Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide, among which non-small cell lung cancer (NSCLC)
accounts for more than 80% of cases (1). Radiotherapy is becoming increasingly
significant since it has been shown to improve local control and
reduce recurrence of NSCLC. However, the use of radiotherapy is
also confronted with dilemmas due to intrinsic radioresistance.
Transducer of erbB2.1 (TOB1) was identified as a
member of the B-cell translocation gene (BTG)/transducer of erbB2
(TOB) anti-proliferative protein family, which was discovered in
1996 (2). Accumulated evidence
confirms that TOB1 is involved in the negative regulation of cell
growth and functions as a tumor suppressor (3). In previous studies in 2007 from our
laboratory, Jiao et al(4)
demonstrated that pre-irradiation treatment with
adenovirus-mediated TOB1 significantly increased the
radiosensitivity of breast cancer cells. However, whether TOB1
radiosensitizes NSCLC cells to ionizing radiation and whether or
not its underlying mechanisms are associated with epidermal growth
factor receptor (EGFR)-dependent signaling pathways has not been
thoroughly elucidated.
In the present study, TOB1-overexpressing and
-suppressed NSCLC cells were used to determine the clonogenic
growth after radiation in order to investigate whether TOB1 affects
the radiosensitivity of lung cancer cells. Radiation-induced
double-strand break (DSB) formation and cell cycle redistribution
were also detected. Western blot analysis was used to investigate
the underlying molecular signaling and to identify the precise
mechanisms of TOB1 in NSCLC cell lines in response to
radiation.
Materials and methods
Cell culture and reagents
The human NSCLC cell lines NCI-H1975 and A549 were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were maintained in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal calf serum (FCS), L-glutamine (5
mmol/l), non-essential amino acids (5 mmol/l), penicillin (100
U/ml) and streptomycin (100 U/ml) (Invitrogen, Carlsbad, CA, USA),
at 37°C in a humidified atmosphere with 5% CO2.
Gefitinib (Iressa®) was purchased from AstraZeneca
(Macclesfield, UK) and the MEK1/2 inhibitor U0126 was obtained from
Cell Signaling Technology, Inc. (Beverly, MA, USA).
Ionizing radiation
A Siemens 6 MV X-ray linear accelerator was used to
deliver a single dose of IR radiation, at a dose rate of 200
cGy/min at room temperature.
Plasmids, small interfering RNAs (siRNAs)
and transfection
The full-length human TOB1 cDNA was derived using
polymerase chain reaction (PCR), using specific primers designed
according to the TOB1 reference sequence from GenBank
(NM_005749.2), and then cloned into the eukaryotic expression
vector pcDNA3.0 (Invitrogen). Three siRNAs that target TOB1 mRNA
and control (scrambled-sequence) siRNA were designed and
synthesized by Invitrogen. The Lipofectamine-introduced plasmid and
siRNA transfection were performed as previously described (5). The expression of TOB1 was determined
by western blot analysis.
Clonogenic survival assay
The cells were plated at different cell densities
and irradiated with 6 Gy X-rays 24 h later. After 12–14 days of
incubation at 37°C, the cells were stained with Giemsa. The number
of colonies per dish was counted and the surviving fractions were
calculated as the ratio of plating efficiencies for irradiated and
unirradiated cells. Plating efficiency was defined as the colony
number divided by the number of cells plated for the unirradiated
controls. Experiments were conducted in triplicate and data are
presented as the means ± standard deviation (SD) from three
independent experiments. All survival fractions were fitted into
the linear quadratic model.
Cell cycle analysis
The cells were removed with trypsin and collected
into centrifuge tubes together with the culture medium. The
detailed methods for flowcytometric analysis have been previously
described (6). The cell cycle
distribution was calculated from 10,000 cells using ModFit LT
software (Becton-Dickinson, San Jose, CA, USA) and FACSCalibur
(Becton-Dickinson).
