Introduction
Gastric cancer ranks as the third and fifth most
common malignancy in males and females, respectively, in the world
(1). Although its morality has
significantly declined in recent years, gastric cancer is still
estimated to account for approximately 10% of invasive cancers with
a high incidence in Japan, China, Korea, Central and South American
(2–5). To date, chemotherapy remains the
primary treatment option for both resectable and advanced gastric
cancer to improve overall survival and the quality of life of
patients (6,7). However, expression of multiple drug
resistance (MDR) genes is a major obstacle to successful
chemotherapy of various blood-related and solid tumors (8), including gastric cancer. It has been
recognized that during or following chemotherapy, MDR occurs, and
the underlying molecular mechanisms have been extensively studied
in vivo and in vitro(9–12). For
example, upregulation of ATP-binding cassette transporters, such as
P-glycoprotein (P-gp) and MDR-associated protein 1 (MRP1) are
mainly responsible for chemotherapy-induced MDR. Recently, several
novel factors acting downstream of the initial drug-induced insult
have been shown to play an important role in the development of
MDR, such as enhanced DNA repair activity, defective apoptosis
pathway, or altered metabolism of drugs (10). The MDR mechanisms in gastric
carcinoma cells have been extensively explored, but there are no
clear answers. Accumulated evidence to date suggests that
mechanisms responsible for MDR in gastric carcinoma are likely to
be extremely intricate. Therefore, there is a need to further
explore the mechanisms involved in MDR and to identify efficient
and low toxicity modulators to be used for the clinical treatment
of gastric cancer.
Inhibitor of growth 4 (ING4) represents a novel
member of the ING tumor-suppressor family, which was isolated and
characterized by Shiseki et al in 2003 (13). The tumor-suppressor protein ING4 can
interact with p53, inhibit cell growth and induce apoptosis in
different types of cells. ING4 protein contains a plant homeodomain
(PHD)-finger motif (14), a
transcriptional regulator that involves chromatin remodeling
(15), in its COOH-terminal region
and a potential bipartite nuclear localization signal (NLS)
(16) in its middle region. More
recently, ING4 has been shown to play a role in the regulation of
DNA repair, tumor growth, angiogenesis, migration and gene
transcription. Furthermore, ING4 can suppress the loss of contact
inhibition induced by myelocytomatosis viral-related oncogene,
neuroblastoma derived (MYCN) and myelocytomatosis viral oncogene
homolog (MYC) family oncogenes (17). In addition, the ING4-dominant mutant
can promote MYC-initiated mouse mammary carcinogenesis (18), whereas overexpression of ING4
protein can apparently enhance tumor growth inhibition in a
p53-dependent and p53-independent manner by induction of cell cycle
alteration and apoptosis (13,19–21)
and inhibition of tumor angiogenesis by repressing nuclear factor
κB and hypoxia-inducible factor-1α (22–24).
In addition, ING4 can enhance radiosensitivity and chemosensitivity
in non-small cell lung cancer SPC-A1 cells and hepatocarcinoma
cells, respectively (19,25). Previous studies have shown that the
combination of radiotherapy, chemotherapy, and other conventional
therapies with gene therapy is a promising practice for the
treatment of cancers (26–28). ING4 has been reported to enhance
chemosensitivity to DNA-damage agents, such as doxorubicin or
etoposide in human hepatocarcinoma cells (19,26).
Our previous data also demonstrated that adenovirus-mediated
(AdVING4) gene therapy sensitized human hepatocarcinoma cells to
cisplatin (CDDP) and enhanced the radiosensitivity of
non-small-cell lung cancer (19,25).
However, reversal of its resistance in human cancers has not been
reported. Therefore, on the basis of prior studies, we hypothesized
that ING4 may affect the MDR phenotype of human gastric carcinoma
cells. In the present study, we investigated the possible role of
ING4 in the reversion of human gastric cancer cell MDR and its
underlying mechanisms.
Materials and methods
Adenoviruses, cell lines, reagents and
mice
The AdVING4 and AdV-green fluorescent protein
(AdVGFP) replication-incompetent Ad5E1- and E3-deleted adenoviruses
were previously constructed in our laboratory (21). A human embryonic kidney cell line
QBI-293A was a kind gift from Professor Jiang Zhong of Fudan
University (Shanghai, China). The human gastric carcinoma cell line
SGC7901 was purchased from the American Type Culture Collection
(ATCC; Rockville, MD, USA). QBI-293A and SGC7901 cell lines were
cultured in RPMI-1640 medium (Gibco-BRL, Shanghai, China)
supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT,
USA). TRIzol reagent and the reverse transcriptase MuMLV were
purchased from Invitrogen (Shanghai, China). The Cell Counting
Kit-8 was purchased from the subsidiary of Dojindo Laboratories
(Shanghai, China). A polyclonal anti-ING4 antibody was purchased
from Abcam (Shanghai, China). The SuperEnhanced chemiluminescence
detection kit was obtained from Applygen Technologies Inc.
(Beijing, China). The antibodies against P-gp, MRP1, Bax, Bcl-2,
and survivin were from Cell Signaling Technology, Inc. (Boston, MA,
USA). An UltraSensitive™ SP kit was obtained from Maixin (Fuzhou,
China). Chemotherapeutical drugs, cisplatin (CDDP), 5-fluorouracil
(5-FU) and adriamycin (ADM), were kindly provided by The First
Hospital Affiliated to Soochow University (Suzhou, China). In
addition, female athymic nude mice were purchased from the Shanghai
Experimental Animal Center (Shanghai, China) and maintained in the
animal facility at Soochow University according to the guidelines
of the Animal Research Committee of Soochow University.
