Introduction
Nasopharyngeal carcinoma (NPC) is a squamous cell
carcinoma originating from epithelial cells of the nasopharynx,
which is a distinctive type of head and neck cancer (HNC) (1). Genetic susceptibility and
environmental factors such as Epstein-Barr virus (EBV) infection
are major causes of NPC (1,2). The neoplasm is endemic in Southern
China and Southeast Asia (3,4),
representing a marked ethnic and geographic distribution. The
annual incidence rate of NPC still remains very high and peaks at
50 cases per 100,000 people in the prevalent regions (3). NPC has the highest metastatic
potential among HNC. The majority of patients have metastasis to
regional lymph nodes or even distant organs at the time of
diagnosis (5). The conventional
treatments for NPC involve radiotherapy, chemotherapy and the
concurrent combination. However, the 5-year overall survival (OS)
rate after treatment is ~70% (6)
and has not been significantly improved. Moreover, 30 to 40%
patients eventually will relapse with locoregional recurrence and
distant metastasis, with poor median survival ranging from 7.2 to
22 months (7,8). Hence, the search for novel therapeutic
modalities to treat patients with metastatic and recurrent NPC is
urgently needed. The intensive multimodality management of standard
and novel therapeutic agents may improve the prognosis of NPC.
Melanoma differentiation-associated gene-7
(mda-7)/interleukin-24 (IL-24) is a unique member of the IL-10
family (9), exhibiting ubiquitous
antitumor activities and cancer-specific cytotoxicities (10). IL-24 can strongly suppress tumor
growth irrespective of p53 status via induction of apoptosis and
cell cycle arrest (10). IL-24 can
also inhibit tumor angiogenesis through direct suppression of
vascular endothelial cell differentiation and migration via
interacting with the IL-22R1/IL-20R2 heterodimeric receptor on
vascular endothelial cells (11),
and indirectly downregulating the production of vascular
endothelial growth factor (VEGF) and IL-8 pro-angiogenic factors
(12). Furthermore, IL-24 as a
pro-Th1 cytokine can display potent immunoactivating property and
enhance antitumor immunity by stimulating the production of
secondary cytokines such as IL-6, tumor necrosis factor (TNF)-α and
interferon (IFN)-γ (13). In
addition, IL-24 can suppress tumor cell invasion and metastasis by
reduction of focal adhesion kinase (FAK) and matrix
metalloproteinase (MMP)-2 and MMP-9 (14). Interestingly, IL-24 can induce
cytotoxic autophagy in tumor cells through endoplasmic reticulum
(ER) stress response and protein kinase R (PKR)-like ER kinase
(PERK) activation (15,16). Notably, IL-24 can sensitize
radiotherapy or chemotherapy of tumors and exert profound bystander
antitumor activities, leading to the augmentation of antitumor
efficacy (10). Thus, IL-24 as a
promising tumor suppressor has been hailed as a ‘magic bullet’ for
cancer.
Gene therapy represents a novel therapeutic strategy
against cancer including NPC (17),
and is based on the introduction of genetic material such as a
tumor-suppressor gene and small short hairpin RNA (shRNA) targeting
an oncogene or a pro-angiogenic factor gene into tumor cells. The
combination of gene therapy and conventional radiotherapy or
chemotherapy can improve antitumor benefits and reduce side-effects
(18,19). IL-24 has been shown to exhibit
striking radiosensitizing effects in a diverse spectrum of tumors
(12,20,21).
We previously demonstrated that adenovirus-mediated IL-24
(AdVIL-24) gene therapy can elicit potential antitumor activity in
human laryngocarcinoma (22).
However, the therapeutic effect of AdVIL-24 alone or combined with
ionizing radiotherapy on human NPC is still elusive. In the present
study, we investigated the therapeutic efficacy of AdVIL-24 gene
therapy combined with ionizing radiation in vitro in CNE-2Z
human NPC (nonkeratinizing carcinoma) cells and in vivo in
an athymic nude mouse CNE-2Z human NPC xenografted tumor model, and
we also elucidated the underlying mechanisms.
Materials and methods
Adenoviruses, cell lines, reagents and
mice
The recombinant replication-incompetent Ad5E1- and
E3-deleted adenovirus AdVIL-24/green fluorescent protein (GFP)
(termed AdVIL-24) expressing both human IL-24 and GFP and its
control blank adenovirus AdVGFP (termed AdV) expressing GFP but no
human IL-24 were constructed in our laboratory (23), at the Cell and Molecular Biology
Institute, College of Medicine, Suzhou University (Suzhou, Jiangsu,
China). The CNE-2Z human NPC cell line was purchased from the
American Type Culture Collection (ATCC, Rockville, MD, USA). The
CNE-2Z tumor cells were cultured in RPMI-1640 (Invitrogen,
Shanghai, China) supplemented with 10% fetal bovine serum (FBS;
HyClone, Logan, UT, USA), respectively. The TRIzol and reverse
transcriptase polymerase MuMLV were purchased from Invitrogen. The
DL2000 DNA marker was purchased from Takara (Shanghai, China). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
kit and mammalian cell lysis kit were purchased from Sigma
(Shanghai, China). The propidium iodide (PI) cell cycle detection
kit and Annexin-PE/7-AAD apoptosis detection kit were purchased
from Nanjing Kaiji Biological Engineering Co., Ltd. (Nanjing,
Jiangsu, China). The antibodies specific for IL-24, P21, P27,
cyclin E, cyclin-dependent kinase 2 (CDK2), Bcl-2, Bax,
caspase-3/cleaved caspase-3, β-actin, CD34 and VEGF were purchased
from Abcam (Shanghai, China). The SuperEnhanced chemiluminescence
detection kit was purchased from Applygen Technologies, Inc.
(Beijing, China). The UltraSensitive™ SP kit was purchased from
Maixin (Fuzhou, Fujian, China). The terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) apoptosis
detection kit was purchased from Beyotime Institute of
Biotechnology (Beijing, China). Male athymic BALB/c nude mice (4-
to 6-week-old) were purchased from the Shanghai Experimental Animal
Center (Shanghai, China) and maintained in the animal facility at
Soochow University according to the Animal Research Committee
Guidelines of Soochow University.
AdVIL-24 gene transfer
To evaluate the optimal multiplicity of infection
(MOI) for a maximal adenoviral infection and human IL-24 transgene
expression in CNE-2Z tumor cells, the CNE-2Z human NPC cells were
infected with recombinant adenovirus AdVIL-24 expressing both human
IL-24 and marker GFP or control blank adenovirus AdV expressing GFP
at various MOIs (0, 10, 25, 50, 100 and 200), respectively. The GFP
expression and adenoviral infection efficiency were then examined
by fluorescence microscopy. Moreover, adenovirus-directed human
IL-24 transgene expression in CNE-2Z NPC cells was determined by
reverse transcription (RT)-PCR and western blot analysis as
described previously (24).
