
Following treatment with curcumin, the expression
levels of Shh and GLI1 were significantly decreased in
TGF-β1-stimulated PANC-1 cells. GLI protein is the downstream
transcription factor and plays a key role in the Hh pathway. GLI1
is also a target gene of the Hh signal. Our results suggest that
curcumin inhibits Hh signaling in TGF-β1-stimulated PANC-1
cells.
The most important changes of EMT are reduced or
lost expression of epithelial markers E-cadherin, and elevated
expression of interstitial cell markers, such as fibronectin,
vimentin (8,9). Our experimental study also found that
the expression levels of Shh and GLI1 were significantly decreased,
as was the expression of vimentin, while the expression of
E-cadherin increased in TGF-β1-stimulated PANC-1 cells treated with
curcumin. Furthermore, cell invasion significantly decreased, and
the scratch wound healed slower in the curcumin-treated group. The
results suggest that curcumin inhibits the cell invasion and
migration of TGF-β1-stimulated PANC-1 cells, and reverses the EMT
of TGF-β1-stimulated PANC-1 cells.
These findings suggest that TGF-β1 induces the EMT
of the pancreatic cancer PANC-1 cells through activation of Hh
signaling. Curcumin inhibits cell proliferation, induces apoptosis,
decreases cell invasion and migration, and reverses the EMT of the
TGF-β1-stimulated PANC-1 cells by inhibiting the Hh signaling
pathway.
Acknowledgements
This study was supported by the Zhejiang Provincial
Natural Science Foundation of China (no. Y2100434).
References
|
1
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nutr Cancer. 62:919–930. 2010.PubMed/NCBI
|
|
2
|
Basnet P and Skalko-Basnet N: Curcumin: an
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 16:4567–4598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pirozzi G, Tirino V and Camerlingo R:
Epithelial to mesenchymal transition by TGF-β1 induction increases
stemness characteristics in primary non small cell lung cancer
line. PLoS One. 6:e215482011.
|
|
4
|
Maitah YM, Ali S, Ahmad A, Gadgeel S and
Sarkar FH: Up-regulation of sonic hedgehog contributes to
TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells.
PLoS One. 6:e160682011.PubMed/NCBI
|
|
5
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011.PubMed/NCBI
|
|
6
|
Uramoto H, Iwata T, Onitsuka T, Shimokawa
H, Hanagiri T and Oyama T: Epithelial-mesenchymal transition in
EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res.
30:2513–2517. 2010.PubMed/NCBI
|
|
7
|
Wells A, Yates C and Shepard CR:
E-cadherin as an indicator of mesenchymal to epithelial reverting
transitions during the metastatic seeding of disseminated
carcinomas. Clin Exp Metastasis. 25:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotz HG, Hotz B and Buhr HJ: Genes
associated with epithelial-mesenchymal transition: possible
therapeutic targets in ductal pancreatic adenocarcinoma? Anticancer
Agents Med Chem. 11:448–454. 2011. View Article : Google Scholar
|
|
9
|
Dai J, Ai K, Du Y and Chen G: Sonic
hedgehog expression correlates with distant metastasis in
pancreatic adenocarcinoma. Pancreas. 40:233–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelleher FC: Hedgehog signaling and
therapeutics in pancreatic cancer. Carcinogenesis. 32:445–451.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katoh Y and Katoh M: Hedgehog target
genes: mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mimeault M and Batra SK: Potential
application of curcumin and its novel synthetic analogs and
nanotechnology-based formulations in cancer prevention and therapy.
Chin Med. 23:312011.PubMed/NCBI
|
|
13
|
Nautiyal J, Banerjee S, Kanwar SS, Yu Y,
Patel BB, Sarkar FH and Majumdar AP: Curcumin enhances
dasatinib-induced inhibition of growth and transformation of colon
cancer cells. Int J Cancer. 128:951–961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teiten MH, Gaascht F, Cronauer M, Henry E,
Dicato M and Diederich M: Anti-proliferative potential of curcumin
in androgen-dependent prostate cancer cells occurs through
modulation of the Wingless signaling pathway. Int J Oncol.
38:603–611. 2011.
|
|
15
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
16
|
Yallapu MM, Maher DM, Sundram V, Bell MC,
Jaggi M and Chauhan SC: Curcumin induces chemo/radio-sensitization
in ovarian cancer cells and curcumin nanoparticles inhibit ovarian
cancer cell growth. J Ovarian Res. 3:112010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aggarwal BB, Banerjee S, Bharadwaj U, Sung
B, Shishodia S and Sethi G: Curcumin induces the degradation of
cyclin E expression through ubiquitin- dependent pathway and
up-regulates cyclin-dependent kinase inhibitors p21 and p27 in
multiple human tumor cell lines. Biochem Pharmacol. 73:1024–1032.
2007. View Article : Google Scholar
|
|
18
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar
|
|
19
|
Ali S, Ahmad A, Banerjee S, Padhye S,
Dominiak K, Schaffert JM, Wang Z, Philip PA and Sarkar FH:
Gemcitabine sensitivity can be induced in pancreatic cancer cells
through modulation of miR-200 and miR-21 expression by curcumin or
its analogue CDF. Cancer Res. 70:3606–3617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jutooru I, Chadalapaka G, Lei P and Safe
S: Inhibition of NFkappaB and pancreatic cancer cell and tumor
growth by curcumin is dependent on specificity protein
down-regulation. J Biol Chem. 285:25332–25344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramachandran C, Resek AP, Escalon E,
Aviram A and Melnick SJ: Potentiation of gemcitabine by Turmeric
Force™ in pancreatic cancer cell lines. Oncol Rep. 23:1529–1535.
2010.PubMed/NCBI
|
|
22
|
Mimeault M and Batra SK: Frequent
deregulations in the hedgehog signaling network and cross-talks
with the epidermal growth factor receptor pathway involved in
cancer progression and targeted therapies. Pharmacol Rev.
62:497–524. 2010. View Article : Google Scholar
|
|
23
|
Morton JP, Mongeau ME, Klimstra DS, Morris
JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M and Lewis BC: Sonic
hedgehog acts at multiple stages during pancreatic tumorigenesis.
Proc Natl Acad Sci USA. 104:5103–5108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nolan-Stevaux O, Lau J, Truitt ML, Chu GC,
Hebrok M, Fernandez-Zapico ME and Hanahan D: GLI1 is
regulated through Smoothened-independent mechanisms in neoplastic
pancreatic ducts and mediates PDAC cell survival and
transformation. Genes Dev. 23:24–36. 2009. View Article : Google Scholar
|
|
25
|
Cao M, Seike M, Soeno C, Mizutani H,
Kitamurak, Minegishi Y, Noro R, Yoshimura A, Cai L and Gemma A:
MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition
by targeting E-cadherin in lung cancer cells. Int J Oncol.
41:865–875. 2012.
|