Introduction
Gliomas, the most common type of primary brain
tumors in adults, are subcategorized by histopathologic evaluation
and clinical criteria into four grades (I–IV), according to the
current World Health Organization (WHO) guidelines (1). Despite attempts at treatment using
surgical resection, radiation and chemotherapy with the alkylating
agent temozolomide, the survival rates of patients with high-grade
gliomas are less than 10% at 5 years (2). The extremely poor prognosis of these
patients are due to the biological characteristics of glioma cells
which include unrestricted proliferation and extensive invasion
(3). Therefore, understanding the
main molecular mechanisms of this malignancy is the key for the
development of novel and effective therapeutic strategies for
gliomas.
The leucine-rich repeat-containing G protein-coupled
receptor 5 (LGR5) was initially identified as an orphan G
protein-coupled receptor, containing a large extracellular domain
with 17 leucine-rich repeats and a seven transmembrane domain
(4). Recently, LGR5 is regarded as
a somatic stem cell marker that plays key functional roles in
normal development (5,6). Studies have demonstrated that LGR5 is
highly expressed during embryonic development but is detected only
in stem cells in postnatal tissues including the intestine,
stomach, hair follicles and kidney (7–10).
Significantly, research has demonstrated that LGR5
plays a role in tumor progression likely due to mutational
activation of the Wnt pathway (11). Accumulating evidence in human cancer
cell lines has now confirmed that LGR5 promotes the growth and
survival of ovarian carcinoma (12), basal cell carcinoma (13), colorectal and gastric carcinoma,
esophageal adenocarcinoma (9,10,14,15)
and liver carcinoma (16). Rcently,
Nakata et al(17)
demonstrated that expression of LGR5 is correlated with WHO grade
in clinical samples of astrocytoma and that depletion of LGR5
induced apoptosis in brain cancer stem-like cells (CSCs). Thus,
there is compelling evidence that LGR5 contributes to cancer
initiation and progression. However, the exact mechanisms of LGR5
in glioma cells have not been elucidated.
In the present study, we confirmed the expression of
LGR5 in human gliomas and its correlation with pathologic grade and
proliferation. Moreover, we explored the role of LGR5 in the
proliferation of U87 cells in vitro and in vivo by
knockdown of LGR5 with RNA interference.
Materials and methods
Patients and specimens
Specimens from 54 patients with glioma who underwent
surgical resection at the Department of Neurosurgery, Peking
University People's Hospital between November of 2009 and May of
2012 were collected. Of the patients, 29 were male and 25 were
female. Ages of the patients at the time of surgery ranged from 21
to 75 years [mean age ± standard deviation (SD), 45.8±14.5 years].
According to the revised WHO criteria for the central nervous
system (18), tumors were
categorized into grade I (n=5), grade II (n=13), grade III (n=13)
and grade IV (n=23). All tumor tissues were obtained from the
initial surgery, and none of the patients had been subjected to
chemotherapy or radiation therapy prior to tumor excision. The
histologic subtypes and pathologic grades of all glioma samples
were confirmed by two pathologists independently. The present study
was approved by the Institutional Review Board, and all
participants provided written informed consent.
Immunohistochemistry
All paraffin-embedded sections were deparaffinized
followed by washing in xylenes and serial dilutions of ethanol.
Endogenous peroxidase was blocked by 3% H2O2
for 12 min. After antigen retrieval, blocks for avidin and biotin
and the Fc receptor were applied. The rabbit anti-LGR5 polyclonal
antibody was used at a 1:150 dilution (Abcam, Cambridge, UK) or the
rabbit anti-human Ki-67 polyclonal antibody was used at a 1:200
dilution (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight
at 4°C in a humidified chamber. The primary antibodies were then
detected using the appropriate labeled streptavidin-biotin (LSAB)
kit (Fuzhou Maixin Biotechnology, Fuzhou, China) according to the
manufacturer's instructions. Immunolabeled sections were visualized
with 3′,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St.
Louis, MO, USA) and counterstained with hematoxylin. As control,
phosphate-buffered saline (PBS) was used instead of the primary
antibody.
Evaluation of the staining results
The staining results for immunohistochemistry were
evaluated by two independent neuropathologists who were blinded to
clinical information. Brown-yellow staining in the cytoplasm and/or
membrane was considered positive for LGR5. Brown-yellow staining in
the nucleus was positive for Ki-67. To measure the LGR5
immunoreactivity score (IRS) and proliferative index (PI), 10
high-power (x400) fields (~1,000 cells) were randomly chosen for
quantification in the most strongly stained tumor area of each
section. The LGR5 staining intensity (LGR5-SI), the percentage of
LGR5-positive tumor cells (LGR5-PP), and the resulting LGR5
immunoreactivity score (LGR5-IRS) were evaluated by a modified
method as previously described (19,20).