Establishment of the CDDP-based MDR
phenotype in the human gastric carcinoma cell line
SGC7901/CDDP
Gastric cancer SGC7901 cells were cultured in
RPMI-1640 supplemented with 10% FBS overnight, and the growth
medium was replaced with medium containing 0.05 μg/ml cisplatin to
induce cells to MDR following a gradual increase in the drug
concentrations to 2 μg/ml. After 6 months of culture, the SGC7901
cells were able to stably grow in the growth medium containing 2
μg/ml cisplatin. To maintain the MDR phenotype, the medium
containing 2 μg/ml cisplatin was used in the subsequent
experiments.
Infection of the AdVING4 virus to induce
ING4 expression in gastric cancer cells
AdVING4 and control AdV adenoviruses were prepared
as described previously (21). The
titer of the purified adenoviruses was determined by using the gene
transfer unit method (GTU) through calculation of the numbers of
the reporter gene GFP-expressing QBI-293A cells in 18 h of
adenoviral infection using fluorescence microscopy. To assess the
optimal ratio of infectious adenovirus (GTU) to target cells, a
multiplicity of infection (MOI), for a maximal infection and
transgene expression, human gastric carcinoma cells SGC7901 and
SGC7901/CDDP were infected with AdVING4 and AdV at various MOIs (0,
10, 25, 50, 100, 150 and 200, respectively) for 24 h. The
adenoviral infection efficiency was detected according to the GFP
expression. Furthermore, the ING4 transgene expression meditated by
the adenoviral infection in SGC7901 and SGC7901/CDDP gastric
carcinoma cells was determined using reverse transcriptase-PCR and
western blot analysis.
RNA isolation, reverse transcriptase-PCR
and qRT-PCR
Total cellular RNA was isolated from AdVING4- or
AdV-infected and uninfected SGC7901 and SGC7901/CDDP cells using
TRIzol and then reversely transcribed into cDNA using oligo
d(T)18 as primer according to the manufacturers’
protocols. PCR amplification was carried out using these cDNA
samples as templates and ING4 primers (5′-GCGTCGA
CATGGATGATGGGATGTATTTGGAAC-3′ and 5′-GCAA
GCTTCTATTTCTTCTTCCGTTCTTGGGAG-3′) under conditions of an initial 1
cycle at 94°C for 2 min and 72°C for 10 min followed by 35 cycles
at 94°C for 50 sec, 58°C for 50 sec and 72°C for 55 sec and a final
extension at 72°C for 10 min. The PCR products were separated on 1%
agarose gel using electrophoresis with ethidium bromide
staining.
To determine the expression levels of MDR1, MRP1,
Bcl-2, Bax and survivin mRNA in the AdVING4-transfected
SGC7901/CDDP cells, qRT-PCR based on SYBR-Green I detection was
performed using MJ Research Opticon™ 2 System (MJ Research).
Briefly, the total cellular RNA was isolated using TRIzol reagent
and reversely transcribed into cDNA (see above). PCR primers were:
MDR1, 5′-ATGCC TTCATCGAGTCACTG-3′ and 5′-TAACAAGGGCACGAG CTATG-3′;
MRP1, 5′-ATGCCTTCATCGAGTCACTG-3′ and 5′-TAACAAGGGCACGAGCTATG-3′;
Bcl-2, 5′-GCCCTGT GGATGACTGAGTA-3′ and 5′-CAGCCAGGAGAAATCAA ACA-3′;
Bax, 5′-ACGGCCTCCTCTCCTACTTT-3′ and 5′-CAGCCCATCTTCTTCCAGAT-3′;
survivin, 5′-TTCTCA AGGACCACCGCATC-3′ and 5′-GCCAAGTCTGGCTCGT
TCTC-3′ and GAPDH, 5′-GCACTCGAGCCATGAATTT TCA-3′ and
5′-GCTTCTAGATCAGAGCTTGT-3′. The qPCR amplification contained a
total volume of 50 ml of 1 ml cDNA, 1 ml of 900 nM of each primer,
25 ml 2X SYBR-Green I PCR Master mix, and 22 ml ddH2O
and was subsequently performed according to the following program:
94°C for 2 min and 72°C for 10 min followed by 35 cycles of 94°C
for 30 sec, 58°C for 30 sec, and 72°C for 30 sec. The level of
expression of each gene was normalized to the internal control gene
GAPDH and calculated using the pooled cDNA from all samples by the
2−ΔΔCT method as described previously (29). The authenticity of the PCR products
was verified by melting curve analysis and agarose gel
electrophoresis. Each sample was analyzed in duplicate in an
independent reaction, and the experiment was repeated thrice.
Protein extraction and western
blotting
Total cellular protein was extracted from the
AdVING4- or AdV-infected and uninfected SGC7901 and SGC7901/CDDP
cells and resolved in 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and subsequently transferred onto a
polyvinylidene difluoride membrane. Subsequently, the membrane was
incubated in 5% (w/v) non-fat dry milk in Tris-buffered saline
containing 0.05% Tween-20 (TBST) for 1 h at 37°C and then further
with a primary antibody of polyclonal goat anti-ING4 (1:1,000) in
blocking solution for 1 h at 37°C. The membrane was washed with
TBST and incubated with a peroxidase horseradish
peroxidase-conjugated secondary antibody of rabbit anti-goat IgG
(1:3,000) in blocking solution for another 1 h at 37°C. After being
washed with TBST thrice, the positive bands were developed using a
SuperEnhanced chemiluminescence detection kit and visualized after
exposure of the membranes to Kodak X-ray film.