Immunofluorescence assay
The immunofluorescence detection of γ-H2AX foci was
applied for the determination of residual DNA DSBs. Cells grown on
coverslips (Fisher Scientific) were fixed in ice cold 4%
paraformaldehyde for 30 min, blocked with 3% bovine serum albumin
(BSA) in phosphate-buffered saline (PBS) and incubated with the
antibody phospho-H2AX (ser139, dilution 1:500; Millipore) for 2 h
at 4°C. After washing with PBS, the secondary FITC-conjugated
antibody was added for 1 h, and the slides were washed with PBS and
mounted with mounting medium containing DAPI. The slides were
mounted with fluorescent mounting medium (Dako, Germany). For each
treatment condition, γ-H2AX foci were determined in ≥50 cells and
the data are presented as the means ± SD from three independent
experiments.
Western blot analysis
Western blot analysis was performed as previously
described (5). The following
primary antibodies were used for immunoblotting: β-actin (C-4),
TOB1 (E-1), EGFR (53A5), NF-κB (P65A), cyclin B1 (D-11), Cdc2 (B-5)
and the secondary antibodies horseradish peroxidase (HRP)-labeled
goat anti-mouse (GAM-007) and goat anti-rabbit (SC-2004) IgG
(dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), as well as phospho-extracellular signal-regulated kinase
(ERK) 1/2 (T202/Y204), ERK1/2, phospho-p38 (T180/Y182), p38 and
phospho-p53 (serine 15) (dilution, 1:1,000; Cell Signaling
Technology, Inc.).
Statistical analysis
The data are presented as the means ± SD.
Statistical comparisons of the experimental results between the
treated and control groups were made using the two-tailed Student’s
t-test. All statistical tests were performed using SPSS version
17.0. P≤0.05 was considered to indicate a statistically significant
result.
Results
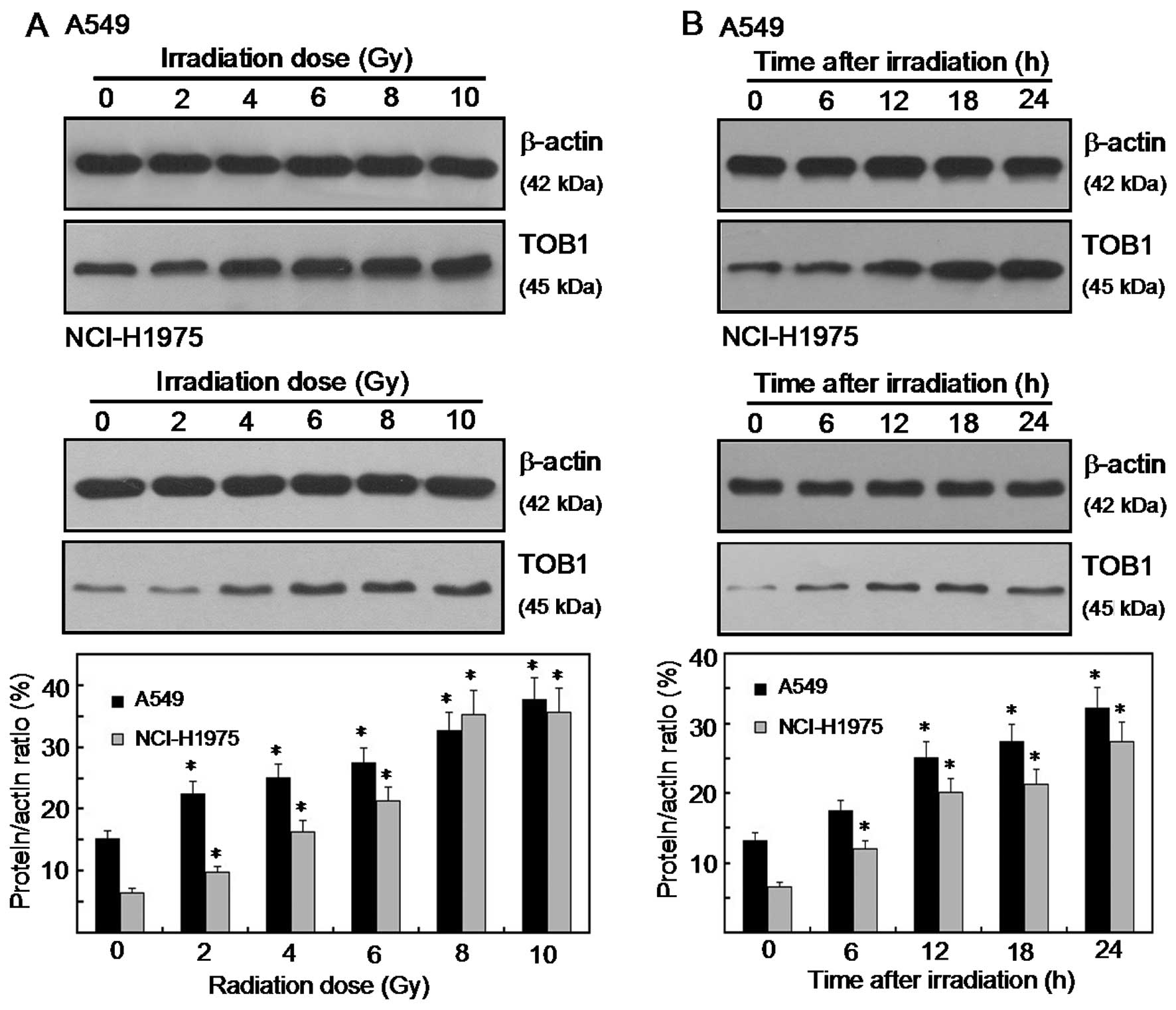
Radiation-induced TOB1 expression in
human lung cancer cell lines
IR radiation stimulated TOB1 expression in NCI-H1975
and A549 lung cancer cell lines. The expression levels of TOB1
following various doses (0–10 Gy) of IR radiation in the two lung
cancer cell lines were determined by western blot and densitometric
analyses. Radiation-induced TOB1 expression was measured 24 h after
radiation. A significant increase in the TOB1 protein level was
observed when a dose of ≥2 Gy was used (Fig. 1A). For the time-response induction
