Introduction
Although the mortality rate for hepatocellular
carcinoma (HCC) has decreased, it is still one of the most common
forms of cancer in China. Even with the advancement in diagnostic
tools, surgical techniques and chemotherapy, a complete cure for
HCC has not yet been achieved. Therefore, new diagnostic tools,
novel therapeutic methods and new molecular markers of prognosis
concerning this disease are urgently needed.
During cancer cell development, genes that encode
oncoproteins, tumor-suppressors, and their regulators can be
stochastically acquired and selectively accumulate mutations.
Receptor tyrosine kinases (RTKs) are important regulators of signal
transduction pathways and promote cell growth, survival, invasion
and motility in tumors (1).
Dysregulation of RTKs through mutation, amplification or
overexpression, can increase kinase activity and ultimately
oncogenic transformation. The generality of this paradigm of
gain-of-function RTK signaling in cancer has been recently
challenged by the discovery of the dual roles of Eph receptors in
both promoting and inhibiting oncogenesis and tumor progression.
Eph receptors constitute the largest subfamily of RTKs by far
(2–4). A strikingly complementary pattern of
expression between Ephs and ephrins in apposed cellular
compartments has been observed in developing rhombomeres (5), along neural crest migration pathways
(6) and in tissues throughout the
entire developing mouse embryo (7).
In contrast to most other RTKs, activation of Eph receptors does
not typically lead to a proliferative response in cells, but rather
to changes in cell shape, movement (2) and cell adhesion (8,9).
Numerous studies have reported that various Eph receptors are
abnormally expressed in cancer. It has been confirmed that EphA1
protein is significantly associated with depth of invasion of
gastric cancer, and patients with EphA1 upregulation experience a
shorter survival time (10). Other
studies suggest that the expression of EphA2 is higher in gastric
cancer than that in normal mucosa, and is positively correlated
with tumor TNM stage (11,12). Overexpression of EphA7 has been
observed more often in advanced gastric cancer, and EphA7 may have
roles in the pathogenesis and development of gastric cancer
(13). Several studies have shown
that EphA3 expression is aberrantly regulated in hepatic cancer,
lung cancer, renal cancer, colorectal cancer, melanoma and sarcoma
(14–17). However, the relationship between
EphA3 expression and survival in HCC patients has not been
explored.
The present study used immunohistochemistry to
investigate EphA3 protein expression and was the first to examine
the potential relationship between EphA3 protein expression and
patient prognosis in HCC. Furthermore, this study explored the role
of EphA3 in the invasion and metastasis of HCC via its effect on
the regulation of VEGF in vitro.
Materials and methods
Patients and tissue specimens
Tissue specimens from HCC and adjacent non-cancerous
hepatic tissues (at least 1.5 cm away from the tumor) were
collected from 101 patients who underwent surgical treatment for
primary HCC at the Department of Hepatobiliary Surgery at Xijing
Hospital (Xi’an, China) between 2003 and 2006. Specimens were
obtained from patients who had not received preoperative treatments
such as chemotherapy, ethanol injection, or transarterial
chemoembolization. The study included 59 male and 42 female
patients with a median age of 51.2 years (range, 32–71 years). The
median size of the tumors was 6.3 cm (range, 2.7–13.1 cm). This
study was approved by the Ethics Committee of the Fourth Military
Medical University and conformed to the ethical guidelines of the
2004 Declaration of Helsinki. Written informed consent was obtained
from each patient or from his/her legal guardians. Before the study
was initiated, histopathological examinations were performed to
confirm that there were enough cancer cells in the tumor samples
and that no cancer cells had contaminated the non-cancerous hepatic
tissues. All specimens were fixed in 10% formalin and embedded in
paraffin, and 4-μm serial sections were examined by
immunohistochemistry. Clinical parameters such as gender, age,
tumor location, tumor size, tumor grade, metastasis, satellite
lesions, tumor number, AJCC TNM stage and α-fetoprotein (AFP) were
collected. The 38 cases diagnosed with metastasis included venous
invasion (n=26), bile duct tumor thrombi (n=9) and lymph node
metastasis (n=3) and were verified by pathological analysis. The
enrolled patients were followed up for 5 years to perform survival
calculations.