Cell viability CCK-8 assay
The in vitro resistance index of human
gastric carcinoma SGC7901/CDDP cells to CDDP was determined using
the CCK-8 assay. Briefly, SGC7901 and SGC7901/CDDP cells were
dispensed into 96-well culture plates at 1×104/well and
incubated for 24 h at 37°C and treated with CDDP, 5-FU, ADM at 7
different concentrations (see Results) for 48 h. The viability of
SGC7901 and SGC7901/CDDP cells was analyzed using the CCK-8 kit
according to the manufacturer’s protocol. Similarly, the cells
infected with AdVING4 or control AdV were also included in the
CCK-8 assay. The SGC7901/CDDP cells were infected with 100 MOI
AdVING4 or AdV or without adenovirus (PBS control) for 24 h and
then treated with CDDP, 5-FU or ADM for 48 h and subjected to CCK-8
assay. The inhibitory rate (%) was calculated using the formula: 1
− (ODexperiment/ODcontrol) × 100%; Resistance
index: IC50(SGC7901/CDDP)/IC50(SGC7901) and
Reversion index: IC50(AdVING4)/IC50(PBS).
Nude mouse xenograft assay
To test the effects of ING4 expression in
vivo, we performed an animal experiment, which divided animals
into 7 groups [PBS + SGC7901 (PBS1), PBS + SGC7901/CDDP
(PBS2), AdV + SGC7901/CDDP (AdV), AdVING4 + SGC7901/CDDP
(AdVING4), CDDP + SGC7901/CDDP (CDDP), AdV + CDDP + SGC7901/CDDP
(AdV + CDDP) and AdVING4 + CDDP + SGC7901/CDDP (AdVING4 + CDDP)].
Briefly, female athymic nude mice were subcutaneously (s.c.)
inoculated in the armpits of their right anterior limbs with
2×106 human gastric carcinoma SGC7901 cells
(PBS1) and SGC7901/CDDP (the other group). After the
tumor mass reached a mean tumor volume of ~100–120 mm3,
AdVING4, AdV or PBS was administered once every 3 days by
intratumoral injection for 36 days. From days 7 to 14 and 21 to 28,
4.5 mg/kg of CDDP was administered weekly via tail vein injection.
Tumor progression and regression were monitored, and tumor volume
was measured with a caliper every 4 days. The tumor volume was
calculated by the formula, ab2/2, where a is the larger
and b is the smaller of the 2 dimensions of the tumor mass. The
tumor-bearing mice were then sacrificed at day 36 after the
treatments and tumor xenograft tissues were removed, weighed, fixed
by 10% neutral formalin, and then embedded in paraffin for
hematoxylin and eosin staining and immunohistochemical
analysis.
Immunohistochemistry
Expression of P-gp, MRP1, Bcl-2, Bax, and survivin
proteins in human gastric carcinoma xenograft tissues was analyzed
using immunohistochemistry with an UltraSensitive™ SP kit according
to the manufacturer’s instructions. The presence of buffy or brown
diaminobenzidine precipitates is indicative of positive reactivity.
The integral optical density (IOD) of immunohistochemical intensity
was analyzed by Image-Pro Plus 6.0 software (Media Cybernetics,
Bethesda, MD, USA).
Statistical analysis
All data are presented as the means ± SD. The
significant difference between 2 groups was evaluated using the
Student’s t-test and one-way or two-way repeated measures analysis
of variance and multiple comparisons with SPSS 10.0 software (SPSS,
Chicago, IL, USA). A value of P<0.05 was considered to indicate
a statistically significant result.
Results
Establishment of the gastric carcinoma
SGC7901/CDDP cell line for CDDP-induced MDR phenotype
To obtain the CDDP-induced MDR phenotype in gastric
cancer SGC7901/CDDP cells, the cells were grown under increasing
cisplatin concentrations from 0.05 to 2 μg/ml during a 6 month
period of time. The CDDP-resistant SGC7901 cells were able to grow
stably in 2 μg/ml cisplatin-containing medium. To maintain the MDR
phenotype, 2 μg/ml of cisplatin-containing medium was used for all
the experiments. Cell viability CCK-8 assay was performed initially
to detect the altered cell viability in the SGC7901/CDDP cells. The
data showed that the SGC7901/CDDP cells acquired 6.68-, 7.53- and
19.92-fold resistance to ADM, 5-FU and CDDP compared to the
parental SGC7901 cell line, respectively (Fig. 1A). Moreover, expression of
MDR-related genes (MDR1, MRP1, and apoptosis-related Bcl-2,
survivin genes) was apparently upregulated while the
apoptosis-related Bax gene was downregulated in the SGC7901/CDDP
cells when compared to the parental SGC7901 cells (Fig. 1B).
Establishment of stable ING4-expressing
gastric cancer SGC7901 cells
To select the optimal MOI for a maximal transgene
expression with minimal adenovirus itself-induced cytotoxicity,
human gastric carcinoma SGC7901 and SGC7901/CDDP cells were
infected with GFP-expressing AdVING4 or AdV at different MOIs and
observed using fluorescence microscopy. More than 90% of GFP
expression was found in the AdVING4- or AdV-infected SGC7901 and
SGC7901/CDDP tumor cells at an MOI of 100 or above, whereas the GFP
expression was not found in the uninfected control SGC7901 and
SGC7901/CDDP tumor cells. Additionally, an adenovirus-elicited
cytotoxic effect was rarely noted in the 100 MOI blank AdV-infected
SGC7901 and SGC7901/CDDP cells (data not shown). Furthermore, the
adenovirus-mediated exogenous ING4 tumor-suppressor gene was
significantly expressed in 100 MOI AdVING4-infected SGC7901 and
SGC7901/CDDP tumor cells but not in the AdV-infected and uninfected
SGC7901 and SGC7901/CDDP control cells (Fig. 2A and B), indicating that the ING4
tumor-suppressor gene was lost or profoundly downregulated in the
human gastric carcinoma SGC7901 and SGC7901/CDDP cells. The results
suggest that 100 MOI can be used as an optimal dose for the
adenovirus-directed ING4 gene induction and transgene expression in
human gastric carcinoma SGC7901 and SGC7901/CDDP cells.