of TOB1, the expression was increased at the earliest time-point
tested (6 h) and was elevated at 24 h following 6 Gy of radiation
(Fig. 1B). Little or no change in
the expression of β-actin was observed in all of the
experiments.
TOB1 regulates the radiosensitivity of
lung cancer cell lines
NCI-H1975 cells were transfected with TOB1
recombinant plasmid to establish stable cells. TOB1-siRNA was
induced into A549 cells to determine whether downregulation of TOB1
expression enhances the radioresistance of lung cancer cells. The
efficacy of TOB1 recombinant plasmid and siRNA was confirmed by
western blot analysis. Clonogenic assay was performed to assess the
cell survival after radiation. TOB1 overexpression was combined
with radiation and was found to reduce the clonogenic growth of
NCI-H1975 cells compared with irradiated control cells. On the
contrary, the number of colonies in the mock-transfection group was
not affected. TOB1-siRNA transfection was combined with radiation
and was found to induce clonogenic growth of A549 cells compared
with irradiated control cells (Fig.
2).
TOB1 regulates cell cycle redistribution
of lung cancer cells after radiation
Most mammalian cells exhibit transient delays in the
G1 and G2 phases of the cell cycle after radiation treatment
allowing the cell to correct possible defects (6). In the present study, we identified the
effects of TOB1 on lung cancer cell cycle progression. After
irradiation with 6 Gy X-rays, all the above-described cells were
analyzed for cell cycle distribution. As shown in Fig. 3A, radiation treatment significantly
increased the percentage of G2/M phase cells. In NCI-H1975/TOB1
cells prior to radiation, the percentage of cells in the G2/M phase
was decreased compared with radiation alone (t=7.75, P<0.05). In
A549/siRNA cells subjected to radiation, the percentage of cells in
the G2/M phase was increased compared with radiation alone (t=6.32,
P<0.05) (Fig. 3B). The
expression levels of several important proteins associated with the
cell cycle were analyzed to investigate the significant cell cycle
changes in TOB1-transfected NCI-H1975 cells. Results revealed that
cyclin B1 was significantly suppressed (Fig. 3C). A549 cells knocked down for TOB1
exhibited opposite effects for the regulation of the cell
cycle-associated proteins (Fig.