Briefly, the immunoreactivity score (LGR5-IRS: negative, 0; weak,
1–3; moderate, 4–6; strong, 8–12) was determined by multiplication
of the value for LGR5 staining intensity (LGR5-SI: 0, no staining;
1, weak staining; 2, moderate staining; 3, strong staining) and the
value for the percentage of LGR5-positive tumor cells (LGR5-PP: 0,
<1%; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4, >75%). Due to the
heterogeneous staining intensity of the tumor cells, the SI was
determined according to the staining intensity noted in the
majority of the cells. The percentage of Ki-67-positive cells was
regarded as the PI of each tumor tissue sample, respectively.
Cell culture
Human malignant glioma cell lines (U118, U87 and
U251) and normal human astrocytes (1800) were obtained from the
Cell Library of the Chinese Academy of Sciences (Shanghai, China).
U118, U87 and U251 cells were cultured at 37°C in 5% CO2
in Dulbecco's modified Eagle's essential medium (DMEM) (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
HyClone, Logan, UT, USA), 2 mM L-glutamine and 100 U/ml
penicillin-streptomycin (Gibco). The normal astroctyes (1800) were
cultured at 37°C in 5% CO2 in modified RPMI-1640
(HyClone) supplemented with 10% FBS, 2 mM L-glutamine and 100 U/ml
penicillin-streptomycin (Gibco). The medium was changed every 3–4
days, and cultures were split using 0.25% trypsin. U87, U87-NC and
U87-KD fluorescent (EGFP)-labeled cells were developed (Shanghai
GeneChem Co, Ltd., Shanghai, China) and an in vivo optical
imaging technique was used.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA was isolated from cells using the RNeasy
Mini kit, including DNase treatment (Qiagen K.K., Tokyo, Japan).
cDNA was synthesized using the PrimeScripts RT reagent kit (Perfect
Real-Time; Takara Bio, Shiga, Japan) and qPCR was performed on a
Thermal Cycler Dice Real-Time System using SYBR Premix Ex Taq™
(Perfect Real-Time). The primer sequences for qPCR were as follows:
GAPDH forward, 5′-ATCATCCCTGCCTCTACTGG-3′ and GAPDH reverse,
5′-TTTCTAGACGGCAGGTCAGGT-3′; LGR5 forward,
5′-GAGGATCTGGTGAGCCTGAGAA-3′ and LGR5 reverse,
5′-CATAAGTGATGCTGGAGCTGGTAA-3′. GAPDH was used as a reference. Fold
induction values were calculated using the 2−ΔΔCt
method. All experiments were performed in triplicate and repeated
at least three times in separate experiments; representative data
are shown.
Western blotting
Cell lysates or the tissues dissolved in SDS sample
buffer were separated by SDS-PAGE and transferred to nitrocellulose
membranes. β-actin was used as a control. Membranes were probed for
the LGR5 antibody (1:1,500; Abcam) or β-actin (1:2,000; Abcam) in
Tris-buffered saline (TBS) containing 1% milk and 0.05% Tween-20
overnight at 4°C. The secondary antibody was horseradish peroxidase
(HRP)-goat anti-rabbit or anti-mouse IgG (1:2,500; Cell Signaling)
and incubation ws carried out for 1 h at room temperature. Blots
were developed with Amersham ECL Western Blotting Detection reagent
(GE Healthcare, Chalfont St. Giles, UK).
siRNA
For knockdown of human LGR5, small hairpin RNA
(shRNA) of the human LGR5 lentivirus gene transfer vector encoding
green fluorescent protein (GFP) sequence was constructed by
Shanghai GeneChem Co., Ltd. (Shanghai, China). The targeting
sequence of the shRNA was 5′-GTCTGCAATCAGTTACCTA-3′. The
recombinant lentivirus of small interference RNA targeting LGR5
(LGR5-RNAi-Lentivirus) was prepared and titered to 109
TU/ml (transfection units/ml). A scrambled short-hairpin RNA
(shRNA) was used as a negative control.
MTT assay
An MTT assay was performed to detect the
anti-proliferative effect of LGR5 RNA interference on U87 cells.