AdVING4 infection reverses the MDR of
SGC7901/CDDP cells in vitro and in vivo
Based on the CDDP-induced MDR phenotype in
SGC7901/CDDP cells, we first investigated the AdVING4-mediated
reversal effects of AdVING4 infection on SGC7901/CDDP tumor cells
in vitro. Gastric carcinoma SGC7901/CDDP cells were infected
with AdVING4 or AdV at an MOI of 100, and tumor cell viability was
determined on day 4 using the CCK-8 assay. The IC50 of
the AdVING4-infected SGC7901/CDDP cells was significantly
decreased, whereas this phenomenon did not occur in the AdV- or
PBS-treated cells (P<0.05) (Fig.
3A), indicating that AdVING4 is a potent modulator for the
CDDP-induced MDR phenotype in gastric carcinoma SGC7901/CDDP cells.
To further address the potential effects of AdVING4 on SGC7901/CDDP
cells in vivo, we injected SGC7901/CDDP cells into athymic
nude mice and further injected AdVING4, AdV (1×108 GTU),
or PBS followed by treatment with or without CDDP. The data showed
that AdVING4 plus CDDP significantly reduced the tumor volume from
days 12 to 36 when compared to the other groups (P<0.05;
Fig. 3B and C). Similarly, the
tumor weight was also reduced in the AdVING4 plus CDDP-treated mice
(Fig. 3D), indicating that AdVING4
markedly modulated MDR of gastric carcinoma SGC7901/CDDP cell
xenografts in vivo in the athymic nude mouse model.
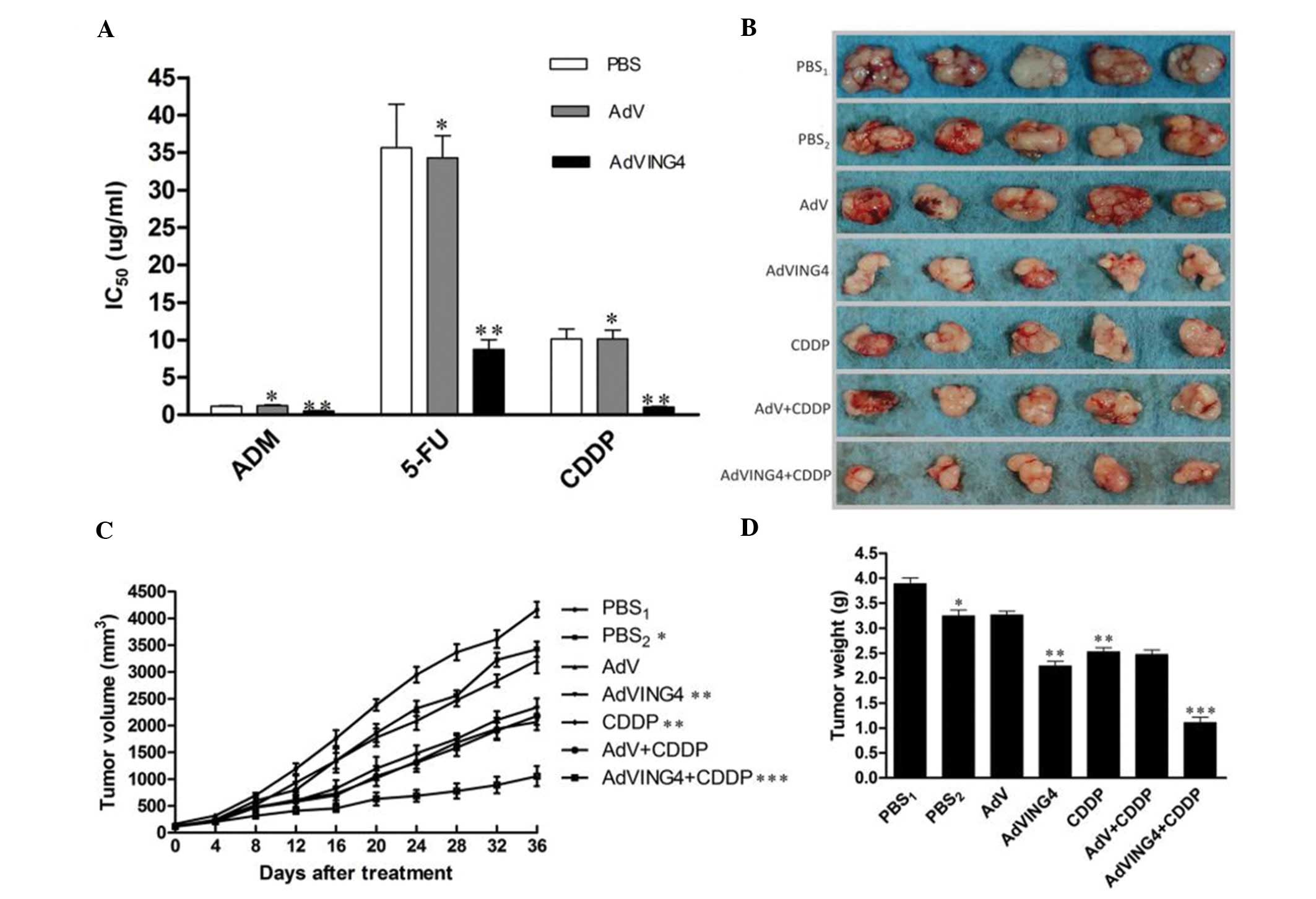 | Figure 3Effects of AdVING4 on the modulation
of MDR in SGC7901/CDDP cells in vitro and in vivo.