Flow cytometric analysis
To select an optimal irradiation dose in the in
vitro combined experiment of AdVIL-24 gene therapy and ionizing
radiotherapy for CNE-2Z tumor cells, the CNE-2Z human NPC cells
were cultured in T25 flasks at 1×106 cells/flask.
Forty-eight hours later, the tumor cells were irradiated at various
doses (2, 4, 6 and 8 Gy) using a 60Co-γ source (1
Gy/min). After another 24-h incubation, the irradiated and
unirradiated CNE-2Z tumor cells were harvested, washed with cold
PBS and then subjected to apoptosis analysis by flow cytometry
using Annexin V-PE (early apoptotic marker) and 7-AAD (late
apoptotic marker) double staining following the manufacturer’s
instructions. Briefly, the tumor cells (1×105) were
incubated in the presence of 5 μl Annexin V-PE and 5 μl 7-AAD in
100 μl of 1X Annexin V binding buffer at room temperature. After a
15-min incubation, 400 μl of 1X binding buffer was added and the
apoptotic cells were then analyzed by flow cytometry. To further
assess the combined effect of AdVIL-24 plus radiotherapy on cell
cycle profiles and apoptosis of CNE-2Z tumor cells, the CNE-2Z
human NPC cells were also cultured in T25 flasks at
1×106 cells/flask. After a 24-h incubation, the CNE-2Z
tumor cells were infected with AdVIL-24 or AdV used as a blank
adenovirus control at the MOI of 100. The medium containing PBS
without the adenovirus was used as a cell control (PBS control).
Twenty-four hours post adenoviral infection, the tumor cells were
further exposed to radiation at the optimal dose of 4 Gy. The
experiments were divided into 5 groups: PBS, AdV, AdVIL-24 alone, 4
Gy alone and AdVIL-24 plus 4 Gy. Twenty-four hours after
irradiation, tumor cells were then processed to analyze apoptosis
by flow cytometry as described above. In addition, the cell cycle
distribution of treated and untreated CNE-2Z tumor cells was
determined using PI staining by flow cytometric analysis. In brief,
these cells were harvested and washed in cold PBS. The cell pellets
were fixed in 70% cold alcohol for more than 24 h at 4°C, washed in
cold PBS, stained with PI solution [50 μg/ml PI, 50 μg/ml RNase A
and 0.1% (v/v) Triton X-100] at 4°C in the dark for 30 min, then
washed and analyzed by flow cytometry.
MTT assay
The in vitro suppressive effect of AdVIL-24
plus ionizing radiation on CNE-2Z human NPC cells was determined by
MTT assay. The CNE-2Z tumor cells were dispensed into 96-well
culture plates at 1×104 cells/well. After a 24-h
incubation, the CNE-2Z tumor cells were infected with 100 MOI
AdVIL-24 or AdV or without the adenovirus (PBS control) followed by
irradiation with an optimal dose of 4 Gy at day 1 after infection.
Before treatment and at different time points after single and/or
combined treatment, the viability of CNE-2Z tumor cells was then
analyzed using the MTT kit according to the manufacturer’s
protocol.
Clonogenic survival assay
The in vitro inhibitory effect of AdVIL-24
plus ionizing radiation on CNE-2Z human NPC cells was also assessed
by clonogenic survival assay. Briefly, 24 h after infection, the
100 MOI AdVIL-24- and AdV-infected CNE-2Z tumor cells or uninfected
control CNE-2Z tumor cells were dispensed into 6-well culture
plates at 200 cells/well, and then irradiated with 4 Gy. After
another 2 weeks of culture, the cells were washed with PBS, fixed
with methanol and stained with 0.1% crystal violet. The number of
colonies was then manually counted under a microscope. The colonies
consisting of 50 or more cells were considered to be survivors.
Western blot analysis
The CNE-2Z human NPC cells were treated with PBS,
AdV (100 MOI), AdVIL-24 (100 MOI) alone, 4 Gy alone and AdVIL-24
(100 MOI) plus 4 Gy as described above. Twenty-four hours after
irradiation, these cells were collected, washed with cold PBS and
lysed in lysis buffer (1×107 cells/1 ml lysis buffer)
for preparation of total cellular lysate using the mammalian cell
lysis kit. The protein concentration was determined by BCA protein
assay using a spectrophotometer. The total cellular lysates (100
μg/lane) were resolved by 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and subsequently transferred onto a
polyvinylidene difluoride (PVDF) membrane for western blot analysis
using a panel of antibodies specific for P21, P27, cyclin E, CDK2,
Bcl-2, Bax and caspase-3 (pro-caspase-3 and cleaved caspase-3),
respectively. The membrane was then washed and developed using the
SuperEnhanced chemiluminescence detection kit. The protein bands
were visualized after exposure of the membranes to Kodak X-ray
film.
Real-time quantitative RT-PCR
analysis
The in vitro expression of P21, P27, cyclin
E, CDK2, Bcl-2 and Bax in the treated and untreated CNE-2Z human
NPC cells was confirmed by SYBR-Green I-based real-time
quantitative RT-PCR analysis using the following primers: P21-F,
5′-ccc gtg agc gat gga ac-3′ and P21-R, 5′-aaa tct gtc atg ctg gtc
tgc-3′; P27-F, 5′-gtc taa cgg gag ccc gag cct gg-3′ and P27-R,
5′-gaa ggc cgg gtt ctt ctt ggg cg-3′; cyclin E–F, 5′-tgg cgt tta
agt ccc ctg ac-3′ and cyclin E–R, 5′-tca gtt ttg agc tcc ccg tc-3′;
CDK2-F, 5′-ctc cag ggc cta gct ttc tg-3′ and CDK2-R, 5′-ttc agg agc
tcg gta cca ca-3′; Bcl-2-F, 5′-tgt ggc ctt ctt tga gtt cg-3′ and
Bcl-2-R, 5′-cta ccc agc ctc cgt tat cc-3′; Bax-F, 5′-gga tgc gtc
cac caa gaa-3′ and Bax-R, 5′-gca ctc ccg cca caa aga-3′; and
β-actin-F, 5′-tgc gtg aca tta agg aga ag-3′ and β-actin-R, 5′-ctg
cat cct gtc ggc aat g-3′. The cDNA quantities were normalized to
the internal control gene β-actin measured in the same samples.
Relative gene expression was calculated using the pooled cDNA from
all samples by the 2−ΔΔCT method as described previously
(25,26). The authenticity of PCR products was
verified by melting curve analysis and agarose gel electrophoresis.
Each sample was analyzed in triplicate in an independent reaction
and the experiment was repeated three times.