U87 cells were seeded in 96-well plates at a density of
2×103/well. After 24 h of incubation, cells were serum
starved overnight. In another experiment, U87-NC-shRNA and
U87-LGR5-shRNA cells were seeded in 96-well plates at a density of
2×103/well and incubated for 24, 48, 72, 96 or 120 h. At
each time-point, 20 ml of 5 mg/ml MTT (Sigma) solution was added to
each well. After 4 h of incubation, the medium was removed from the
wells by aspiration, and the formazan crystals were dissolved in
150 ml of dimethyl sulfoxide (DMSO; Sigma). Color intensity was
measured at 490 nm with an enzyme linked immunosorbent assay plate
reader (Bio-Rad Laboratories, Hercules, CA, USA). Cell growth
curves were determined using the average absorbance at 490 nm from
triplicate samples of three independent experiments.
Cell cycle analysis
Cells were cultured in 25-ml flasks and incubated
until they reached 60–70% confluence in DMEM containing 10% FBS.
The cells were collected and washed twice with ice-cold PBS, and
then fixed overnight with 70% ethanol at 4°C. Following incubation
with 50 mg/ml RNase A at room temperature for 30 min, the cells
were stained with 20 mg/ml propidium iodide (PI; Sigma) for an
additional 30 min. DNA content and cell cycle distribution were
analyzed by flow cytometry (FACSCalibur; Becton-Dickinson, San
Jose, CA, USA) and the results were interpreted using ModFit and
CellQuest software. All of the samples were assayed in
triplicate.
Plate colony formation assay
Cells (800 cells/plate) were cultured in 3 ml of
DMEM supplemented with 10% FBS and 800 mg/ml G418 in a 6-well
plate. After 2 weeks, colonies were rinsed with PBS, fixed with
methanol for 5 min, and stained with Giemsa (Sigma) for 20 min.
Clearly visible colonies (>50 mm in diameter) were counted as
positive for growth.
Tumorsphere formation assays
For tumorsphere formation, single-cell suspensions
were suspended in Dulbecco's modified Eagle's medium/F12 (DMEM/F12;
HyClone) supplemented with B-27 (1X, Gibco), 20 ng/ml epidermal
growth factor (EGF; PeproTech Inc., Rocky Hill, NJ, USA) and 20
ng/ml basic fibroblast growth factor (bFGF; Peprotech) and then
plated in 24-well ultra-low attachment plates (Corning
Incorporated, Corning, NY, USA) at a concentration of 1,000
cells/well. Plates were analyzed 7–10 days later for tumorsphere
formation, which was quantified using an inverted microscope
(Olympus) at ×100 magnification.
In vivo tumorigenicity assays
In an orthotopic model, 6-week-old BALB/C nude mice
(Cancer Institute of the Chinese Academy of Medical Science) were
randomly divided into three groups (three mice per group). All
experiments were performed following approval of the Animal Studies
Ethics Committee of the Peking University People's Hospital. A
small burr hole, 2 mm in diameter was made (1 mm to the midline and
0.5 mm anterior to the bregma) using a microskull drill. A trochar
packed with donor tissue was navigated to a depth of 2.5 mm via the
skull hole. Cells (5×105) (U87, Si-NC and Si-LGR5 cells)
suspended in 5 μl PBS were slowly and smoothly injected into the
subcortex of the mouse brain. The skull hole was sealed with bone
wax and the scalp was sutured. Tumor growth in mice was detected by
the Kodak In-Vivo FX Pro system (Kodak, Rochester, NY, USA). The
tumor volume of the xenografts was detected every 5 days over the
course of the study using fluorescence signaling.
Prior to the in vivo imaging, the mice were
anesthetized with phenobarbital sodium. Fluorescence imaging was
carried out with an excitation wavelength of 490 nm and emission
wavelength of 535 nm. Exposure times ranged from 1 to 2 min. Mice
were sacrificed and examined 5 weeks after the implantation. LGR5
was detected by immunohistochemistry.
Statistical analysis
The experiments were performed in triplicate and
repeated three times independently. SPSS 16.0 was used for all
statistical analysis. Comparisons among all groups were performed
using one-way analysis of variance (ANOVA). The t-test was used for
comparison of differences between two groups. Correlation
coefficients of LGR5 IRS with the PI were evaluated using Pearson
correlation analysis. Values of P<0.05 were considered to
indicate statistically significant results in all cases.
Results
Expression of LGR5 and its association
with pathologic grade and proliferation index (PI) in glioma
In the present study, LGR5 protein was overexpressed
in human glioma specimens. Positive tumor cells showed primarily
cytoplasmic and/or membranous labeling under a light microscope.