(A) Cell viability CCK-8 assay. The IC50 values of
SGC7901/CDDP cells infected with AdV or AdVING4 and then treated
with ADM, 5-FU and CDDP were evaluated by CCK-8.
*P>0.05 compared to the PBS control cells,
**P<0.05 compared to PBS and AdV groups,
respectively, using one-way repeated ANOVA measures and multiple
comparisons. n=3 replicates/condition. (B) Nude mouse xenograft
assay. Images of tumor masses in the different groups. (C and D)
Tumor volume and weight. The athymic nude mice bearing gastric
carcinoma SGC7901/CDDP cell xenograft tumors were intratumoral
injected with AdVING4 (1×108 GTU), AdV (1×108
GTU), PBS or CDDP alone, or CDDP plus an intratumoral injection of
AdVING4 (1×108 GTU) or AdV as described in Materials and
methods. (C) The tumor volume before and after treatment and (D)
the tumor weight at 36 days after treatment.*P<0.05
compared to PBS1 group; **P<0.05 compared
to PBS2 and AdV groups; ***P<0.05 compared
to AdVING4, CDDP and AdV+CDDP groups, respectively
(1×108 GTU) using one-way and two-way repeated ANOVA
measures and multiple comparisons (n=5 mice/condition). Data shown
are representative of 3 independent experiments. The data showed
that AdVING4 reversed MDR and consequently AdVING4 plus CDDP
induced synergistic tumor inhibition in vivo. MDR, multiple
drug resistance; ADM, adriamycin; 5-FU, 5-fluorouracil; CDDP,
cisplatin. |
Effect of AdVING4 on MDR and expression
of apoptosis-related genes
To further address the underlying molecular events
that may be responsible for the AdVING4-mediated effects in
reversing MDR, expression levels of MDR-related genes (MDR1 and
MRP1) and apoptosis-related genes (Bcl-2, Bax and survivin) in
these cells were analyzed. The data showed that levels of MDR1,
MRP1, Bcl-2 and survivin were apparently downregulated, whereas
expression of apoptosis-promoting gene, Bax, was upregulated in
AdVING4 and AdVING4 plus CDDP-treated SGC7901/CDDP cells compared
to the PBS, AdV, CDDP or AdV plus CDDP groups (P<0.01).
Furthermore, expression of MDR-related proteins, P-gp and MRP1, and
apoptosis-related proteins Bcl-2, Bax and survivin was also
modulated in the nude mouse xenograft tissues (P<0.05; Fig. 4A and B). The results indicate that
AdVING4 may play an important role in reversing MDR in SGC7901/CDDP
cells by regulating expression of P-gp, MRP1, Bcl-2, Bax and
survivin.
 | Figure 4Effect of AdVING4 on the expression
of MDR-related and apoptosis-related genes. (A) qRT-PCR. Relative
quantification of MDR1, MRP1, Bcl-2, Bax and survivin mRNA in cells
following various treatments was analyzed using qRT-PCR. The data
were normalized to levels of GAPDH expression.
*P<0.01 compared to PBS and AdV cells;
**P<0.01 compared to CDDP and AdV+CDDP-treated cells;
***P>0.05 compared to AdVING4-infected cells by using
one-way repeated ANVOA measures and multiple comparisons (n=3
replicates/condition, n=2 replicates/sample). (B) Representative
images of immunohistochemical detection of P-gp, MRP1, Bcl-2, Bax
and survivin in gastric carcinoma SGC7901/CDDP xenograft tissues.
Consequently, the integral optical density (IOD) of the
immunostaining intensity was quantified using Image-Pro Plus 6.0
software. *P<0.05 compared to PBS1;
**P<0.05 compared to PBS2 and AdV groups;
***P<0.05 compared to CDDP and AdV+CDDP groups;
***P>0.05 compared to AdVING4 group by using one-way
repeated ANOVA measures and multiple comparisons (n=6
replicates/condition, n=5 observations/representative section).
Original magnification, ×400. Data shown are representative of 3
independent experiments. MDR, multiple drug resistance; CDDP,
cisplatin. |
Discussion
In the present study, we investigated the MDR of
CDDP-induced gastric cancer cells and found that MDR promoted the
resistance of gastric cancer cells 40-fold to CDDP treatment or
other chemotherapeutic drugs, including 5-FU or ADM. We further
determined the effects of AdVING4-induced ING4 expression on these
tumor cells and found that induction of ING4 expression
significantly sensitized these gastric cancer cells to chemotherapy
in vitro and in nude mouse xenografts. At the molecular
level, ING4 expression inhibited expression of MDR-related proteins
(P-gp and MRP1) and apoptosis-suppressing proteins (Bcl-2 and
survivin), but induced expression of pro-apoptosis Bax protein
in vitro and in tumor xenograft tissues. These data indicate
that AdVING4 warrants further verification as a feasible adjuvant
chemotherapy for gastric carcinoma patients.