Animal studies
The male athymic BALB/c nude mice were
subcutaneously (s.c.) inoculated in the armpit of the right
anterior limb with 2×106 CNE-2Z human NPC cells and then
monitored daily for tumor growth. Tumor volume was measured with a
caliper and calculated using the formula, Tumor size =
ab2/2, where a is the larger and b
is the smaller of the two dimensions. When tumors grew to a mean
tumor volume of ~100 mm3, the CNE-2Z human NPC s.c.
xenografted tumor-bearing mice were subjected to AdVIL-24 human
gene therapy by multi-point intratumoral injection of AdVIL-24
(1×108), AdV (1×108) or PBS every other day
for a total of 6 times, respectively. Following the second AdVIL-24
gene therapy, the mice bearing CNE-2Z xenografted tumors were
further assigned to receive single radiotherapy at a dose of 10
Gy/tumor using a 60Co-γ source (1 Gy/min) as reported
previously (27). All the mice for
radiation were anesthetized with 10% chloral hydrate (3 μl/g body
weight) and positioned in the radiation field so that only the
tumor xenograft implanted on the armpit of the right anterior limb
was exposed to the irradiation beam and the rest of the mouse’s
body was shielded by a lead block. Tumor progression and regression
were monitored, and the tumor volume was measured daily. In
addition, the tumor-bearing mice were sacrificed 2 weeks after
treatment, and the CNE-2Z human NPC s.c. xenografted tumors were
removed, weighed, fixed with 10% neutral formalin and embedded in
paraffin for hematoxylin and eosin (H&E) staining and
immunohistochemistry analysis.
Immunohistochemistry
The in vivo expression of P21, P27, cyclin E,
CDK2, Bcl-2, Bax, cleaved caspase-3, CD34 and VEGF in the treated
and untreated CNE-2Z human NPC s.c. xenografted tumors was examined
by immunohistochemistry using the UltraSensitive™ SP kit following
the manufacturer’s instructions. The presence of buffy or brown
diaminobenzidine precipitates is indicative of positive reactivity.
The integral optical density (IOD) of immunohistochemical intensity
was analyzed by Image-Pro Plus 6.0 software (Media Cybernetics,
Bethesda, MD, USA). In addition, the microvessel density (MVD) was
assessed by CD34 immunostaining as described previously (28). Any endothelial cell cluster
immunoreactive for CD34 clearly separated from adjacent
microvessels was considered as a single countable vessel. To detect
the apoptotic cells in vivo in the CNE-2Z human NPC
xenografted tumors, tumor sections were further analyzed for
apoptosis using the TUNEL apoptosis detection kit according to the
manufacturer’s protocols. Each value represents IOD, microvessels
or apoptotic cells counted at a high-power view (x200) by
microscopy. The mean value represents the average number derived
from five high-power fields of each case.
Statistical analysis and evaluation of
combinatorial interaction
All data are presented as the mean ± standard
deviation (SD). The significance of the difference between groups
was evaluated by one-way or two-way repeated measure analysis of
variance (ANOVA) and multiple comparisons with SPSS 10.0 software
(SPSS, Chicago, IL, USA). A value of P<0.05 was considered
statistically significant. The interactive effects of AdVIL-24 and
radiotherapy were evaluated by Q-value (29), Q = F(A + B)/FA + (1 - FA)FB, where
F(A + B) represents the fraction affected by combined treatment
with AdVIL-24 plus radiotherapy compared to the untreated control
group, FA represents the fraction affected by AdVIL-24 alone and FB
represents the fraction affected by radiotherapy alone. A value of
Q>1.15 indicates a synergistic effect between AdVIL-24 and
radiotherapy, Q<0.85 indicates an antagonistic effect and Q
between 0.85 and 1.15 indicates an additive effect.
Results
Transgene IL-24 overexpression
To assess the optimal MOI for a maximal
adenovirus-directed IL-24 transgene expression with minimal
adenovirus itself-induced cytotoxic effect, CNE-2Z human NPC cells
were infected with AdVIL-24 or AdV at various MOIs, and then
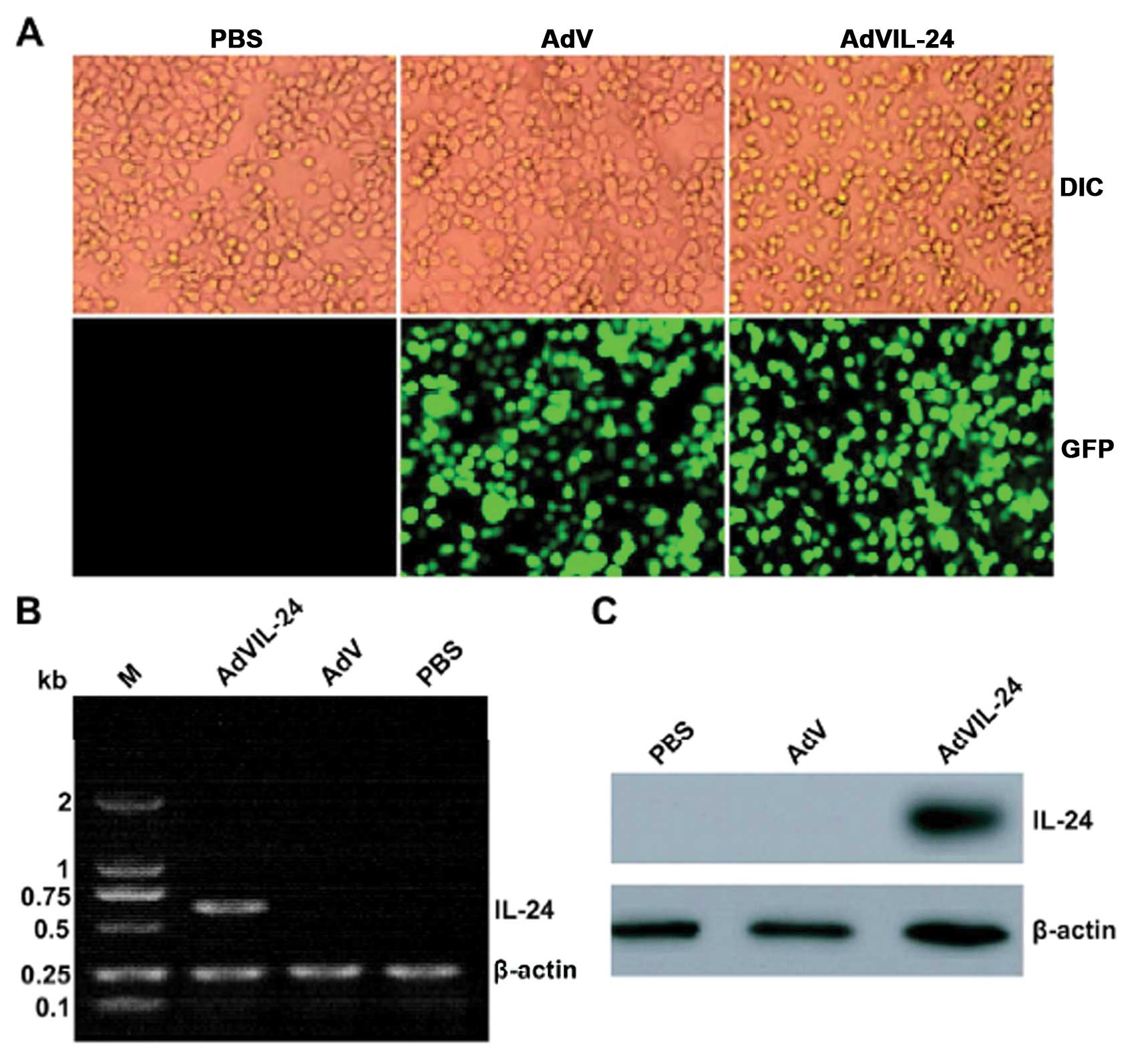
examined under fluorescence microscopy. As shown in Fig. 1A, more than 90% of GFP expression
was observed in the CNE-2Z tumor cells treated with AdVIL-24 or AdV
at a MOI of 100 or above (data not shown), whereas the GFP
expression was not found in the untreated control CNE-2Z tumor
cells. In addition, there was almost no adenovirus-elicited
cytotoxicity in the 100 MOI blank AdV-infected CNE-2Z tumor cells
(Fig. 1A). Therefore, we selected a
MOI of 100 as an optimal dose for the adenoviral infection of the
CNE-2Z tumor cell line in the following experiments. To further
determine AdVIL-24 expression, the AdVIL-24- or AdV-treated and
untreated CNE-2Z tumor cells were subjected to RT-PCR (Fig. 1B) and western blotting (Fig. 1C) analysis, respectively. These
results indicated that exogenous IL-24 transgene mediated by the
adenoviral transfer was efficiently expressed in the
AdVIL-24-infected CNE-2Z tumor cells at both the transcriptional
and translational levels.