However, normal brain tissues had exceedingly weak immunoreactivity
for this protein (data not shown). The LGR5 IRS was 4.60±2.57 for
54 cases of tumor specimens. The LGR5 IRS was positively and
markedly correlated with increasing WHO grade (Fig. 1I; Table
I). There were significant differences in LGR5 IRS between
grade I and grade III (P<0.05), grade I and grade IV
(P<0.005), and grade II and grade IV (P<0.05) gliomas,
respectively. Representative images of LGR5 immunostaining are
shown in Fig. 1A–D, and the related
results are provided in Table I.
The results of the western blot analysis and RT-PCR were coincident
with that of immunohistochemistry. The results showed that mRNA and
protein expression levels of LGR5 markedly increased with an
increase in pathologic grade of the brain gliomas (P<0.05,
Fig. 1K and L).
 | Figure 1Expression of LGR5 is associated with
pathologic grade and PI in glioma. (A–D) Immunohistochemical
expression of LGR5 in glioma specimens of different grade. LGR5
immunoreactivity shows homogeneous brown-yellow staining in the
cytoplasm of tumor cells; hematoxylin counterstain. (E–H)
Immunohistochemical expression of Ki-67 in glioma specimens of
different grade. Ki-67 immunoreactivity shows brown-yellow staining
in the nucleus of tumor cells; hematoxylin counterstain. (A and E)
Grade I; (B and F) grade II; (C and G) grade III; (D and H) grade
IV. Original magnification, ×400. With increasing pathologic grade,
the LGR5 IRS (I) and PI (J) in human gliomas were significantly
increased (P<0.05; *compared with grade I;
Δcompared with grade II). (K) Western blot analysis
revealed a high level of expression of LGR5 in gliomas. β-actin was
assessed as a loading control. Lanes NB, human normal brain tissue;
lanes I, II, III, IV represent glioma grades, respectively. (L)
qRT-PCR analysis revealed a high level of expression of LGR5 in
gliomas. N, normal brain tissue; I, II, III, IV represent glioma
grades, respectively. (P<0.05; *compared with grade
I; Δcompared with N). (M) Scatterplots showing the
correlation of LGR5 IRS with PI in human gliomas. A trend line is
provided in each plot, which represents the ‘best fit’ as
determined by simple linear regression. With increased LGR5 IRS,
the PI was significantly increased (P<0.05). |
 | Table ILGR5 IRS and PI in human gliomas of
different pathologic grade. |
Table I
LGR5 IRS and PI in human gliomas of
different pathologic grade.
| Pathologic
classification | n | LGR5 IRS | PI (%) |
|---|
| Grade I | 5 | 2.58±0.30 | 11.90±2.28 |
| Grade II | 13 | 3.52±0.87 | 26.15±4.22 |
| Grade III | 13 | 5.14±1.47 | 36.44±13.5 |
| Grade IV | 23 | 7.89±1.84 | 36.44±13.5 |
| Total | 54 | 4.60±2.57 | 47.11±10.1 |
| P-valuea | | <0.01 | <0.01 |
| P-valueb | | <0.01 | <0.01 |
Pathologic grade has been linked to cell
proliferation. Therefore, we sought to determine whether high
expression of LGR5 might also be associated with proliferation. To
address this we first compared LGR5 expression with proliferation.
The proliferative index (PI) of tumor cells was evaluated by Ki-67
staining. The cell proliferation marker Ki-67 was expressed in all
tumor specimens (Fig. 1E–H). With
increasing pathologic grade of glioma, PI increased markedly
(Fig. 1J; Table I). Moreover, the PI was positively
correlated with LGR5 IRS (r=0.886, P<0.0001) (Fig. 1M).