3D).
TOB1 regulates γ-H2AX focus formation in
lung cancer cells following irradiation
The induction of DNA-DSBs analyzed by the formation
of γ-H2AX foci was measured 1 h after irradiation of lung cancer
cells, non-transfected or transfected with TOB1 recombinant plasmid
or TOB1-siRNA. This procedure aimed to identify the molecular
mechanisms of radiosensitization of TOB1 and investigate its
effects on the initial DNA damage response to IR radiation. Results
showed that the radiation-induced γ-H2AX focus formation was
significantly increased in TOB1-transfected NCI-H1975 cells 2 h
post-radiation compared with the control cells (t=11.20, P<0.05)
(Fig. 4A), suggesting inhibition of
DNA repair. TOB1 knockdown demonstrated opposite effects in the
siRNA-transfected A549 cells (t=7.12, P<0.05) (Fig. 4B).
TOB1 is involved in serine 15
phosphorylation of p53 via the mitogen-activated protein kinase
(MAPK)/ERK signaling pathway after irradiation
Western blot analysis was performed to identify the
targets of TOB1 that are involved in the enhancement of
radiosensitivity. EGFR, which is frequently involved in the
pathogenesis of human epithelial tumors, adversely affects
prognosis and treatment outcome due to EGFR-mediated therapy
resistance (7). The results of this
study revealed that TOB1 overexpression did not significantly
inhibit EGFR expression in the EGFR-mutated NCI-H1975 cells.
However, TOB1 knockdown enhanced EGFR expression in the
siRNA-tranfected A549 cells. In EGFR downstream effectors, TOB1
augmented a significantly suppressed radiation-induced
phosphorylation of ERK1/2 and p38, while it had no obvious effects
on the expression of these proteins. Phosphorylation and subsequent
activation of p53, as well as its transcriptional induction, are
key initial responses to cell cycle distribution and DNA damage
(8). The specific signaling cascade
involved in this response was investigated in the present study
using mitogen-activated or extracellular signal-regulated protein
kinases 1 and 2 (MEK1/2) inhibitor (U0126) specific to the MAPK/ERK
pathway. The increased phospho-p53 in A549 cells knocked down for
TOB1 was inhibited by MEK1/2 inhibitor (Fig. 5C), suggesting that TOB1 mediates the
phosphorylation of p53 at serine 15 by modulating the MAPK/ERK
pathway.
Discussion
More than 70% of cancer patients currently receive
radiotherapy during their treatment course (9–11).
However, conquering the intrinsic radioresistance and increasing
the precision of dose delivery to target tumor remain difficult for
radiotherapy (12). To the best of
our knowledge, the results of the present study indicated for the
first time that irradiation stimulated TOB1 expression in the lung
cancer cell lines A549 and NCI-H1975. A significant increase in the
TOB1 protein level was observed when a dose of ≥2 Gy was used. The
time-dependent induction of TOB1 was increased 6 h after radiation.
Thus, TOB1 is potentially involved in the regulation of
radiosensitivity in lung cancer cells.
In the present study, we investigated whether the
combination of the regulation of TOB1 expression and IR radiation
affects the kinetics of cell death compared with cells treated with
radiation alone. Using the clonogenic survival assay, we found that
TOB1 overexpression reduces the clonogenic growth of NCI-H1975
cells after irradiation compared with the parental and mock-treated
cells. On the contrary, TOB1 knockdown protects A549 cells from
radiation when compared with the control cells.