Enhanced tumor suppression by AdVIL-24
plus radiation
Before performing experiments involving a
combination of AdVIL-24 gene therapy plus ionizing radiotherapy, we
first carried out a radiation dose-assessing study in vitro
in the CNE-2Z human NPC cells by flow cytometric analysis of
apoptosis. As shown in Fig. 2A and
B, irradiation with various doses resulted in dose-dependent
apoptosis in the CNE-2Z tumor cells. A slight induction of
apoptosis in the CNE-2Z tumor cells was observed at 2 Gy (14.51%
early apoptosis). Moderate apoptotic induction was observed at 4 Gy
(26.13%), and significant apoptotic induction was observed starting
at 6 Gy (41.46%) with maximum induction observed at 8 Gy (61.75%).
To avoid the serious side-effects of a high dose of radiation and
in order to leave a window for the observation of combined effect,
we chose 4 Gy as the radiation dose in the in vitro combined
treatment with AdVIL-24 gene therapy for CNE-2Z human NPC cells. To
examine the combined antitumor effect of AdVIL-24 plus radiotherapy
in vitro on the CNE-2Z tumor cells, CNE-2Z human NPC cells
were treated with AdVIL-24 (100 MOI), AdV (100 MOI), PBS or 4 Gy
alone, or AdVIL-24 (100 MOI) plus 4 Gy. The tumor cell viability
was determined daily for 4 days by MTT assay. As shown in Fig. 2C, the combined treatment with
AdVIL-24 gene therapy plus 4 Gy radiotherapy additively inhibited
the in vitro CNE-2Z tumor cell growth in a time-dependent
manner, compared with the single AdVIL-24- and 4 Gy-treated group
(P<0.05; Q=0.916 and 1.050, at day 3 and 4 after treatment,
respectively). The in vitro combined tumor-suppressive
effect of AdVIL-24 gene therapy plus ionizing radiotherapy on
CNE-2Z human NPC cells was confirmed by clonogenic survival assay
(Fig. 2D) (P<0.05; Q=1.074). To
further explore whether the combination of AdVIL-24 plus
radiotherapy exerts an in vivo enhanced antitumor effect,
the athymic nude mice bearing CNE-2Z human NPC s.c. xenografted
tumors were intratumorally injected with AdVIL-24, AdV or PBS
alone, or irradiated with 10 Gy alone or in combination with
intratumoral injection of AdVIL-24. The tumor growth in vivo
was monitored daily, and tumor volume and weight were measured. As
shown in Fig. 2E–G, the tumor
growth was more significantly retarded in the AdVIL-24 plus 10 Gy
group, compared with single AdVIL-24- and 10 Gy-treated groups
(P<0.05; Qvolume =1.099, 1.098, 0.981, 1.127 and
1.053, at day 6, 8, 10, 12 and 14 after treatment, and
Qweight =1.004, respectively), indicating that the
combination treatment with AdVIL-24 plus 10 Gy markedly suppressed
CNE-2Z human NPC s.c. xenografted tumor growth in vivo in
the athymic nude mice with an overlapping effect.
 | Figure 2Adenovirus-mediated interleukin-24
(AdVIL-24) plus radiotherapy induces enhanced tumor suppression. (A
and B) The effect of radiation alone on CNE-2Z tumor cells by flow
cytometry. The CNE-2Z human NPC cells were irradiated with various
doses of radiation. (A) Twenty-four hours later, the tumor cells
were harvested, stained with Annexin V-PE and 7-AAD and then
analyzed by flow cytometry. (B) The Annexin V single-positive cells
in the total cell population represented early apoptotic cells. (C
and D) AdVIL-24 combined with radiation induces enhanced growth
inhibition in vitro. The CNE-2Z human NPC cells were treated
with PBS, AdV, AdVIL-24, 4 Gy or AdVIL-24 plus 4 Gy for the
indicated time periods. (C) The surviving cells were evaluated at
day 0, 1 (i.e. 24 h after AdVIL-24 infection), 2 (i.e. 24 h after 4
Gy irradiation), 3 and 4 after treatment by MTT assay.