Clinically, the effectiveness of chemotherapy on
gastric cancer or other types of cancer is modest or disappointing
as evidenced by tumor cell chemoresistance, while a high-dose
treatment of chemotherapeutic drugs induces severe side-effects,
such as an immunocompromised state, bone marrow suppression,
digestive disturbances, renal toxicity, ototoxicity or ascites.
Although much effort has been made to explore the causes of tumor
chemoresistance, the underlying molecular mechanisms responsible
for chemoresistance remain uncler. Previously studies have shown
that MDR is a major cause for the failure of many modes of
chemotherapy (8) and limits the
efficacy of cancer treatment. Upregulated expression of ATP-binding
cassette transporters, such as P-gp and MRP1, may play a central
role in acquired drug resistance as these proteins act as ‘pumps’
to lower the intracellular drug concentration and reduce drug
efficacy in tumor cells (30).
Therefore, since the early 1980’s, various compounds have been
identified to overcome P-gp- and MRP1-mediated MDR. However, they
have achieved only limited success in clinical trials. Therefore,
the characterization of signaling pathways sustaining MDR is thus
essential for designing rational novel therapies for MDR. Recently,
cancer gene therapy represents a promising novel therapeutic
modality for cancers, particularly for the treatment of cancers of
the digestive system (31). Various
strategies combining gene or molecular therapy with chemotherapy
have been applied to radiosensitize or chemosensitize tumor cells
to chemotherapy or radiation therapy and to reduce
treatment-associated toxicity (25,26,32).
To date limited success has been reported. For example, the
adenoviral p53 gene has been in adjuvant use together with
conventional chemotherapy, radiation therapy, and surgery for lung,
and head and neck cancers (33).
In the present study, we focused on the ING4 gene as
ING4 is a tumor-suppressor gene and plays a role in suppression of
oncogenesis, tumor cell growth, angiogenesis, migration and gene
transcription. A number of studies have shown that ING4 expression
is frequently downregulated or deleted in a large variety of
cancer, including gastric cancer (22,34–37).
Moreover, restoration of ING4 expression was found to suppress
tumor growth in a p53-dependent and p53-independent manner, induce
tumor cell cycle arrest and apoptosis (13,19–21),
and inhibit tumor angiogenesis by repressing nuclear factor-κB and
hypoxia-inducible factor-1α (22–24).
ING4 protein can also enhance radiosensitivity and chemosensitivity
in non-small-cell lung cancer and hepatocarcinoma cells (19,25).
In the present study, we first successfully established the
CDDP-induced MDR phenotype in a gastric carcinoma cell line for
assessing the effects of the AdVING4 gene on the reversion of the
MDR phenotype in gastric carcinoma cells in vitro and in
vivo. We demonstrated that AdVING4-mediated ING4 expression
decreased the IC50 of CDDP, 5-FU and ADM, accelerated
apoptosis in the CDDP-induced MDR phenotype of gastric carcinoma
cells. Moreover, AdVING4 plus CDDP also synergistically inhibited
the growth of gastric carcinoma xenograft tumors in athymic nude
mice. This finding suggests that ING4 may be useful for the
clinical adjuvant therapy of gastric cancer.
Furthermore, the results showed that AdVING4 was
able to inhibit expression of P-gp, MRP1, Bcl-2 and survivin while
increasing Bax expression in vitro and in vivo, which
molecularly supports that ING4 could regulate the CDDP-induced MDR
of gastric cancer cells. Previous studies have shown that P-gp is
only expressed in the proximal tubular epithelial cells of normal
human kidney and not in other cell types (38), but P-gp expression is enhanced by
long-term chemotherapy in tumor cells (39). Thus, suppression of P-gp expression
could sensitize tumor cells to chemotherapy (40). Indeed, the present study showed that
P-gp was significantly increased in CDDP-induced MDR gastric
carcinoma cells and that overexpression of ING4 protein
significantly decreased P-gp expression and AdVING4 plus CDDP
induced significant apoptosis in gastric carcinoma xenografts in
athymic nude mice. This finding demonstrated that P-gp protein
could mediate MDR in gastric cancer cells. Moreover, MRP1 protein
is usually localized in proximal tubular epithelial cells of normal
renal tissue, and the basolateral localization of the protein is
important for efflux of its substrates into blood (41). Another study showed that MRP1
expression was significantly correlated with the survival of
patients with neuroblastoma (42).
The present study showed that MRP1 expression was also upregulated
following CDDP-induced MDR in SGC7901/CDDP cells, while AdVING4
downregulated MRP1 expression in the gastric carcinoma cells in
vitro and in vivo. Molecularly, ING4 is involved in the
p53-dependent regulatory pathway for its antitumor activity
(19), and a previous study showed
that p53 plays a decisive role in the regulation of P-gp and MRP1
expression in chemoresistance (43,44).
Thus, the downregulation of P-gp and MRP1 expression by ING4 may be
through a p53-dependent pathway.
In addition, the Bcl-2 protein family is known to be
a pivotal regulator of apoptosis and an important determinant of
cellular fate (45). A previous
study showed that apoptosis-related genes (such as Bax, Bcl-2 and
survivin) are key factors responsible for MDR (25,32,46).
The ratio of anti- to pro-apoptotic Bcl-2 family molecules, such as
Bcl-2/Bax, constitutes a rheostat that sets the threshold of
susceptibility to apoptosis for the intrinsic apoptosis pathway,
which promotes pore formation in the mitochondrial outer membrane,
loss of mitochondrial integrity, and the release into the cytosol
of cytochrome c followed by the cleavage of caspase-9,
leading to the activation of the intrinsic apoptotic pathway.
