Introduction
Breast cancer is one of the leading causes of
cancer-related mortality among females in the developed world.
Although therapies for human breast cancer have been developed, the
mortality rate of breast cancer patients has remained unchanged.
Therefore, the development of new strategies for more effective
treatment is highly desirable, and chemotherapeutic use of
phytochemicals as anticancer agents has received recent attention
due to their low cytotoxicities and the low cost of plant-derived
raw materials (1,2).
Since malignancy of tumors is generally attributed
to their invasive and metastatic capacity, a therapeutic agent that
only possesses the ability to induce apoptosis might not be
effective against metastatic tumors. Metastasis is a multi-step
process that involves the detachment of cancer cells from the
primary tumor as well as their migration, adhesion and invasion
into lymphatic vessels or blood. Next, extravasation from the
vessels is mediated by the action of extracellular proteases, among
which the matrix metalloproteinases (MMPs) have been demonstrated
to play crucial roles. In particular, type IV collagenases or
gelatinases (e.g., MMP-2 and MMP-9) are major enzymes in the
degradation of a variety of extracellular matrix (ECM) components,
leading to tumor migration, invasion and metastasis in numerous
types of cancer (3,4). COX-2 is overexpressed throughout
breast cancer progression. The upregulation of COX-2 and
PGE2 might be involved in cancer cell invasion by
stimulating the expression of MMPs. In fact, both MMP-2 and MMP-9
are overexpressed in breast cancer and are closely associated with
metastasis, poor prognosis and a high mortality rate in breast
cancer patients. Therefore, inhibition of MMP activity and
expression is important for blocking the metastatic ability of
breast cancer cells (3).
In general, MMP-2 is constitutively overexpressed in
highly metastatic tumors, whereas MMP-9 can be stimulated by the
inflammatory cytokine tumor necrosis factor (TNF)-α, the epidermal
growth factor and phorbol esters through the activation of distinct
intracellular signaling pathways. Moreover, stimulators, including
cytokines and 12-O-tetradecanoylphorbol-13-acetate (TPA),
regulate the expression of MMP-9 and COX-2 by controlling the
activation of transcription factors, such as the nuclear factor
(NF)-κB and the activator protein (AP)-1, since the promoter
regions of MMP-9 and COX-2 possess NF-κB-binding sites (5,6). Two
transcription factors, NF-κB and AP-1, regulate the expression of a
number of genes and of the products associated with inflammation,
tumorigenesis and metastasis. In fact, NF-κB and AP-1 are major
transcription factors involved in the activation of genes encoding
inflammatory cytokines, such as IL-1β and TNF-α. Additionally,
NF-κB can induce the activation of COX-2 and MMP-9. Several reports
have indicated that the early suppression of MMP-9 and COX-2 enzyme
activity or expression could be used for preventing invasion and
cancer metastasis (6–8). Therefore, agents possessing the
ability to inhibit the expression of MMP-9 or COX-2 warrant
investigation with regards to treatment of cancer cell invasion and
metastasis.
HO-1 is an inducible enzyme that catalyzes the
rate-limiting step for the oxidative degradation of cellular heme
into carbon monoxide, biliverdin and free iron (9). HO-1 and its enzymatic byproducts
provide host defense mechanisms such as antioxidant and
anti-inflammatory effects. Experimental evidence has established
HO-1 as a critical component of multiple signaling pathways that
regulate proliferation and metastasis. Increased HO-1 expression
likely plays an important role in the development and progression
of breast cancer (10,11). Therefore, HO-1 might be an important
therapeutic target for the treatment of human breast cancer.
In traditional Chinese medicine, Macleaya
cordata (plume poppy) has long been used as a painkiller and an
anti-inflammatory agent in humans. Macleaya cordata is a
plant of the Papaveraceae family, which includes abundant bioactive
compounds, mostly isoquinoline alkaloids such as allocryptopine,
angoline, berberine, bocconine, bocconoline, chelerythrine,
heleritrine, macarpine, protopine and sanguinarine (12,13).