*P<0.05 compared with AdVIL-24 and 4 Gy group;
Q=0.916 and 1.050, at day 3 and 4 after treatment, respectively,
two-way repeated measure ANOVA and multiple comparisons, n=4
replicates/condition. (D) The survival fraction in the clonogenic
survival assay was calculated by comparison with the PBS-treated
control group. *P<0.05 compared with AdVIL-24 and 4
Gy group; Q=1.074, one-way repeated measure ANOVA and multiple
comparisons, n=3 replicates/condition. (E-G) AdVIL-24 combined with
radiation induces enhanced growth inhibition in vivo. The
athymic nude mice bearing CNE-2Z human NPC subcutaneously
xenografted tumors were intratumorally injected with AdVIL-24, AdV
or PBS every other day for a total of 6 times, respectively. The
mice were further subjected to single radiotherapy at a dose of 10
Gy/tumor at day 2 post AdVIL-24 gene therapy (i.e. after the second
AdVIL-24 gene therapy). (E) Tumor volume before and after
treatment, and (F) tumor weight of the CNE-2Z xenografted tumors
removed 14 days after treatment were measured. (G) Tumor growth
inhibition rate in vivo was further calculated according to
tumor weight. *P<0.05 compared with AdVIL-24 and 10
Gy group; Qvolume =1.099, 1.098, 0.981, 1.127 and 1.053,
at day 6, 8, 10, 12 and 14 after treatment, and Qweight
=1.004, respectively; two-way and one-way repeated measure ANOVA
and multiple comparisons, n=6 mice/condition. Data shown are
representative of three independent experiments. |
Alteration in cell cycle distribution and
enhanced induction of apoptosis by AdVIL-24 plus radiation
To explore the mechanism by which combination
therapy with AdVIL-24 and radiation additively suppresses tumor
cell growth, the in vitro cell cycle profiles and apoptosis
of the CNE-2Z cells treated with AdVIL-24 or 4 Gy alone, or
AdVIL-24 plus 4 Gy for 48 h were analyzed using PI single staining,
and Annexin V-PE/7-AAD double staining by flow cytometry,
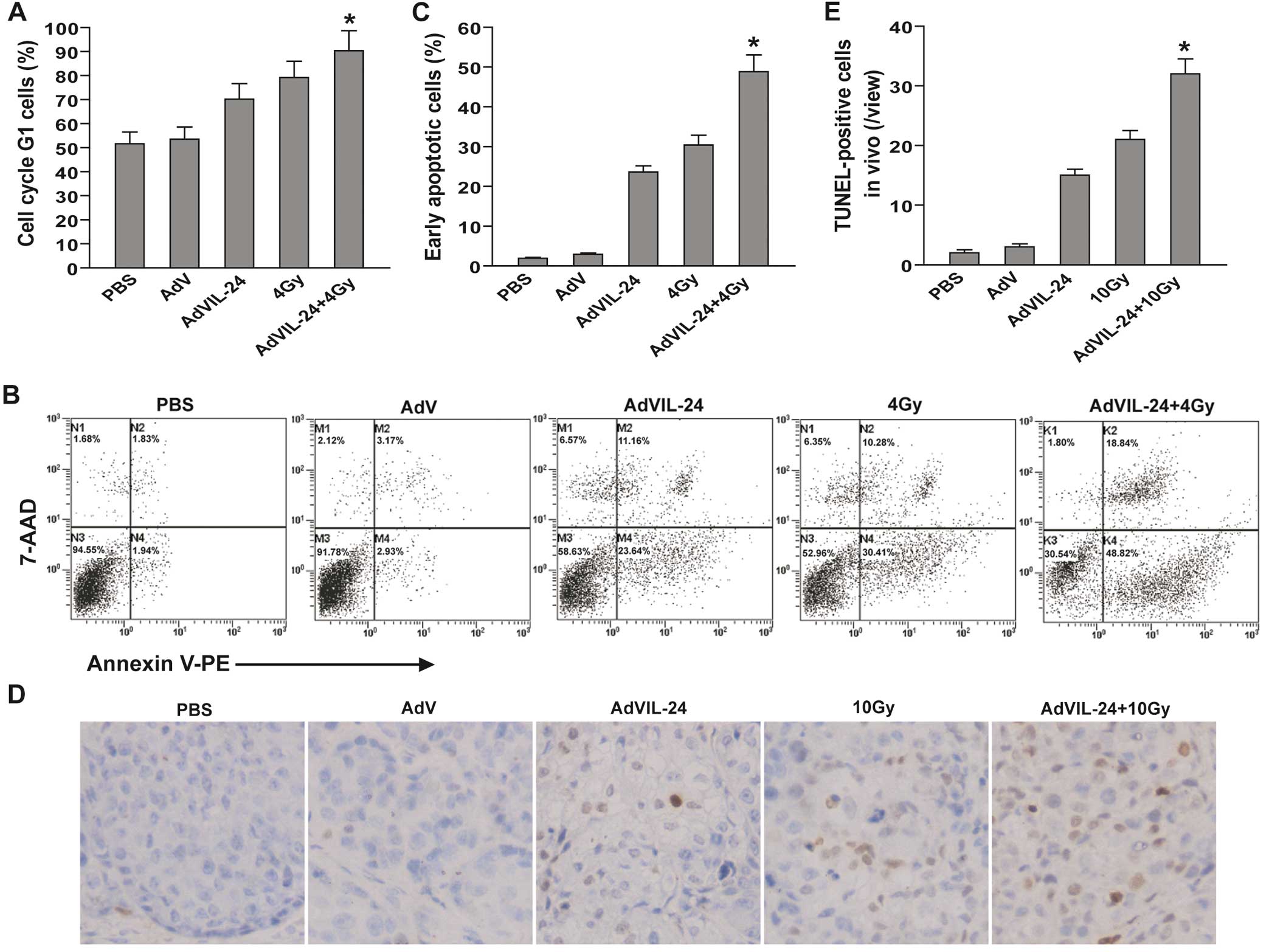
respectively. As shown in Fig. 3A,
compared with the PBS (51.61%) and AdV (53.52%) control group, a
significant increase in the G1 phase population was observed in the
AdVIL-24 (70.22%), 4 Gy (79.24%) and AdVIL-24 plus 4 Gy (90.37%)
groups (P<0.05). Compared with the single AdVIL-24- and 4
Gy-treated groups, AdVIL-24 plus 4 Gy additively induced CNE-2Z
tumor cell cycle G1 phase arrest (P<0.05; Q=0.943). Furthermore,
AdVIL-24 plus radiation induced 48.82% early apoptosis in CNE-2Z
tumor cells, whereas there was only 1.94, 2.93, 23.64 and 30.41%
CNE-2Z cells undergoing early apoptosis in the PBS, AdV, AdVIL-24
and 4 Gy groups (Fig. 3B and C).
Compared with the single AdVIL-24- and 4 Gy-treated groups,
treatment with AdVIL-24 plus 4 Gy more efficiently elicited CNE-2Z
tumor cell apoptosis with an overlapping effect (P<0.05;
Q=1.042). To further assess the induction of apoptosis in
vivo, we performed a TUNEL assay in the treated and untreated
CNE-2Z human NPC s.c. xenografted tumors by immunohistochemistry
analysis (Fig. 3D and E).
Consistent with the flow cytometric results in vitro,
AdVIL-24 plus radiation also had an additive effect on the in
vivo apoptosis induction of CNE-2Z human NPC cells s.c.
implanted in the athymic nude mice (P<0.05; Q=0.974).
 | Figure 3Adenovirus-mediated interleukin-24
(AdVIL-24) plus radiotherapy enhances G1 phase arrest and
apoptosis. (A) In vitro cell cycle analysis by flow
cytometry. The CNE-2Z human NPC cells were treated with PBS, AdV,
AdVIL-24, 4 Gy or AdVIL-24 plus 4 Gy. Forty-eight hours after
infection (i.e. 24 h after irradiation), the tumor cells were
harvested, fixed in 70% cold alcohol, stained with propidium iodide
and then analyzed by flow cytometry. The G1 phase cells in the
total cell population are presented. *P<0.05 compared
with AdVIL-24 and 4 Gy groups; Q=0.943, one-way repeated measure
ANOVA and multiple comparisons, n=3 replicates/condition. (B and C)
In vitro apoptosis analysis by flow cytometry. The CNE-2Z
tumor cells were treated with PBS, AdV, AdVIL-24, 4 Gy or AdVIL-24
plus 4 Gy. (B) Forty-eight hours after infection (i.e. 24 h after
irradiation), the tumor cells were harvested, stained with Annexin
V-PE and 7-AAD and then analyzed by flow cytometry. (C) The Annexin
V single-positive cells in the total cell population represented
early apoptotic cells. *P<0.05 compared with AdVIL-24
and 4 Gy group; Q=1.042, one-way repeated measures ANOVA and
multiple comparisons, n=3 replicates/condition. (D and E) In
vivo apoptosis analysis by TUNEL assay. (D) Representative
immunohistochemical images of TUNEL analysis of the CNE-2Z human
NPC subcutaneously xenografted tumors are shown. (E) The
TUNEL-positive cells represented in vivo apoptotic cells.