Cell culture and reagents
The human liver non-tumor cell line (HL-7702) and
the human HCC cell lines (HepG2, Huh-7, SMMC-7721 and MHCC97H) were
cultivated in DMEM supplemented with 10% fetal calf serum
(Sigma-Aldrich Chemical Co., St. Louis, MO, USA). The HCC cells
were seeded into 6-well cell culture plates at a density of
1×105 cells/well. Primary antibodies against EphA3, VEGF
and GAPDH were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). All secondary antibodies were obtained from Pierce
Biotechnology, Inc. (Rockford, IL, USA). An SP immunostaining kit
was purchased from Zymed (ZSGB; Beijing, China). EphA3 small
interfering RNA (siRNA) and siRNA controls were obtained from Santa
Cruz Biotechnology, Inc. Lipofectamine 2000 was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). All other
chemicals and solutions were purchased from Sigma-Aldrich unless
otherwise indicated.
Immunohistochemistry and evaluation of
staining
Immunohistochemistry was performed using the
avidin-biotin-peroxidase method for all tissues. All sections were
deparaffinized in xylene and dehydrated through a graded alcohol
series prior to the blockade of endogenous peroxidase activity
using 0.5% H2O2 in methanol for 10 min.
Nonspecific binding was blocked by incubating the sections with 10%
normal goat serum in PBS for 1 h at room temperature. Without
washing, the sections were incubated with an anti-EphA3 antibody
(1:50) in PBS at 4°C overnight in a humidified chamber.
Biotinylated IgG (1:200; Sigma) was then added, and the sections
were incubated for 2 h at room temperature. Detection was performed
using a streptavidin-peroxidase complex. The brown color indicative
of peroxidase activity was obtained by incubating with 0.1%
3,3-diaminobenzidine (Sigma) in PBS with 0.03%
H2O2 for 10 min at room temperature. The
tissue specimens were scored independently by two pathologists, who
were blinded to the clinicopathological results and patient
outcome, using a previously described immunoreactivity scoring
system (18). Based on the score,
we divided all HCC specimens into 2 subgroups: the low expression
group (score of 0–4) and the high expression group (score of
5–12).
Small interfering RNA transfection
According to the protocol supplied with the
Lipofectamine 2000, HepG2 and MHCC97H cells were transfected with
either EphA3 siRNA or control siRNA. siRNA-transfected cells were
seeded into 6-well cell culture plates at a density of
1×105 cells/well. The cells were allowed to grow for an
additional 24 h and were then harvested for further analysis.
Real-time reverse transcription-PCR
Total RNA was extracted and reverse-transcribed. The
primers used for the PCR reaction were as follows: EphA3 forward
primer (5′-CCA TGGACTGCCCAGCTGCC-3′) and reverse primer (5′-CCT
TGCGGCTGCACTGGTGA-3′); and GAPDH forward primer
(5′-AAATCCCATCACCATCTTCC-3′) and reverse primer
(5′-TCACACCCATGACGAACA-3′). The primer sequences were verified by
running a virtual PCR, and the primer concentrations were optimized
to avoid primer-dimer formation. Additionally, dissociation curves
were evaluated to avoid nonspecific amplification. Real-time PCR
amplifications were performed using an Mx4000 Multiplex QPCR System
(Stratagene, La Jolla, CA, USA) with 2X SYBR-Green PCR Master Mix
(Applied Biosystems). Data were analyzed according to the
comparative Ct method and were normalized to GAPDH
expression in each sample.
Protein extraction and western
blotting
The cells were lysed in lysis buffer [50 mmol/l Tris
(pH 7.5), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton
X-100, 2.5 mmol/l sodium orthovanadate, 10 μl/ml protease inhibitor
cocktail, and 1 mmol/l PMSF] by incubating for 20 min at 4°C. The
protein concentration was determined using the Bio-Rad assay system
(Bio-Rad, Hercules, CA, USA). Total proteins were fractionated
using SDS-PAGE and transferred onto nitrocellulose membranes. The
membranes were blocked with 5% nonfat dried milk or bovine serum
albumin in 1X TBS buffer containing 0.1% Tween-20 and then
incubated with the appropriate primary antibodies. Horseradish
peroxidase-conjugated anti-rabbit or anti-mouse IgG was used as the
secondary antibody, and the protein bands were detected using the
enhanced chemiluminescence detection system (Amersham Pharmacia
Biotech). Quantification of the western blotting was performed
using laser densitometry, and relative protein expression was then
normalized to GAPDH levels.