Radiation induces a complex cellular response, which
activates and coordinates cell cycle checkpoints and damages repair
(13). Most mammalian cells exhibit
transient delays in the G1 and G2 phases after irradiation allowing
the cell to correct possible defects (6). In the present study it was shown that
TOB1 overexpression decreases the radiation-induced NCI-H1975 cell
accumulation in the G2 phase. TOB1 knockdown demonstrated opposite
effects in A549 cells, suggesting that TOB1 regulates the cell
cycle redistribution of lung cancer cells after radiation. We
analyzed the expression levels of several cell cycle-associated
proteins and found that the expression levels of cyclin B1 and Cdc2
were significantly suppressed in TOB1-transfected NCI-H1975 cells.
TOB1 knockdown demonstrated opposite effects in siRNA-transfected
A549 cells.
Phosphorylation of H2AX plays an important role in
the recruitment of repair- and damage-signalling factors to the
site of DNA breaks (14–16). γ-H2AX is widely used in monitoring
the extent of DSB induction and analyzing the effectiveness of
novel biological therapies (17).
TOB1 produced a higher amount of γ-H2AX foci in TOB1-transfected
NCI-H1975 cells after irradiation. Radiation-induced γ-H2AX was
significantly inhibited in A549 cells knocked down for TOB1. Based
on these results, it is suggested that the phenotypes of increased
radiosensitivity may thus reflect distinct and differentially
regulated factors of the DNA damage response.
Extensive evidence indicates that EGFR signaling
plays an important role in mediating the radiosensitivity and
activation of multiple signaling pathways (18). Principle data suggest that EGFR
heterodimer or homodimer formation induced by ligand binding
activates the intracellular tyrosine kinase domain, induces
additional downstream pathways and affects radiosensitivity
(19). Inhibition of the MAPK
pathway may allow inhibition of signaling by multiple upstream
receptors and intermediates, such as EGFR (20,21).
Our results revealed that TOB1 overexpression did not inhibit EGFR
expression in EGFR-mutated NCI-H1975 cells, while TOB1 knockdown
enhanced EGFR expression in siRNA-transfected A549 cells. Regarding
the EGFR downstream signaling pathway, we examined the expression
of the phosphorylation of ERK1/2 and p38, which is mainly
associated with radiosensitivity. Results revealed that TOB1
overexpression significantly inhibited the activation of MAPK,
while TOB1 suppression enhanced MAPK activation. This suggests that
TOB1 affects the phosphorylation of MAPK via an EGFR-independent
pathway.
The MAPK pathway directly affects the
phosphorylation of p53 at multiple sites, which are key initial
responses to cell cycle distribution and DNA damage (22–24).
Our results revealed ed that TOB1 knockdown strongly enhanced
radiation-induced phosphorylation of p53 in A549 cells. This result
suggests that these cells possess a more robust DNA damage
surveillance and possibly a repair mechanism that helps them either
adapt to or overcome critical radiation-induced DNA damage. The
inhibitor specific to MAPK/ERK (U0126) was used following
radiation. The ability of the MEK1/2 inhibitor to affect the serine
15 phosphorylation of p53 indicates the direct role of MAPK/ERK in
modulating this particular cellular radiation response in
siRNA-transfected A549 cells, while gefitinib did not affect the
specific event (data not shown).
In conclusion, this study suggests that TOB1 may be
a novel molecular target of irradiation. TOB1 was shown to modulate
the radiosensitivity of lung cancer cells through the MAPK/ERK
signaling pathway via an EGFR-independent pathway. Gene therapeutic
approaches may enhance the radiotherapeutic benefit and overcome
the resistance of EGFR inhibitors in lung cancer therapy by
increasing TOB1 expression.
Acknowledgements
This study was supported by grants from the Doctoral
Fund of the Ministry of Education of China (grant no.
20103201120016), the College Nature Science Foundation of Jiangsu
Province (grant no. SZ126821), the Social Development Projects of
Kunshan City (grant no. KS1224) and the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Matsuda S, Kawamura-Tsuzuku J, Ohsugi M,
et al: Tob, a novel protein that interacts with p185erbB2, is
associated with anti-proliferative activity. Oncogene. 12:705–713.