*P<0.05 compared with AdVIL-24 and 10 Gy group;
Q=0.974, one-way repeated measure ANOVA and multiple comparisons,
n=6 mice/condition, n=5 observations/representative section. Data
shown are representative of three independent experiments. |
Enhanced upregulation of P21 and P27 CDK
inhibitors (CDIs) and Bax/Bcl-2 as well as downregulation of cyclin
E and CDK2 following treatment with AdVIL-24 plus radiation
To further elucidate the molecular mechanism
underlying the antitumor effect mediated by combined therapy of
AdVIL-24 plus radiation, the expression levels of cell cycle
regulatory molecules such as P21 and P27 CDIs, cyclin E and CDK2
and apoptosis-related proteins including Bcl-2, Bax and caspase-3
in the CNE-2Z cells after different treatments were analyzed by
western blotting. As shown in Fig.
4A, the expression of P21, P27, and Bax in the AdVIL-24, 4 Gy
and AdVIL-24 plus 4 Gy groups was significantly increased, whereas
the expression of cyclin E, CDK2 and Bcl-2 was decreased. In
addition, cleavage of caspase-3 was noted in the AdVIL-24, 4 Gy and
AdVIL-24 plus 4 Gy groups but not in the PBS and AdV groups.
Moreover, the combined treatment of AdVIL-24 plus radiation
resulted in an enhanced effect on the upregulation of P21 and P27
CDIs and the ratio of pro-apoptotic to anti-apoptotic molecules
Bax/Bcl-2, downregulation of cyclin E and CDK2 and activation of
caspase-3. The effect of AdVIL-24 plus radiation on in vitro
expression of P21, P27, cyclin E, CDK2 and Bax/Bcl-2 in CNE-2Z
tumor cells and in vivo expression of P21, P27, cyclin E,
CDK2, Bax/Bcl-2 and cleaved caspase-3 in CNE-2Z NPC s.c.
xenografted tumors was further confirmed by real-time quantitative
RT-PCR analysis (Fig. 4B)
(P<0.05; QP21 =0.959, QP27 =0.956,
Qcyclin E =1.078, QCDK2 =1.046 and
QBax/Bcl-2 =0.995) and immunohistochemistry (Fig. 4C) (p<0.05; QP21
=0.920, QP27 =0.937, Qcyclin E =1.060,
QCDK2 =1.019, QBax/Bcl-2 =0.982 and
Qcleaved caspase-3 =0.927).
 | Figure 4Adenovirus-mediated interleukin-24
(AdVIL-24) plus radiotherapy enhances the upregulation of P21 and
P27 cyclin-dependent kinase (CDK) inhibitors and downregulation of
cyclin E and CDK2 as well as activation of intrinsic apoptosis. (A)
Western blot analysis of cell cycle regulatory molecules and
apoptosis-related proteins. The total cellular lysates derived from
CNE-2Z human NPC cells treated with PBS, AdV, AdVIL-24, 4 Gy or
AdVIL-24 plus 4 Gy were subject to western blot analysis using a
panel of antibodies specific for P21, P27, cyclin E, CDK2, Bcl-2,
Bax, caspase-3 and β-actin. (B) Real-time quantitative reverse
transcription (RT)-PCR analysis of cell cycle regulatory molecules
and apoptosis-related proteins. The total cellular RNAs derived
from CNE-2Z tumor cells after different treatments were subjected
to SYBR-Green I-based real-time quantitative RT-PCR analysis using
primer sets specific for P21, P27, cyclin E, CDK2, Bcl-2, Bax and
β-actin. In each case, the transcriptional expression of P21, P27,
cyclin E, CDK2 and Bax/Bcl-2 was normalized to the expression level
of β-actin, and the relative change was expressed as a ratio, with
1 being the value for PBS-treated control cells.
*P<0.05 compared with the AdVIL-24 and 4 Gy group,
QP21 =0.959, QP27 =0.956, Qcyclin
E =1.078, QCDK2 =1.046 and QBax/Bcl-2
=0.995, respectively; one-way repeated measures ANOVA and multiple
comparisons, n=3 replicates/condition, n=3 replicates/sample. (C)
Immunohistochemical analysis of cell cycle regulatory molecules and
apoptosis-related proteins. The immunostaining intensity of P21,
P27, cyclin E, CDK2, Bcl-2, Bax and cleaved caspase-3 was
quantified as integral optical density by Image-Pro Plus 6.0
software. *P<0.05 compared with the AdVIL-24 and 10
Gy groups, QP21 =0.920, QP27 =0.937,
Qcyclin E =1.060, QCDK2 =1.019,
QBax/Bcl-2 =0.982 and Qcleaved caspase-3
=0.927, respectively; one-way repeated measures ANOVA and multiple
comparisons, n=6 replicates/condition, n=5
observations/representative section. Data shown are representative
of three independent experiments. |
Enhanced reduction of MVD by AdVIL-24
plus radiation
Tumor angiogenesis plays a critical role in cancer
progression. To examine the combined effect of AdVIL-24 plus
radiation on tumor angiogenesis in vivo, the MVD in CNE-2Z
human NPC s.c. xenografted tumors was analyzed by CD34
immunohistochemical analysis. The positive expression of CD34 was
mainly presented as brownish yellow or brownish granules in
vascular endothelial cells of the CNE-2Z human NPC s.c. xenografted
tumors (Fig. 5A). Compared with the
PBS and AdV groups, the CD34 expression of tumor vascular
endothelial cells in the AdVIL-24, 10 Gy and AdVIL-24 plus 10 Gy
groups was weaker or less (Fig. 5A and
B) (P<0.05). In addition, the MVD (Fig. 5C) determined in the AdVIL-24, 10 Gy
and AdVIL-24 plus 10 Gy groups was significantly less than that in
the PBS and AdV groups (P<0.05). Moreover, AdVIL-24 gene therapy
plus 10 Gy radiotherapy had an overlapping effect on the
downregulation of CD34 and the reduction in MVD in the CNE-2Z human
NPC s.c. xenografted tumors (p<0.05; QCD34 =0.990 and
QMVD =0.988), which may be responsible for the AdVIL-24
and 10 Gy combined therapy-induced enhanced growth suppression of
the in vivo CNE-2Z human NPC s.c. xenografted tumors in the
athymic nude mice.