Invasion assays
Cell invasion was analyzed using Matrigel-coated
Transwell cell culture chambers (8-μm pore size) (Millipore,
Billerica, MA, USA). Briefly, the treated cells (5×104
cells/well) were serum-starved for 24 h and plated in the upper
insert of a 24-well chamber in serum-free medium. Medium containing
10% serum as a chemoattractant was then added to the wells, and the
cells were incubated for 24 h. Cells on the upper side of the
filters were mechanically removed using a cotton swab, after which
the membrane was fixed with 4% formaldehyde for 10 min at room
temperature, and stained with 0.5% crystal violet for 10 min.
Finally, invasive cells were counted at ×200 magnification from 10
different fields in each filter.
ELISA assay
The enzyme-linked immunosorbent assay (ELISA)
technique (Amersham, Buckinghamshire, UK) was used to quantify the
activity of VEGF. The samples were thawed on ice, and all reagents
were equilibrated to room temperature. All assays were carried out
according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Each experiment was
repeated at least 3 times, and all data were summarized and
presented as the means ± SDs. The differences between means were
statistically analyzed using a t-test. The χ2 test for
proportions was used to analyze the relationship between EphA3
expression and various clinicopathologic factors. Survival curves
were calculated using the Kaplan-Meier method and compared using
the log-rank test. Cox proportional hazard analysis was used for
univariate and multivariate analysis to explore the effect of
clinicopathological factors and the EphA3 expression on survival.
P-values <0.05 were considered to indicate statistically
significant results.
Results
EphA3 immunohistochemistry
EphA3 expression was mainly localized within the
cytoplasm and at the cell membrane. No significant EphA3 expression
was noted in the adjacent non-cancerous hepatic tissues, with only
weak staining for EphA3 at the cell membrane and in the cytoplasm.
As shown in Fig. 1, the expression
of EphA3 differed between HCC tissues. EphA3 staining was negative
in 19 samples of HCC, whereas weak positive staining was detected
in 27 samples of HCC, moderate positive staining was detected in 23
samples of HCC, and strong positive staining was detected in 32
samples of HCC.
Relationship between EphA3 expression and
clinicopathological characteristics
The pathological factors examined for 101 cases of
HCC included gender, age, tumor location, tumor size, tumor grade,
metastasis, tumor number, AJCC TNM stage and AFP. In cases
diagnosed with metastasis, we also analyzed vascular invasion. For
this analysis, we divided the 101 patients into 2 subgroups: a high
EphA3 expression group (n=55) and a low EphA3 expression group
(n=46). The relationship between EphA3 expression and the
clinicopathological factors is summarized in Table I. The results demonstrated that high
EphA3 expression was strongly correlated with tumor size (P=0.006),
tumor grade (P=0.003), metastasis (P=0.003), venous invasion
(P=0.008) and AJCC TNM stage (P<0.001). However, there were no
significant associations between EphA3 expression and the other
pathological factors examined (P>0.05). These results indicate
that EphA3 may be involved in the differentiation, invasion and
metastasis of HCC.
 | Table IAssociation of EphA3 expression with
clinicopathologic factors of the HCC patients. |
Table I
Association of EphA3 expression with
clinicopathologic factors of the HCC patients.