Meanwhile, survivin can inhibit apoptosis by interacting with
cyclin kinase cdk4, p34 and cdc2 and block apoptotic signal
transduction. Survivin is barely expressed in normal adult tissues
but is weakly expressed in the placenta and thymus (47); however, it is widely expressed in
tumor cells (48). Expression of
survivin in cancer was shown to be closely associated with
malignant phenotypes, poor prognosis, tumor recurrence and drug
resistance (47). In the present
study, Bcl-2 and survivin mRNA and protein were significantly
upregulated while Bax was downregulated in the SGC7901/CDDP cells
and xenograft tumors when compared to their levels in the parental
cells. AdVING4 elicited an encouraging effect on the upregulation
of Bax but downregulated Bcl-2 and survivin expression to activate
the apoptotic pathways. However, the present study is just a
proof-of-principle, and further study is required to investigate
the safety issue of AdVING4 for clinical use in cancer patients. In
addition, we will investigate the underlying molecular gene
pathways that mediated ING4-reduced MDR in gastric cancer
cells.
Acknowledgements
This study was supported by the Project of the
Nature Science Foundation of China (81201905), the Shanghai
Postdoctoral Scientific Program of China (13R21415200), the Nature
Science Research Grants in University of Jiangsu Province of China
(12KJB320009) and the Science and Technology Research Project of in
Science and Technology Bureau of Suzhou City of China (YJS0916 and
SYS201220).
References
|
1
|
Forman D and Burley VJ: Gastric cancer:
global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roder DM: The epidemiology of gastric
cancer. Gastric Cancer. 5(Suppl 1): S5–S11. 2002. View Article : Google Scholar
|
|
3
|
Shin HR, Jung KW, Won YJ and Park JG; 139
KCCR-affiliated Hospitals. 2002 annual report of the Korea Central
Cancer Registry: based on registered data from 139 hospitals.
Cancer Res Treat. 36:103–114. 2004. View Article : Google Scholar
|
|
4
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun XD, Mu R, Zhou YS, et al: Analysis of
mortality rate of stomach cancer and its trend in twenty years in
China. Zhonghua Zhong Liu Za Zhi. 26:4–9. 2004.(In Chinese).
|
|
6
|
Cunningham D, Allum WH, Stenning SP, et
al: Perioperative chemotherapy versus surgery alone for resectable
gastroesophageal cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rivera F, Vega-Villegas ME and López-Brea
MF: Chemotherapy of advanced gastric cancer. Cancer Treat Rev.
33:315–324. 2007. View Article : Google Scholar
|
|
8
|
Arceci RJ: Tumor cell survival and
resistance to therapy. Curr Opin Hematol. 3:279–287. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Filipits M: Mechanisms of cancer:
multidrug resistance. Drug Discov Today Dis Mech. 1:229–234. 2004.
View Article : Google Scholar
|
|
10
|
Xia L, Zhang D, Du R, et al: miR-15b and
miR-16 modulate multidrug resistance by targeting BCL2 in human
gastric cancer cells. Int J Cancer. 123:372–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Zhai H, Wang X, et al: Ribosomal
proteins S13 and L23 promote multidrug resistance in gastric cancer
cells by suppressing drug-induced apoptosis. Exp Cell Res.
296:337–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, You H, Liu F, et al:
Differentially expressed gene profiles between multidrug resistant
gastric adenocarcinoma cells and their parental cells. Cancer Lett.
185:211–218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiseki M, Nagashima M, Pedeux RM, et al:
p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity.
Cancer Res. 63:2373–2378. 2003.PubMed/NCBI
|
|
14
|
He GH, Helbing CC, Wagner MJ, Sensen CW
and Riabowol K: Phylogenetic analysis of the ING family of PHD
finger proteins. Mol Biol Evol. 22:104–116. 2005.PubMed/NCBI
|
|
15
|
Aasland R, Gibson TJ and Stewart AF: The
PHD finger: implications for chromatin-mediated transcriptional
regulation. Trends Biochem Sci. 20:56–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Wang KS, Wang ZQ, et al: Nuclear
localization signal of ING4 plays a key role in its binding to p53.
Biochem Biophys Res Commun. 331:1032–1038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim S, Chin K, Gray JW and Bishop JM: A
screen for genes that suppress loss of contact inhibition:
identification of ING4 as a candidate tumor suppressor gene in
human cancer. Proc Natl Acad Sci USA. 101:16251–16256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim S, Welm AL and Bishop JM: A dominant
mutant allele of the ING4 tumor suppressor found in human
cancer cells exacerbates MYC-initiated mouse mammary
tumorigenesis. Cancer Res. 70:5155–5162. 2010.
|
|
19
|
Zhang X, Xu LS, Wang ZQ, et al: ING4
induces G2/M cell cycle arrest and enhances the chemosensitivity to
DNA-damage agents in HepG2 cells. FEBS Lett. 570:7–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Unoki M, Shen JC, Zheng ZM and Harris CC:
Novel splice variants of ING4 and their possible roles in the
regulation of cell growth and motility. J Biol Chem.