AdVIL-24 suppresses radiation-induced
expression of VEGF, a pro-angiogenic factor
Tumors can express pro-angiogenic factors, which
promote the formation of tumor blood vessels and consequently
facilitate tumor growth in vivo. To investigate whether
AdVIL-24, radiation, or their combination affectes the production
of pro-angiogenic factors, we assessed VEGF expression in
vivo in the CNE-2Z human NPC s.c. xenografted tumors by
immunohistochemical analysis. As shown in Fig. 5A and B, some constitutive expression
of VEGF was apparent in the PBS or AdV groups. AdVIL-24 alone
significantly inhibited the expression levels of VEGF (P<0.05).
However, radiation alone substantially enhanced its expression
(P<0.05). Of note, AdVIL-24 potentially blocked
radiation-induced enhancement of VEGF expression, indicating that
AdVIL-24 gene therapy is capable of impairing the
radiation-elicited pro-angiogenic activity via suppressing the
production of pro-angiogenic factors such as VEGF.
Discussion
NPC is a major malignant tumor of the head and neck
region, with high a incidence in Southern China and Southeast Asia.
Radiotherapy or combination with chemotherapy is currently the
mainstay of proven treatment strategies. However, the local
recurrence and distant metastasis noted in NPC remains a
significant obstacle to therapeutic strategies, which is a main
reason for treatment failure. Thus, the development of novel
therapeutic approaches for NPC is of paramount importance. Gene
therapy is a promising treatment modality for cancer. Preclinical
studies and clinical trials have shown that AdV p53 gene therapy
alone or combined with ionizing radiotherapy and chemotherapy
exerts strong antitumor potential in NPC (18,30–32).
Extensive studies have demonstrated that IL-24 can specifically
induce apoptosis in a large variety of tumor cells, suppress tumor
angiogenesis, stimulate immune responses, promote bystander
antitumor activity and enhance radio- and chemo-sensitivity.
Therefore, this prompted us to extend previous research and examine
the therapeutic effect of AdVIL-24 gene therapy combined with
ionizing radiation on NPC. In this study, we found that AdVIL-24
gene therapy combined with ionizing radiotherapy induced enhanced
growth suppression, G1 phase arrest and apoptosis in vitro
in CNE-2Z human NPC cells. Moreover, AdVIL-24 plus radiotherapy
also additively inhibited in vivo CNE-2Z human NPC s.c.
xenografted tumor growth in athymic nude mice.
Cyclins, CDKs and CDIs are necessary for cell cycle
progression. The activities of these cyclin/CDK complexes are
negatively regulated by CDIs by directly preventing CDK
phosphorylation and inhibiting their activity. P21 and P27 as
important members of the CDIs belong to the Cip/Kip family and
suppress the activation of cyclin E-CDK2, cyclin D-CDK4 and cyclin
A-CDK2 complexes, leading to cell cycle G1 phase arrest (33,34).
Initiation of apoptosis induced by irreparable cellular damage is a
key mechanism by which ionizing radiation kills cancer cells.
Members of the Bcl-2 protein family are known to be key regulators
of apoptosis and crucial determinants of cellular fate (35). Bcl-2 is an important anti-apoptotic
protein containing four conserved Bcl-2 homology (BH) domains,
which heterodimerizes pro-apoptotic protein Bax and its
overexpression protects cells from apoptosis induced by different
stimuli. The ratio of pro-apoptotic to anti-apoptotic molecules
such as Bax/Bcl-2 constitutes a rheostat that sets the threshold of
susceptibility to apoptosis for the intrinsic pathway, including
pore formation in the mitochondrial outer membrane, loss of
mitochondrial integrity and the release of cytochrome c into
the cytosol followed by the cleavage of caspase-9 and -3 (35). Thus, overlapping effect of AdVIL-24
plus radiation on the upregulation of P21 and P27 CDK inhibitors
and Bax/Bcl-2 ratio, downregulation of cyclin E and CDK2 and
activation of caspase-3 may be responsible for the enhanced
induction of G1 phase arrest and apoptosis, and consequent additive
growth suppression in NPC.
Tumor angiogenesis is a prerequisite for successful
tumor growth and formation of metastasis, and is regulated by the
balance of tumor-derived pro-angiogenic and anti-angiogenic
factors. Thus, tumor angiogenesis is a potential therapeutic target
in anticancer therapy. It has been shown that VEGF is a crucial
pro-angiogenic factor for tumor angiogenesis (36). Accumulating evidence also suggests
that ionizing radiation can induce VEGF production and subsequently
facilitate the formation of tumor vessels, leading to tumor
regrowth and accelerated metastasis (37,38).
Combined therapy with radiotherapy and an angiogenesis inhibitor
can override the ionizing radiation-elicited pro-angiogenic
activity, thereby exhibiting enhanced antitumor activity. In our
study, we demonstrated that AdVIL-24 gene therapy plus ionizing
radiotherapy additively inhibited CD34 expression and reduced MVD
in CNE-2Z human NPC s.c. xenografted tumors, which may be another
important mechanism involved in AdVIL-24 plus radiation-mediated
in vivo enhanced growth inhibition of CNE-2Z human NPC
xenografted tumors in athymic nude mice. In agreement with a
previous report (37), ionizing
radiation alone abundantly elevated the levels of the
pro-angiogenic factor VEGF. Notably, AdVIL-24 efficiently blocked
the upregulation of VEGF induced by radiation, likely contributing
to the enhanced anti-angiogenic effect.
Taken together, AdVIL-24 gene therapy plus ionizing
radiotherapy induced enhanced growth inhibition, cell cycle G1
phase arrest and apoptosis in vitro in CNE-2Z human NPC
cells and in vivo in CNE-2Z xenografted tumors s.c.
implanted in athymic nude mice. Mechanistically, AdVIL-24 combined
with ionizing radiation led to the substantial upregulation of P21
and P27 CDK inhibitors, ratio of pro-apoptotic to anti-apoptotic
molecules Bax/Bcl-2 and cleaved caspase-3 as well as downregulation
of cyclin E and CDK2 in vitro and in vivo in CNE-2Z
human NPC cells. Furthermore, AdVIL-24 plus radiation additively
reduced the tumor vessel CD34 expression and MVD in vivo.
More importantly, AdVIL-24 potentially blocked the
radiation-induced enhancement of VEGF. The enhanced antitumor
activity against NPC elicited by AdVIL-24 gene therapy combined
with ionizing radiotherapy was closely associated with the enhanced
induction of G1 phase arrest and apoptosis via the additive
modulation of cell cycle regulatory molecules and activation of the
intrinsic apoptotic pathway, and the overlapping inhibition of
tumor angiogenesis. Thus, our results suggest that AdVIL-24 gene
therapy combined with ionizing radiotherapy may be a novel and
effective treatment strategy for human NPC.