| | EphA3 | | |
|---|
| |
| | |
|---|
| Tumor
characteristics | n | High, n (%)(5–12
score) | Low, n (%)(0–4
score) | P-value | χ2 |
|---|
| All cases | 101 | 55 (54.5) | 46 (45.5) | | |
| Gender |
| Male | 59 | 34 (57.6) | 25 (42.4) | 0.448 | 0.575 |
| Female | 42 | 21 (50.0) | 21 (50.0) | | |
| Age (years) |
| ≤50 | 50 | 30 (60.0) | 20 (40.0) | 0.268 | 1.227 |
| >50 | 51 | 25 (49.0) | 26 (51.0) | | |
| Tumor location |
| Left | 53 | 29 (54.7) | 24 (45.3) | 0.956 | 0.003 |
| Right | 48 | 26 (54.2) | 22 (45.8) | | |
| Tumor size
(cm) |
| ≤5 | 38 | 14 (36.8) | 24 (63.2) | 0.006 | 7.620 |
| >5 | 63 | 41 (65.1) | 22 (34.9) | | |
| Tumor grade
(differentiation) |
| Well | 33 | 25 (75.8) | 8 (24.2) | 0.003 | 8.968 |
| Moderately or
poorly | 68 | 30 (44.1) | 38 (55.9) | | |
| Metastasis |
| Yes | 38 | 28 (73.7) | 10 (26.3) | 0.003 | 9.082 |
| No | 63 | 27 (42.9) | 36 (57.1) | | |
| Venous
invasion |
| + | 26 | 20 (76.9) | 6 (23.1) | 0.008 | 7.126 |
| − | 75 | 35 (46.7) | 40 (53.3) | | |
| Satellite
lesions |
| + | 29 | 18 (62.1) | 11 (37.9) | 0.330 | 0.951 |
| − | 72 | 37 (51.4) | 35 (48.6) | | |
| Tumor number |
| Solitary | 68 | 35 (51.5) | 33 (48.5) | 0.387 | 0.748 |
| Multiple | 33 | 20 (60.6) | 13 (39.4) | | |
| AJCC TNM stage |
| I and II | 19 | 1 (5.3) | 18 (74.7) | <0.001 | 22.834 |
| III and IV | 82 | 54 (65.9) | 28 (34.1) | | |
| AFP (ng/ml) |
| ≤400 | 71 | 18 (60.0) | 1 (40.0) | 0.467 | 0.529 |
| >400 | 30 | 37 (52.1) | 34 (47.9) | | |
Correlation between EphA3 expression and
prognosis of HCC patients
Since the level of EphA3 expression was correlated
with tumor size, tumor grade, metastasis, venous invasion and AJCC
TNM stage, we further speculated that the level of EphA3 expression
may affect the prognosis of HCC patients. Kaplan-Meier
postoperative survival curves were used to evaluate the overall
survival rates of patients with HCC in comparison to their levels
of EphA3 expression. The log-rank test showed that survival time
was significantly different between the low and high EphA3
expression groups (P<0.001). The low EphA3 expression group
demonstrated increased survival, whereas the high EphA3 expression
group demonstrated reduced survival (Fig. 2). The cumulative 5-year survival
rate was 31.2% in the low EphA3 expression group, whereas this rate
was only 13.6% in the high EphA3 expression group.
A univariate Cox regression analysis also found that
tumor grade, metastasis, venous invasion, satellite lesions, AJCC
TNM stage and EphA3 protein expression were significantly
associated with overall survival (Table II). Furthermore, to evaluate the
potential of high EphA3 expression to serve as an independent
predictor for overall survival among HCC patients, multivariate Cox
regression analyses were performed. The results indicated that only
metastasis and EphA3 expression could predict overall survival
among HCC patients (Table II).
 | Table IIUnivariate and multivariate analysis
for overall survival of the HCC patients. |
Table II
Univariate and multivariate analysis
for overall survival of the HCC patients.
| Tumor
characteristics | Relative risk (95%
CI) | P-value |
|---|
| Univariate |
| Gender | 1.024
(0.674–1.556) | 0.911 |
| Age (years) | 1.075
(0.711–1.625) | 0.732 |
| Tumor
location | 0.948
(0.628–1.432) | 0.800 |
| Tumor size | 1.328
(0.857–2.058) | 0.204 |
| Tumor grade
(differentiation) | 0.578
(0.370–0.902) | 0.016 |
| Metastasis | 11.292
(6.203–20.557) | <0.001 |
| Venous
invasion | 10.904
(5.925–20.066) | <0.001 |
| Satellite
lesions | 2.080
(1.306–3.314) | 0.002 |
| Tumor number | 1.357
(0.881–2.090) | 0.166 |
| AJCC TNM
stage | 3.694
(2.022–6.748) | <0.001 |
| AFP (ng/ml) | 0.739
(0.470–1.162) | 0.191 |
| EphA3 | 2.927
(1.876–4.569) | <0.001 |
| Multivariate |
| Tumor grade
(differentiation) | 1.194
(0.725–1.965) | 0.486 |
| Metastasis | 5.917
(2.762–12.676) | <0.001 |
| Venous
invasion | 2.516
(1.240–5.108) | 0.11 |
| AJCC TNM
stage | 1.961
(0.981–3.920) | 0.057 |
| EphA3 | 1.960
(1.181–3.252) | 0.009 |
Downregulated expression of EphA3 by
siRNA reduces the invasiveness of HCC cells
Since high expression of EphA3 was strongly
correlated with metastasis (P=0.003) and venous invasion (P=0.008),
we next sought to determine whether EphA3 was involved in invasion
and metastasis in HCC. We first examined the expression levels of
EphA3 in different HCC cells with different invasive capabilities.