1996.PubMed/NCBI
|
|
3
|
Jia S and Meng A: Tob genes in development
and homeostasis. Dev Dyn. 236:913–921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiao Y, Ge CM, Meng QH, et al:
Adenovirus-mediated expression of Tob1 sensitizes breast cancer
cells to ionizing radiation. Acta Pharmacol Sin. 28:1628–1636.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiao Y, Sun KK, Zhao L, et al: Suppression
of human lung cancer cell proliferation and metastasis in vitro by
the transducer of ErbB-2.1 (TOB1). Acta Pharmacol Sin. 33:250–260.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahsan A, Hiniker SM, Davis MA, et al: Role
of cell cycle in epidermal growth factor receptor
inhibitor-mediated radiosensitization. Cancer Res. 69:5108–5114.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wheeler DL, Dunn EF and Harari PM:
Understanding resistance to EGFR inhibitors - impact on future
treatment strategies. Nat Rev Clin Oncol. 7:493–507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shieh SY, Ikeda M, Taya Y, et al: DNA
damage-induced phosphorylation of p53 alleviates inhibition by
MDM2. Cell. 91:325–334. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang P: Epidemiology of lung cancer
prognosis: quantity and quality of life. Methods Mol Biol.
471:469–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molina JR, Yang P, Cassivi SD, et al:
Non-small cell lung cancer: epidemiology, risk factors, treatment,
and survivorship. Mayo Clin Proc. 83:584–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed KM and Li JJ: NF-kappa B-mediated
adaptive resistance to ionizing radiation. Free Radic Biol Med.
44:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerber DE: EGFR inhibition in the
treatment of non-small cell lung cancer. Drug Dev Res. 69:359–372.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meyn RE, Munshi A, Haymach JV, et al:
Receptor signaling as a regulatory mechanism of DNA repair.
Radiother Oncol. 92:316–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahaney BL, Meek K and Lees-Miller SP:
Repair of ionizing radiation-induced DNA double-strand breaks by
non-homologous end-joining. Biochem J. 417:639–650. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morgan MA, Parsels LA, Maybaum J, et al:
Improving gemcitabine-mediated radiosensitization using molecularly
targeted therapy: a review. Clin Cancer Res. 14:6744–6750. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chastel C, Jiricny J and Jaussi R:
Activation of stress-responsive promoters by ionizing radiation for
deployment in targeted gene therapy. DNA Repair (Amst). 3:201–215.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He HT, Fokas E, You A, et al: Siah1
proteins enhance radiosensitivity of human breast cancer cells. BMC
Cancer. 10:4032010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toulany M, Kasten-Pisula U, Brammer I, et
al: Blockage of epidermal growth factor
receptor-phosphatidylinositol 3-kinase-AKT signaling increases
radiosensitivity of K-RAS mutated human tumor cells in vitro by
affecting DNA repair. Clin Cancer Res. 12:4119–4126. 2006.
View Article : Google Scholar
|
|
19
|
Chung EJ, Brown AP, Asano H, et al: In
vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an
inhibitor of mitogen-activated protein kinase/extracellular
signal-regulated kinase 1/2 kinase. Clin Cancer Res. 15:3050–3057.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li MW, Mruk DD and Cheng CY:
Mitogen-activated protein kinases in male reproductive function.
Trends Mol Med. 15:159–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim Y, Coppey M, Grossman R, et al: MAPK
substrate competition integrates patterning signals in the
Drosophila embryo. Curr Biol. 20:446–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arany PR, Flanders KC, DeGraff W, et al:
Absence of Smad3 confers radioprotection through modulation of
ERK-MAPK in primary dermal fibroblasts. J Dermatol Sci. 48:35–42.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
She QB, Chen N and Dong Z: ERKs and p38
kinase phosphorylate p53 protein at serine 15 in response to UV
radiation. J Biol Chem. 275:20444–20449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dumaz N and Meek DW: Serine15
phosphorylation stimulates p53 transactivation but does not
directly influence interaction with HDM2. EMBO J. 18:7002–7010.
1999. View Article : Google Scholar : PubMed/NCBI
|