281:34677–34686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie Y, Zhang H, Sheng W, Xiang J, Ye Z and
Yang J: Adenovirus-mediated ING4 expression suppresses lung
carcinoma cell growth via induction of cell cycle alteration and
apoptosis and inhibition of tumor invasion and angiogenesis. Cancer
Lett. 271:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garkavtsev I, Kozin SV, Chernova O, et al:
The candidate tumour suppressor protein ING4 regulates brain tumour
growth and angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J and Li G: Cell cycle regulator ING4
is a suppressor of melanoma angiogenesis that is regulated by the
metastasis suppressor BRMS1. Cancer Res. 70:10445–10453. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Colla S, Tagliaferri S, Morandi F, et al:
The new tumor-suppressor gene inhibitor of growth family member 4
(ING4) regulates the production of proangiogenic molecules by
myeloma cells and suppresses hypoxia-inducible factor-1 α (HIF-1α)
activity: involvement in myeloma-induced angiogenesis. Blood.
110:4464–4475. 2007.PubMed/NCBI
|
|
25
|
Ling C, Xie Y, Zhao D, Zhu Y, Xiang J and
Yang J: Enhanced radiosensitivity of non-small-cell lung cancer
(NSCLC) by adenovirus-mediated ING4 gene therapy. Cancer Gene Ther.
19:697–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie Y, Sheng W, Miao J, Xiang J and Yang
J: Enhanced antitumor activity by combining an adenovirus harboring
ING4 with cisplatin for hepatocarcinoma cells. Cancer Gene Ther.
18:176–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishikawa T, Ramesh R, Munshi A, Chada S
and Meyn RE: Adenovirus-mediated mda-7 (IL24) gene therapy
suppresses angiogenesis and sensitizes NSCLC xenograft tumors to
radiation. Mol Ther. 9:818–828. 2004.
|
|
28
|
Zhang X, Cheung RM, Komaki R, Fang B and
Chang JY: Radiotherapy sensitization by tumor-specific TRAIL
gene targeting improves survival of mice bearing human non-small
cell lung cancer. Clin Cancer Res. 11:6657–6668. 2005.PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong D, Ma S, Liang B, et al: The
different regulatory effects of p53 status on multidrug resistance
are determined by autophagy in ovarian cancer cells. Biomed
Pharmacother. 66:271–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Touchefeu Y, Harrington KJ, Galmiche JP
and Vassaux G: Review article: gene therapy, recent developments
and future prospects in gastrointestinal oncology. Aliment
Pharmacol Ther. 32:953–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu W, Shan X, Wang T, Shu Y and Liu P:
miR-181b modulates multidrug resistance by targeting BCL2 in human
cancer cell lines. Int J Cancer. 127:2520–2529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moon C, Oh Y and Roth JA: Current status
of gene therapy for lung cancer and head and neck cancer. Clin
Cancer Res. 9:5055–5067. 2003.PubMed/NCBI
|
|
34
|
Gunduz M, Nagatsuka H, Demircan K, et al:
Frequent deletion and down-regulation of ING4, a candidate tumor
suppressor gene at 12p13, in head and neck squamous cell
carcinomas. Gene. 356:109–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fang F, Luo LB, Tao YM, Wu F and Yang LY:
Decreased expression of inhibitor of growth 4 correlated with poor
prognosis of hepatocellular carcinoma. Cancer Epidemiol Biomarkers
Prev. 18:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang QS, Li M, Zhang LY, et al:
Down-regulation of ING4 is associated with initiation and
progression of lung cancer. Histopathology. 57:271–281. 2010.
|
|
37
|
Tapia C, Zlobec I, Schneider S, et al:
Deletion of the inhibitor of growth 4 (ING4) tumor
suppressor gene is prevalent in human epidermal growth factor 2
(HER2)-positive breast cancer. Hum Pathol. 42:983–990. 2011.
|
|
38
|
Hodorová I, Rybárová S, Vecanová J, Solár
P, Plank L and Mihalik J: Relation between expression pattern of
wild-type p53 and multidrug resistance proteins in human
nephroblastomas. Acta Histochem. 115:273–278. 2013.PubMed/NCBI
|
|
39
|
Camassei FD, Arancia G, Cianfriglia M, et
al: Nephroblastoma: multidrug-resistance P-glycoprotein expression
in tumor cells and intratumoral capillary endothelial cells. Am J
Clin Pathol. 117:484–490. 2002. View Article : Google Scholar
|
|
40
|
Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY
and Fan DM: Suppression of P-gp induced multiple drug resistance in
a drug resistant gastric cancer cell line by overexpression of Fas.
World J Gastroenterol. 6:664–670. 2000.PubMed/NCBI
|
|
41
|
Sarkadi B, Ozvegy-Laczka C, Német K and
Váradi A: ABCG2 - a transporter for all seasons. FEBS Lett.
567:116–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Norris MD, Bordow SB, Marshall GM, Haber
PS, Cohn SL and Haber M: Expression of the gene for
multidrug-resistance-associated protein and outcome in patients
with neuroblastoma. N Engl J Med. 334:231–238. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sakaeda T, Nakamura T, Horinouchi M, et
al: MDR1 genotype-related pharmacokinetics of digoxin after single
oral administration in healthy Japanese subjects. Pharm Res.
18:1400–1404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hait WN and Yang JM: The individualization
of cancer therapy: the unexpected role of p53. Trans Am Clin
Climatol Assoc. 117:85–101. 2006.PubMed/NCBI
|
|
45
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li K, Lu Y, Liang J, et al: RhoE enhances
multidrug resistance of gastric cancer cells by suppressing Bax.
Biochem Biophys Res Commun. 379:212–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
48
|
Altieri DC: The molecular basis and
potential role of survivin in cancer diagnosis and therapy. Trends
Mol Med. 7:542–547. 2001. View Article : Google Scholar : PubMed/NCBI
|


