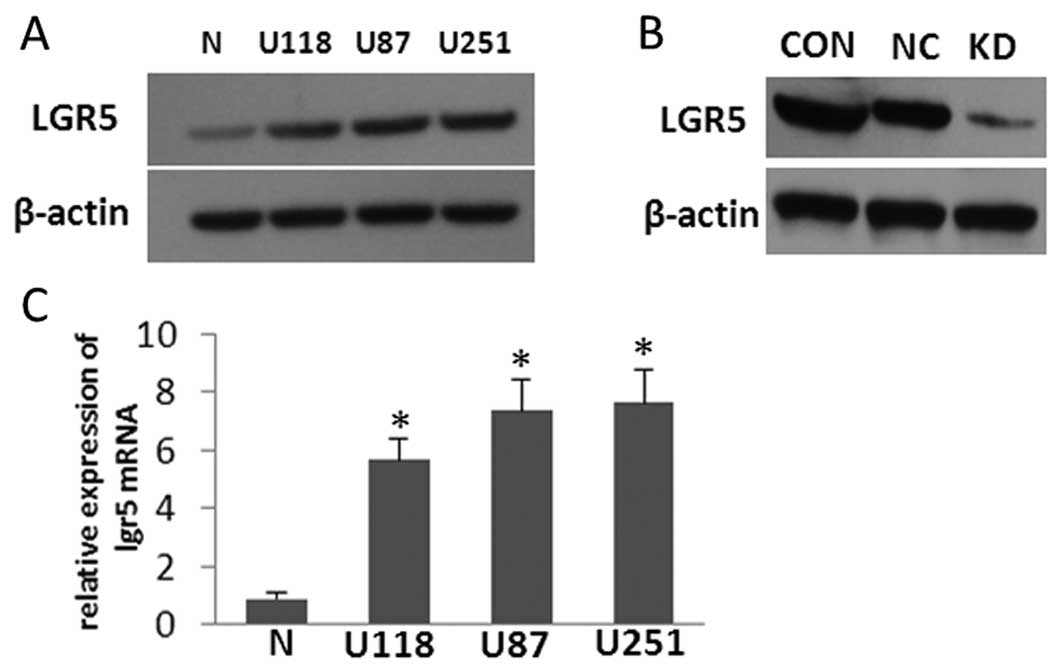
LGR5 is overexpressed in glioma cell
lines, and LGR5 interference in U87 cells markedly reduces its
expression
The expression levels of LGR5 mRNA and protein in
several high grade glioma-derived cell lines (U118, U87 and U251)
cultured in vitro were compared to the expression levels in
normal human cultured primary astrocytes by western blot analysis
and qRT-PCR (Fig. 2A and C). The
results showed that LGR5 was highly expressed in the three glioma
cell lines when compared with that in the normal astrocytes; U87
cells were randomly used as an appropriate in vitro model
for assessing LGR5 function in the subsequent experiments. To study
the role of LGR5 in the malignant progression of glioma, we
established a stably transfected U87 cell line expressing shRNA
against LGR5. The protein level of LGR5 was confirmed to be
dramatically downregulated in the U87-KD cells when compared to the
parental U87 and U87-NC cells. In addition, there was no obvious
difference in LGR5 expression between the control cell lines
(Fig. 2B). These data indicate that
transfection of LGR5 shRNA significantly and specifically inhibited
the endogenous LGR5 expression in U87-KD human glioma cells.
Knockdown of LGR5 inhibits U87 cell
growth in vitro
To evaluate the effect of LGR5 on the growth of U87
cells, viability curves for U87, U87-NC and U87-KD cells were
determined by MTT assay. As shown in Fig. 3, the growth of U87-KD cells was
obviously inhibited when compared with that in the other groups,
with this effect being most obvious from day 3 to day 7
(P<0.01). However, there were no significant differences in cell
growth between the U87 and U87-NC cells (P>0.05). These results
indicate that downregulation of LGR5 expression by RNAi markedly
inhibited the growth of U87 cells.
Knockdown of LGR5 inhibits cell cycle
progression of U87 cells in vitro
To investigate the effect of LGR5 knockdown on cell
cycle progression, flow cytometry was performed to determine the
cell cycle distribution (Fig. 4A).
Compared with parental U87 and U87-NC cells, U87-KD cells
accumulated in the G0/G1 phase (P<0.05), whereas the percentage
of cells distributed in the S phase was decreased sharply
(P<0.05). There was no obvious difference in cell cycle
distribution between the parental U87 and U87-NC cells (P>0.05;
Fig. 4B). These results suggest
that reduction in LGR5 expression in U87 cells by RNAi delays cell
cycle progression and decreases cell proliferation.
Knockdown of LGR5 inhibits colony
formation in vitro
To analyze the effect of LGR5 downregulation on the
anchorage-dependent growth potential of U87 cells, plate colony
formation assays were performed for parental U87, U87-NC and U87-KD
cells (Fig. 5A). Compared to the
control cells, the number and size of colonies for U87-KD cells
were significantly decreased (P<0.05; Fig. 5B). In contrast, there were no
obvious differences in colony-forming ability between the control
cell lines (P>0.05). These data indicate that reduction in LGR5
expression decreased the colony-formation ability of U87 cells
in vitro.
Knockdown of LGR5 negatively regulates
tumorsphere formation
To examine the self-renewal potential of U87 cells
with or without LGR5 knockdown, we undertook tumorsphere formation
culture of shRNA-LGR5/U87, shRNA-Ctr/U87 and untransfected U87
cells in a special ultra-low attachment culture plate with
conditional medium for tumorsphere formation. After 7–10 days,
plates were analyzed for tumorsphere formation which was quantified
using an inverted microscope. As shown in Fig. 6, significantly fewer and smaller
tumorspheres were observed in the shRNA-LGR5/U87 cells than those
in the U87 and shRNA-Ctr/U87 cells (P<0.05; Fig. 6).