As another study reported, the invasive capacity of HepG2 cells was
the lowest, whereas the invasive capacity of MHCC97H cells was the
highest (19). RT-PCR and western
blot analysis showed that the expression levels of EphA3 mRNA and
protein exhibited similar increased tendencies related to the
invasive capability (Fig. 3A and B)
in HCC cells. In HCC cells, siRNA was used to effectively decrease
the expression of EphA3 mRNA and protein (Fig. 4A–C). Using Transwell cell culture
chambers, we measured the invasiveness of EphA3 siRNA-transfected
cells. As illustrated in Fig. 4D,
the number of EphA3 siRNA-transfected HepG2 cells that migrated
through the Transwell was significantly less than the number of
control siRNA-transfected cells that migrated. In addition, the use
of MHCC97H cells showed similar results (Fig. 4D). Thus, these data indicated that
the downregulation of EphA3 by siRNA reduced the invasive capacity
of HCC cells.
Downregulation of EphA3 decreases the
protein expression and proteolytic activity of VEGF
To determine the potential mechanism for the role of
EphA3 in HCC cell invasion, we examined the effect of decreased
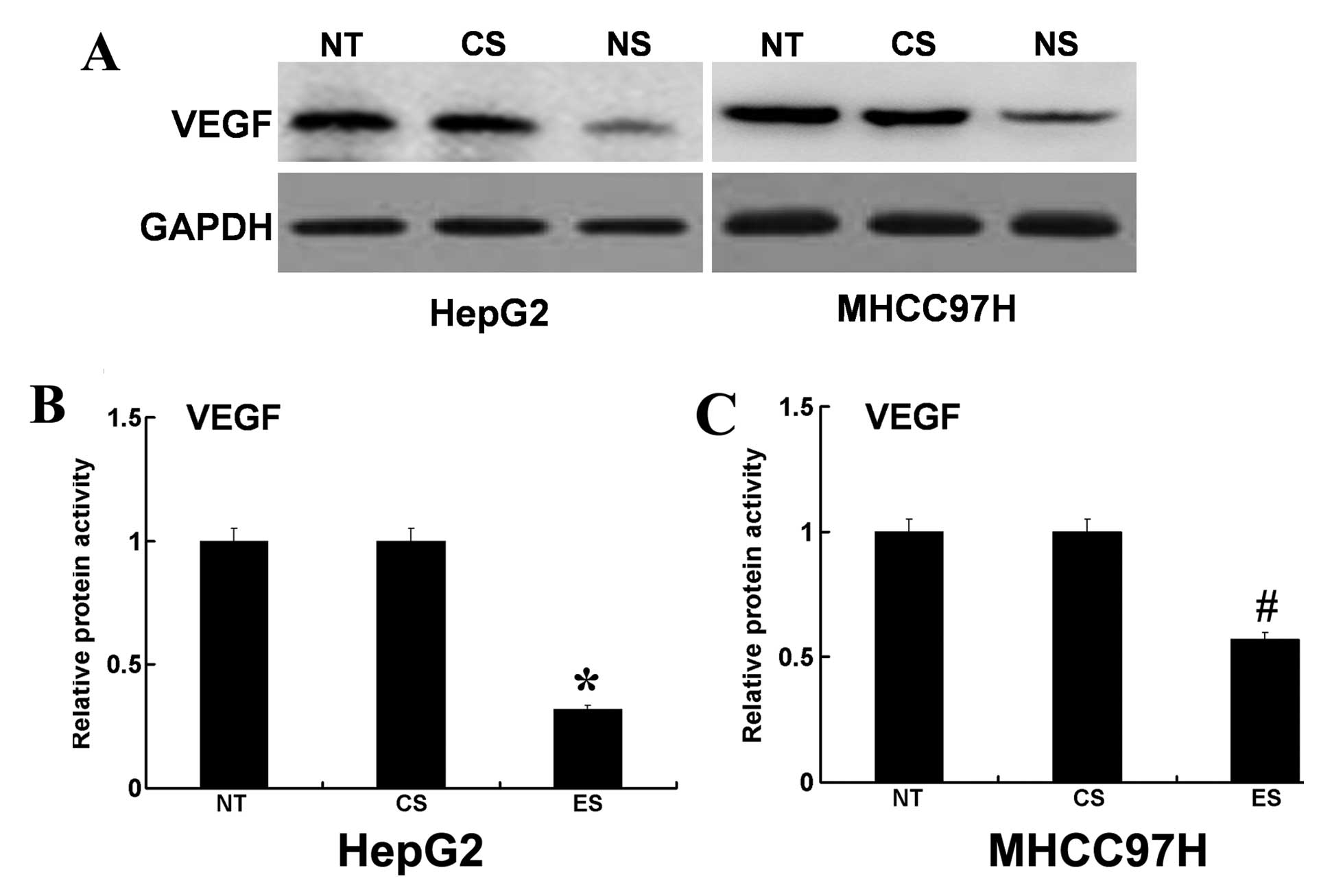
EphA3 on VEGF. Using western blotting and ELISA, we found that the
protein expression levels and proteolytic activity of VEGF were
decreased in EphA3 siRNA-transfected HepG2 cells (Fig. 5A and B). In addition, the use of
MHCC97H cells showed similar results (Fig. 5A and C). These results indicated
that EphA3 may participate in HCC cell invasion by regulating the
expression and activity of VEGF. However, additional studies are
needed to address why EphA3 can regulate protein expression levels
and proteolytic activity of VEGF.