Acknowledgements
This research was supported by grants from the
National Natural Science Foundation of China (no. 81001016) and the
Medicine Research Foundation of the Board of Health of Suzhou City
(no. SYS201014).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young LS and Rickinson AB: Epstein-Barr
virus: 40 years on. Nat Rev Cancer. 4:757–768. 2004.PubMed/NCBI
|
|
3
|
Spano JP, Busson P, Atlan D, Bourhis J,
Pignon JP, Esteban C and Armand JP: Nasopharyngeal carcinomas: an
update. Eur J Cancer. 39:2121–2135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guigay J: Advances in nasopharyngeal
carcinoma. Curr Opin Oncol. 20:264–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AW, Yau TK, Wong DH, et al: Treatment
of stage IV(A–B) nasopharyngeal carcinoma by induction-concurrent
chemoradiotherapy and accelerated fractionation. Int J Radiat Oncol
Biol Phys. 63:1331–1338. 2005.
|
|
7
|
Le QT, Tate D, Koong A, et al: Improved
local control with stereotactic radiosurgical boost in patients
with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
56:1046–1054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee AW, Sze WM, Au JS, et al: Treatment
results for nasopharyngeal carcinoma in the modern era: the Hong
Kong experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sauane M, Gopalkrishnan RV, Sarkar D, et
al: MDA-7/IL-24: novel cancer growth suppressing and apoptosis
inducing cytokine. Cytokine Growth Factor Rev. 14:35–51. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fisher PB: Is mda-7/IL-24 a ‘magic bullet’
for cancer? Cancer Res. 65:10128–10138. 2005.
|
|
11
|
Ramesh R, Mhashilkar AM, Tanaka F, et al:
Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a
novel ligand that regulates angiogenesis via the IL-22 receptor.
Cancer Res. 63:5105–5113. 2003.PubMed/NCBI
|
|
12
|
Nishikawa T, Ramesh R, Munshi A, Chada S
and Meyn RE: Adenovirus-mediated mda-7 (IL24) gene therapy
suppresses angiogenesis and sensitizes NSCLC xenograft tumors to
radiation. Mol Ther. 9:818–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caudell EG, Mumm JB, Poindexter N, et al:
The protein product of the tumor suppressor gene, melanoma
differentiation-associated gene 7, exhibits immunostimulatory
activity and is designated IL-24. J Immunol. 168:6041–6046. 2002.
View Article : Google Scholar
|
|
14
|
Ramesh R, Ito I, Gopalan B, Saito Y,
Mhashilkar AM and Chada S: Ectopic production of MDA-7/IL-24
inhibits invasion and migration of human lung cancer cells. Mol
Ther. 9:510–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park MA, Yacoub A, Sarkar D, et al:
PERK-dependent regulation of MDA-7/IL-24-induced autophagy in
primary human glioma cells. Autophagy. 4:513–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yacoub A, Hamed HA, Allegood J, et al:
PERK-dependent regulation of ceramide synthase 6 and thioredoxin
play a key role in mda-7/IL-24-induced killing of primary human
glioblastoma multiforme cells. Cancer Res. 70:1120–1129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hughes J, Alusi G and Wang Y: Gene therapy
and nasopharyngeal carcinoma. Rhinology. 50:115–121. 2012.
|
|
18
|
Li JH, Huang D, Sun BF, Zhang X,
Middeldorp J, Klamut H and Liu FF: Efficacy of ionizing radiation
combined with adenoviral p53 therapy in EBV-positive nasopharyngeal
carcinoma. Int J Cancer. 87:606–610. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie Y, Sheng W, Miao J, Xiang J and Yang
J: Enhanced antitumor activity by combining an adenovirus harboring
ING4 with cisplatin for hepatocarcinoma cells. Cancer Gene Ther.
18:176–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su ZZ, Lebedeva IV, Sarkar D, et al:
Ionizing radiation enhances therapeutic activity of mda-7/IL-24:
overcoming radiation- and mda-7/IL-24-resistance in prostate cancer
cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2.
Oncogene. 25:2339–2348. 2006. View Article : Google Scholar
|
|
21
|
Emdad L, Sarkar D, Lebedeva IV, et al:
Ionizing radiation enhances adenoviral vector expressing
mda-7/IL-24-mediated apoptosis in human ovarian cancer. J Cell
Physiol. 208:298–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Sheng W, Xie Y, Shan Y, Miao J,
Xiang J and Yang J: The in vitro and in vivo antitumor activity of
adenovirus-mediated interleukin-24 expression for laryngocarcinoma.
Cancer Biother Radiopharm. 25:29–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Ye Z, Zhong J, Xiang J and Yang J:
Adenovirus-mediated Il-24 expression suppresses hepatocellular
carcinoma growth via induction of cell apoptosis and cycling arrest
and reduction of angiogenesis. Cancer Biother Radiopharm. 22:56–63.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Y, Lv H, Sheng W, Miao J, Xiang J and
Yang J: Synergistic tumor suppression by adenovirus-mediated
inhibitor of growth 4 and interleukin-24 gene cotransfer in
hepatocarcinoma cells. Cancer Biother Radiopharm. 26:681–695. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
26
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Madhusoodhanan R, Natarajan M,
Veeraraghavan J, Herman TS and Aravindan N: NFkappaB activity and
transcriptional responses in human breast adenocarcinoma cells
after single and fractionated irradiation. Cancer Biol Ther.
8:765–773. 2009. View Article : Google Scholar
|
|
28
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar
|
|
29
|
Wang W, Qin SK, Chen BA and Chen HY:
Experimental study on antitumor effect of arsenic trioxide in
combination with cisplatin or doxorubicin on hepatocellular
carcinoma. World J Gastroenterol. 7:702–705. 2001.PubMed/NCBI
|
|
30
|
Li JH, Li P, Klamut H and Liu FF:
Cytotoxic effects of Ad5CMV-p53 expression in two human
nasopharyngeal carcinoma cell lines. Clin Cancer Res. 3:507–514.
1997.PubMed/NCBI
|
|
31
|
Pan JJ, Zhang SW, Chen CB, et al: Effect
of recombinant adenovirus-p53 combined with radiotherapy on
long-term prognosis of advanced nasopharyngeal carcinoma. J Clin
Oncol. 27:799–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weinrib L, Li JH, Donovan J, Huang D and
Liu FF: Cisplatin chemotherapy plus adenoviral p53 gene therapy in
EBV-positive and -negative nasopharyngeal carcinoma. Cancer Gene
Ther. 8:352–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toyoshima H and Hunter T: p27, a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: a critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: role of reoxygenation, free radicals, and stress granules.
Cancer Cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Camphausen K, Moses MA, Beecken WD, Khan
MK, Folkman J and O’Reilly MS: Radiation therapy to a primary tumor
accelerates metastatic growth in mice. Cancer Res. 61:2207–2211.
2001.PubMed/NCBI
|