LGR5 siRNA significantly inhibits
tumorigenesis in vivo
To evaluate the effect of LGR5 on glioma in
vivo, we injected shRNA-LGR5/U87, shRNA-Ctr/U87 and
untransfected U87 cells into nude mice to develop orthotopic
tumors. Tumor growth in the mice was monitored by a live imaging
system detecting the fluorescent signals. During the course of the
study, shRNA-LGR5/U87 cells demonstrated significantly inhibited
tumor growth (Fig. 7A). All data
were presented as the mean ± SD and as an average of three
measurements from a representative experiment. The luciferase
signal from shRNA-LGR5/U87 cells was significantly less than that
from shRNA-Ctr/U87 and non-transfected U87 cells (P<0.05;
Fig. 7B). Immunohistochemical
analysis is shown in Fig. 7C)
Discussion
LGR5 is a downstream target gene of the Wnt
signaling pathway. The Wnt signaling pathway comprises a vast
number of protein interactions and plays a critical role in
tumorigenesis (6,21). Recently, studies have demonstrated
the expression of LGR5 in several tumors including colorectal,
gastric carcinoma, esophageal adenocarcinoma (8,9,15),
liver (16), ovarian carcinoma
(12) and Ewing sarcoma (22) and have shown that the overexpression
of LGR5 is associated with poor prognosis. Our data demonstrated
that the expression of LGR5 as detected by immunohistology was
positively and markedly correlated with increasing WHO grade in the
gliomas, which is consistent with the results of a previous report
(17). It is well known that
pathologic grade is linked to the degree of cell proliferation, and
Ki-67 expression is associated with gene-regulated cellular growth
and proliferation (23). We aimed
to ascertain whether LGR5 expression is associated with the
proliferation of glioma cells. The correlation between LGR5
expression and the proliferative index (PI) of tumor cells by Ki-67
staining was evaluated. Our results revealed that PI was positively
related to LGR5 expression indicating LGR5 may be involved in cell
proliferation in gliomas.
To confirm this hypothesis, we explored the role of
LGR5 in three representative glioma cell lines. The data showed
that LGR5 was highly expressed in all of the glioma cell lines at
the protein and mRNA levels but the results did not achieve a
significant difference. In addition, LGR5 expression was obviously
decreased by RNA interference. U87 cells were randomly chosen for
further examination. Downregulation of LGR5 resulted in suppression
of cell proliferation, arrest of the cell cycle and reduction in
clone formation. This evidence suggests a potential role for LGR5
in the regulation of glioma cell growth and proliferation which are
in agreement with previous studies concerning basal cell carcinoma
(13) and Ewing sarcoma (22). However, one recent report showed
that suppression of LGR5 expression in colorectal cancer cells
enhanced tumor formation with increased cell motility, while cells
overexpressing LGR5 tended to grow with tight cell-to-cell contact
and exhibited reduced cell motility (24). They considered that loss of LGR5
upregulates Wnt response genes and key EMT pathway genes. This
paradoxical phenomenon warrants further study in order to
investigate the gene function of LGR5 in different tissues and
cells.
Recently, accumulating evidence supports the
existence of glioma stem cells (GSCs), which have a high
tumorigenic potential and are resistant to chemotherapy and
irradiation (25,26). Researchers have suggested that GSCs
might be responsible for tumor development and recurrence (27,28).
As LGR5 is not only a target gene of the Wnt signaling pathway, but
is also a stem cell marker in the intestine, stomach and hair
follicles in the skin (5,29), we also detected the capability of
tumorsphere formation by deregulating the expression of LGR5 in a
glioma cell line. Consistent with a previous study (17), which showed that LGR5 knockdown
suppresses viability and induces apoptosis of brain cancer stem
cells, our data revealed that LGR5 knockdown inhibited tumorsphere
formation. These results indicate that high levels of LGR5
expression may confer some of the properties of stem cells to tumor
cells.
Additionally, to investigate the function of LGR5 in
glioma in vivo, siRNA-transfected and parental U87 cells
were orthotopically transplanted into nude mice. The results
revealed that LGR5 depletion significantly suppressed tumor growth
in nude mice. To the best of our knowledge, this is the first
report showing the potent activity of LGR5 on glioma growth in an
orthotopic xenograft model. Our result is consistent with a
previous report which showed that LGR5-overexpressing HaCaT cells
resulted in tumor formation when transplanted into nude mice
(13). Moreover, Fukuma et
al(16) reported that
overexpression of LGR5 in hepatocellular carcinoma cells
contributes to obvious nodular tumors. We deduce that, as LGR5 is a
marker of GSCs (17), knockdown of
LGR5 may resulted in the reduction of the capability of
tumorigenesis in vivo which is the most prominent property
of GSCs.