Discussion
Tyrosine kinases, which are the major regulators of
signal transduction pathways, are associated with cellular
proliferation, apoptosis and tumorigenesis (20). Eph receptor tyrosine kinases (Ephs)
and their membrane-anchored ephrin ligands (ephrins) form a
cell-cell system that is associated with cell-to-cell adhesion or
migration, angiogenesis and tumor vasculature in various human
carcinomas (21,22). During adulthood, many Ephs and their
ligands (ephrins) are expressed in malignant tissues and are
thought to participate in tumor invasion and metastasis (23,24).
EphA3 is a component of the Eph/ephrin tyrosine kinase system,
which participates in blood vessel development (25) and play a potentially significant
role in tumor angiogenesis (26).
Changes in EphA3 are likely to produce particular morphological and
biological characteristics, such as cell growth and viability, loss
of cell adhesion to fibronectin, cell migration and anti-apoptosis.
Considerable evidence indicates that aberrant regulation of EphA3
and its genetic alterations are implicated in the development and
progression of various types of cancers (27–30).
Studies have shown that EphA3 expression is detected in B- and
T-lymphoid tumor cell lines and in primary leukemias (31) and is associated with B- and T-cell
malignancies (32–34). Furthermore, it has been reported
that EphA3 is overexpressed in a range of tumors, such as lung
cancer, renal cancer, colorectal cancer, melanoma and sarcoma
(14,15,17).
EphA3 expression was significantly higher in colorectal carcinoma
than that in normal mucosal tissue and was associated with patient
survival (17).
However, the relationship between EphA3 expression
and survival in patients with HCC remains unknown. In the present
study, we examined the expression of EphA3 by immunohistochemistry
in HCC samples. Our results revealed that high levels of EphA3
expression in HCC tumor tissues were correlated with tumor size,
tumor grade, metastasis, venous invasion and TNM stage, each of
which is an indication of advanced tumor status. These results
strongly suggest that EphA3 may play a key role in the progression
of human HCC. Prognostic molecular biomarkers are invaluable for
evaluating patient status and promoting tumor control. Kaplan-Meier
analysis of the survival curves from patients in the present study
showed a significantly worse overall survival rate for patients
whose tumors had high EphA3 expression levels (log-rank test,
P<0.001), indicating that high levels of EphA3 protein may serve
as a marker of poor prognosis for patients with HCC. Moreover, the
multivariate analysis found EphA3 expression to be an indicator of
worse patient outcome, independently of known clinical prognostic
indicators such as TNM stage. These data suggest that high EphA3
expression is correlated with worse patient outcome and may serve
as an independent prognostic factor for patients with HCC.
One significant finding of the present study was
that metastasis and venous invasion were detected more frequently
in EphA3-positive tumors compared to EphA3-negative cases. Invasion
and metastasis are the processes by which tumors spread from the
location of the primary tumor to distant locations in the body.
These processes include a series of sequential steps, including
tumor-induced angiogenesis, tumor invasion and establishment of
metastatic foci at the secondary site involving various molecules
(35,36). One important molecule involved in
tumor cell invasion and metastasis is VEGF. The expression of VEGF
is commonly found to be upregulated in tumors and there is a trend
toward an association between expression of VEGF and distant
metastasis. Investigations by other laboratories have shown that
VEGF promotes migration and invasion of tumor cells (37,38).
Angiogenesis plays an important role in tumors from the initial
stage of carcinogenesis to the end stage of metastatic disease
(39). The development of
neovasculature in the tumor provides essential functions for
growth, invasion and metastasis. VEGF is one of the isolated
angiogenic peptides and is the most well-studied angiogenic factor
to date. Moreover, VEGF is known to play a vital role in
tumor-associated invasion (40,41).
In the present study, we showed that the invasive capabilities of
EphA3 siRNA-transfected HCC cells were decreased and that the
downregulation of EphA3 decreased the protein expression and
proteolytic activity of VEGF. These results suggest that in HCC
cells, the EphA3-VEGF axis may participate in tumor cell invasion.
However, further study is necessary to elucidate the mechanism of
the EphA3-VEGF interaction in HCC.
In summary, our findings strongly suggest that high
levels of EphA3 expression significantly correlate with tumor
progression and an unfavorable patient prognosis. In vitro,
downregulation of EphA3 expression decreased the invasiveness of
HCC cells via the regulation of VEGF. Therefore, EphA3 may be
regarded as not only a novel candidate marker for prognosis but
also a molecular target for HCC therapy. However, the underlying
mechanisms responsible for these observations require further
elucidation.
References
|
1
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klein R: Excitatory Eph receptors and
adhesive ephrin ligands. Curr Opin Cell Biol. 13:196–203. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noren NK and Pasquale EB: Eph
receptor-ephrin bidirectional signals that target Ras and Rho
proteins. Cell Signal. 16:655–666. 2004. View Article : Google Scholar
|
|
4
|
Wilkinson DG: Eph receptors and ephrins:
regulators of guidance and assembly. Int Rev Cytol. 196:177–244.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooke JE and Moens CB: Boundary formation
in the hindbrain: Eph only it were simple. Trends Neurosci.
25:260–267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krull CE, Lansford R, Gale NW, et al:
Interactions of Eph-related receptors and ligands confer
rostrocaudal pattern to trunk neural crest migration. Curr Biol.
7:571–580. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gale NW, Holland SJ, Valenzuela DM, et al:
Eph receptors and ligands comprise two major specificity subclasses
and are reciprocally compartmentalized during embryogenesis.
Neuron. 17:9–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davy A and Robbins SM: Ephrin-A5 modulates
cell adhesion and morphology in an integrin-dependent manner. EMBO
J. 19:5396–5405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lawrenson ID, Wimmer-Kleikamp SH, Lock P,
et al: Ephrin-A5 induces rounding, blebbing and de-adhesion of
EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated
signalling. J Cell Sci. 115:1059–1072. 2002.PubMed/NCBI
|
|
10
|
Wang J, Dong Y, Wang X, et al: Expression
of EphA1 in gastric carcinomas is associated with metastasis and
survival. Oncol Rep. 24:1577–1584. 2010.PubMed/NCBI
|
|
11
|
Nakamura R, Kataoka H, Sato N, et al:
EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci.
96:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan W, Chen Z, Wu S, et al: Expression of
EphA2 and E-cadherin in gastric cancer: correlated with tumor
progression and lymphogenous metastasis. Pathol Oncol Res.
15:473–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Li G, Ma H, et al: Differential
expression of EphA7 receptor tyrosine kinase in gastric carcinoma.
Hum Pathol. 38:1649–1656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hafner C, Schmitz G, Meyer S, et al:
Differential gene expression of Eph receptors and ephrins in benign
human tissues and cancers. Clin Chem. 50:490–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wimmer-Kleikamp SH and Lackmann M:
Eph-modulated cell morphology, adhesion and motility in
carcinogenesis. IUBMB Life. 57:421–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae HJ, Song JH, Noh JH, et al: Low
frequency mutation of the Ephrin receptor A3 gene in hepatocellular
carcinoma. Neoplasma. 56:331–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi HQ and Zhao P: Clinicopathological
significance and prognostic value of EphA3 and CD133 expression in
colorectal carcinoma. J Clin Pathol. 64:498–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chu D, Li Y, Wang W, et al: High level of
Notch1 protein is associated with poor overall survival in
colorectal cancer. Ann Surg Oncol. 17:1337–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and -9 and vascular endothelial growth factor.