In the present study, we found that LGR5
transcriptional levels were upregulated in gliomas. The mechanisms
by which oncogenic mutations and alterations in signaling pathways
lead to the upregulation of LGR5 protein expression is an important
issue concerning glioma cells. Recently, some researchers noted
that the depletion of LGRs abrogated the synergistic effects of
R-spondins on Wnt signaling, indicating that activation of the Wnt
signaling pathway by R-spondins internalized together with LGR5 may
contribute to the upregulation of LGR5 (5,30).
However, the exact mechanisms behind this transcriptional
upregulation of LGR5 and its promotion of proliferation in glioma
remain to be illuminated.
In conclusion, our data demonstrated a correlation
between LGR5 expression and the proliferation index in glioma, and
knockdown of LGR5 resulted in suppression of glioma cell
proliferation in vitro and in vivo. Therefore, LGR5
may be a valuable biomarker for the molecular diagnosis and a novel
target for gene therapy of malignant gliomas.
Acknowledgements
The present study was supported by the Peking
University People's Hospital Research and Development Funds (no.
RDB2011-14). The authors thank Mrs. Danhua Shen, Mrs. Ying Wang and
Mrs. Peiying He for their helpful discussion. The authors also
thank Dr Yidong Niu, Dr Geng Guo, Dr Yanfang Pan and Dr Zejun Lu
for the excellent technical assistance.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD and
Cavenee WK: WHO Classifcation of Tumours of the Central Nervous
System. 4th edition. IARC Press; Lyon: 2007
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, et al;
European Organisation for Research and Treatment of Cancer Brain
Tumour and Radiation Oncology Groups; National Cancer Institute of
Canada Clinical Trials Group. Effects of radiotherapy with
concomitant and adjuvant temozolomide versus radiotherapy alone on
survival in glioblastoma in a randomised phase III study: 5-year
analysis of the EORTC-NCIC trial. Lancet Oncol. 10:459–466.
2009.
|
|
3
|
Mrugala MM: Advances and challenges in the
treatment of glioblastoma: a clinician's perspective. Discov Med.
15:221–230. 2013.PubMed/NCBI
|
|
4
|
Glinka A, Dolde C, Kirsch N, Huang YL,
Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM and Niehrs C:
LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and
Wnt/PCP signalling. EMBO J. 12:1055–1061. 2011.PubMed/NCBI
|
|
5
|
Schuijers J and Clevers H: Adult mammalian
stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J.
31:2685–2696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Lau W, Barker N, Low TY, Koo BK, Li VS,
Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M,
Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y,
Nishimori K, Mohammed S, Heck AJ and Clevers H: Lgr5 homologues
associate with Wnt receptors and mediate R-spondin signalling.
Nature. 476:293–297. 2011.PubMed/NCBI
|
|
7
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ and Clevers H: Identification of stem cells in small
intestine and colon by marker gene LGR5. Nature. 449:1003–1007.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barker N and Clevers H: Leucine-rich
repeat-containing G-protein-coupled receptors as markers of adult
stem cells. Gastroenterology. 138:1681–1696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barker N, Huch M, Kujala P, van de
Wetering M, Snippert HJ, van Es JH, et al: Lgr5+vestem
cells drive self-renewal in the stomach and build long-lived
gastric units in vitro. Cell Stem Cell. 6:25–36. 2010.
|
|
10
|
Barker N, Rookmaaker MB, Kujala P, Ng A,
Leushacke M, Snippert H, et al: Lgr5+vestem/progenitor
cells contribute to nephron formation during kidney development.
Cell Rep. 2:540–552. 2012.
|
|
11
|
Yui S, Nakamura T, Sato T, Nemoto Y,
Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K,
Clevers H and Watanabe M: Functional engraftment of colon
epithelium expanded in vitro from a single adult
Lgr5+stem cell. Nat Med. 18:618–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McClanahan T, Koseoglu S, Smith K, Grein
J, Gustafson E, Black S, Kirschmeier P and Samatar AA:
Identification of overexpression of orphan G protein-coupled
receptor GPR49 in human colon and ovarian primary tumors. Cancer
Biol Ther. 5:419–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanese K, Fukuma M, Yamada T, Mori T,
Yoshikawa T, Watanabe W, Ishiko A, Amagai M, Nishikawa T and
Sakamoto M: G-protein-coupled receptor GPR49 is up-regulated in
basal cell carcinoma and promotes cell proliferation and tumor
formation. Am J Pathol. 173:835–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Sousa E, Melo F, Colak S, Buikhuisen J,
Koster J, Cameron K, de Jong JH, Tuynman JB, Prasetyanti PR,
Fessler E, van den Bergh SP, Rodermond H, Dekker E, van der Loos
CM, Pals ST, van de Vijver MJ, Versteeg R, Richel DJ, Vermeulen L
and Medema JP: Methylation of cancer-stem-cell-associated Wnt
target genes predicts poor prognosis in colorectal cancer patients.