Oncol Rep. 28:874–882. 2012.
|
|
20
|
Raspollini MR, Amunni G, Villanucci A,
Baroni G, Boddi V and Taddei GL: Prognostic significance of
microvessel density and vascular endothelial growth factor
expression in advanced ovarian serous carcinoma. Int J Gynecol
Cancer. 14:815–823. 2004. View Article : Google Scholar
|
|
21
|
Dodelet VC and Pasquale EB: Eph receptors
and ephrin ligands: embryogenesis to tumorigenesis. Oncogene.
19:5614–5619. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clevers H and Batlle E: EphB/EphrinB
receptors and Wnt signaling in colorectal cancer. Cancer Res.
66:2–5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamoto M and Bergemann AD: Diverse roles
for the Eph family of receptor tyrosine kinases in carcinogenesis.
Microsc Res Tech. 59:58–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brantley-Sieders D, Schmidt S, Parker M
and Chen J: Eph receptor tyrosine kinases in tumor and tumor
microenvironment. Curr Pharm Des. 10:3431–3442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Héroult M, Schaffner F and Augustin HG:
Eph receptor and ephrin ligand-mediated interactions during
angiogenesis and tumor progression. Exp Cell Res. 312:642–650.
2006.PubMed/NCBI
|
|
26
|
Xi HQ, Wu XS, Wei B and Chen L: Aberrant
expression of EphA3 in gastric carcinoma: correlation with tumor
angiogenesis and survival. J Gastroenterol. 47:785–794. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davies H, Hunter C, Smith R, et al:
Somatic mutations of the protein kinase gene family in human lung
cancer. Cancer Res. 65:7591–7595. 2005.PubMed/NCBI
|
|
28
|
Bardelli A, Parsons DW, Silliman N, et al:
Mutational analysis of the tyrosine kinome in colorectal cancers.
Science. 300:9492003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonifaci N, Gorski B, Masojc B, et al:
Exploring the link between germline and somatic genetic alterations
in breast carcinogenesis. PLoS One. 5:e140782010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JS and Thorgeirsson SS: Comparative
and integrative functional genomics of HCC. Oncogene. 25:3801–3809.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boyd AW, Ward LD, Wicks IP, et al:
Isolation and characterization of a novel receptor-type protein
tyrosine kinase (hek) from a human pre-B cell line. J Biol Chem.
267:3262–3267. 1992.PubMed/NCBI
|
|
32
|
Wicks IP, Wilkinson D, Salvaris E and Boyd
AW: Molecular cloning of HEK, the gene encoding a receptor tyrosine
kinase expressed by human lymphoid tumor cell lines. Proc Natl Acad
Sci USA. 89:1611–1615. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dottori M, Down M, Hüttmann A, Fitzpatrick
DR and Boyd AW: Cloning and characterization of EphA3 (Hek) gene
promoter: DNA methylation regulates expression in hematopoietic
tumor cells. Blood. 94:2477–2486. 1999.PubMed/NCBI
|
|
34
|
Fox BP, Tabone CJ and Kandpal RP:
Potential clinical relevance of Eph receptors and ephrin ligands
expressed in prostate carcinoma cell lines. Biochem Biophys Res
Commun. 342:1263–1272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
36
|
Weiss L: Metastasis of cancer: a
conceptual history from antiquity to the 1990s. Cancer Metastasis
Rev. 19:I–XI. 193–383. 2000.PubMed/NCBI
|
|
37
|
Wey JS, Fan F, Gray MJ, et al: Vascular
endothelial growth factor receptor-1 promotes migration and
invasion in pancreatic carcinoma cell lines. Cancer. 104:427–438.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
39
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Joo YE, Sohn YH, Lee WS, et al: Expression
of vascular endothelial growth factor and p53 in pancreatic
carcinomas. Korean J Intern Med. 17:153–159. 2002.PubMed/NCBI
|
|
41
|
Zeng H, Datta K, Neid M, Li J, Parangi S
and Mukhopadhyay D: Requirement of different signaling pathways
mediated by insulin-like growth factor-I receptor for
proliferation, invasion, and VPF/VEGF expression in a pancreatic
carcinoma cell line. Biochem Biophys Res Commun. 302:46–55. 2003.
View Article : Google Scholar : PubMed/NCBI
|