Cell Stem Cell. 9:476–485. 2011.PubMed/NCBI
|
|
15
|
Simon E, Petke D, Böger C, Behrens HM,
Warneke V, Ebert M and Röcken C: The spatial distribution of LGR5
cells correlates with gastric cancer progression. PloS One.
7:e354862012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuma M, Tanese K, Effendi K, Yamazaki K,
Masugi Y, Suda M and Sakamoto M: Leucine-rich repeat-containing G
protein-coupled receptor 5 regulates epithelial cell phenotype and
survival of hepatocellular carcinoma cells. Exp Cell Res.
319:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakata S, Campos B, Bageritz J, Bermejo
LJ, Becker N, Engel F, et al: LGR5 is a marker of poor prognosis in
glioblastoma and is required for survival of brain cancer stem-like
cells. Brain Pathol. 23:60–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buckner JC, Brown PD, O'Neill BP, Meyer
FB, Wetmore CJ and Uhm JH: Central nervous system tumors. Mayo Clin
Proc. 82:1271–1286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Remmele W, Hildebrand U, Hienz HA, Klein
PJ, Vierbuchen M, Behnken LJ, Heicke B and Scheidt E: Comparative
histological, histochemical, immunohistochemical and biochemical
studies on oestrogen receptors, lectin receptors, and Barr bodies
in human breast cancer. Virchows Arch A Pathol Anat Histopathol.
409:127–147. 1986. View Article : Google Scholar
|
|
20
|
Guo G, Liu B, Zhong C, Zhang X, Mao X, et
al: FRAT1 expression and its correlation with pathologic grade,
proliferation, and apoptosis in human astrocytomas. Med Oncol.
28:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carmon KS, Lin Q, Gong X, Thomas A and Liu
Q: LGR5 interacts and cointernalizes with Wnt receptors to modulate
Wnt/β-catenin signaling. Mol Cell Biol. 32:2054–2064.
2012.PubMed/NCBI
|
|
22
|
Scannell CA, Pedersen EA, Mosher JT, Krook
MA, Nicholls LA, Wilky BA, Loeb DM and Lawlor ER: LGR5 is expressed
by Ewing sarcoma and potentiates Wnt/β-catenin signaling. Front
Oncol. April 15–2013. View Article : Google Scholar
|
|
23
|
Yamashita Y, Kasugai I, Sato M, Tanuma N,
Sato I, Nomura M, et al: CDC25A mRNA levels significantly correlate
with Ki-67 expression in human glioma samples. J Neurooncol.
100:43–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker F, Zhang HH, Odorizzi A and Burgess
AW: LGR5 is a negative regulator of tumourigenicity, antagonizes
Wnt signalling and regulates cell adhesion in colorectal cancer
cell lines. PloS One. 6:e227332011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rath BH, Fair JM, Jamal M, Camphausen K
and Tofilon PJ: Astrocytes enhance the invasion potential of
glioblastoma stem-like cells. PLoS One. 8:e547522013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Li Y, Yu TS, McKay RM, Burns DK,
Kernie SG and Parada LF: A restricted cell population propagates
glioblastoma growth after chemotherapy. Nature. 488:522–526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gopinath S, Malla R, Alapati K, Gorantla
B, Gujrati M, Dinh DH and Rao JS: Cathepsin B and uPAR regulate
self-renewal of glioma-initiating cells through GLI-regulated Sox2
and Bmi1 expression. Carcinogenesis. 34:550–559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, et al: Glioma stem cells promote radioresistance by
preferential activation of the DNA damage response. Nature.
444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leushacke M and Barker N: Lgr5 and Lgr6 as
markers to study adult stem cell roles in self-renewal and cancer.
Oncogene. 31:3009–3022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carmon KS, Gong X, Lin Q, Thomas A and Liu
Q: R-spondins function as ligands of the orphan receptors LGR4 and
LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci USA.
108:11452–11457. 2011.PubMed/NCBI
|