The capsule of M. cordata contains the highest level of
sanguinarine and chelerythrine. Moreover, the highest amount of
protopine and allocryptopine was found in the footstalks. In
addition, M. cordata is recorded in the European Food Safety
Authority (EFSA) list of plants utilized as ingredients of feed
additives in animal production due to their anti-inflammatory
activity. Sanguinarine and chelerythrine are biologically active
components of these extracts (13–15).
The chemical name of sanguinarine is 13-methyl[1,3]
benzodioxole[5,6-c]-1,3-dioxolan[4,5-i]
phenanthridinium. In particular, sanguinarine is noted for its
pharmacological activity, e.g., its antihypertensive, cardiac and
antitumor properties (14,16,17).
In the present study, we investigated the inhibitory activity of
sanguinarine on the TPA-induced upregulation of MMP-9 and COX-2 in
human MCF-7 breast cancer cells. Here, we provide evidence that
sanguinarine inhibits TPA-induced MMP-9 and COX-2 expression by
blocking NF-κB, Akt and ERK1/2 signaling. Furthermore, we showed
that sanguinarine inhibits the migration and invasion of breast
cancer cells. Sanguinarine exhibits an anti-invasive activity
related to the induction of HO-1 expression. These findings provide
new insights into the mechanism by which sanguinarine mediates its
anti-invasive activity and might thus be useful for developing
novel therapeutic strategies to target breast cancer
metastasis.
Materials and methods
Materials and reagents
Sanguinarine and other chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA). BioCoat™ Matrigel™ invasion
chambers were obtained from BD Biosciences (San Jose, CA, USA).
Antibodies against phosphorylated p38 (p-p38), p-JNK, p-ERK,
p-IκB-α, MMP-2 and MMP-9 were purchased from Cell Signaling
Technology (Beverly, MA, USA). HO-1 small interfering RNA (siRNA)
and antibodies against COX-1, COX-2, ERK, JNK, p38, c-Jun, c-Fos,
NF-κB and TBP were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Cell culture medium RPMI-1640 and fetal bovine
serum (FBS) were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). The FuGENE-6 transfection reagent and the
X-tremeGENE siRNA transfection reagent were purchased from Roche
Diagnostics (Indianapolis, IN, USA).
Cell cultures
Human breast cancer cell line MCF-7 was obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). Cells were grown in RPMI supplemented with 10%
heat-inactivated FBS and 1% penicillin-streptomycin at 37°C in a
humidified incubator in a 5% CO2 atmosphere.
Cell invasion assay
The cell invasion assay was conducted using BioCoat™
Matrigel™ invasion chambers according to the manufacturer's
instructions. Briefly, the Matrigel coating was rehydrated in 0.5
ml of Dulbecco's modified Eagle's medium (DMEM) for 30 min
immediately before the experiments. Cells (5×104)
suspended in 0.5 ml of serum-free medium were added to the upper
chamber of the Matrigel-coated filter inserts. Following treatment
with sanguinarine for 1 h, 0.5 ml of serum-free medium containing
50 nM TPA was added to the bottom well as a chemoattractant. The
chambers were then incubated for 24 h. Following incubation, cells
on the upper side of the chamber were removed using cotton swabs,
and cells that had migrated were fixed and stained with 2% ethanol
containing 0.2% crystal violet powder. Invading cells were
enumerated under a light microscope at ×10 magnification.
In vitro wound-healing repair assay
For the in vitro wound-healing repair assay
(cell migration assay), the cells were seeded in a 24-well culture
dish until they reached 90% confluence. The cells were then
maintained in serum-free medium for 12 h. The monolayers were
carefully scratched using a 200-μl pipette tip. Cellular debris was
removed by washing with phosphate-buffered saline (PBS), and the
cells were incubated in serum-free medium. The migrating cells were
then fixed with cold 75% methanol for 30 min and washed three times
with PBS. The cultures were photographed at 0 and 24 h to monitor
the migration of cells into the wounded area, and then the closure
of the wounded area was calculated.
Gelatin zymography assay
The enzyme activities of MMP-2 and MMP-9 in
conditioned medium were determined using the gelatin zymography
protease assay. Briefly, cells (2×105) were seeded in
6-well plates and allowed to grow at 80% confluence. The cells were
then maintained in serum-free medium for 12 h prior to treatment
with sanguinarine and TPA for 24 h. Conditioned media were
collected, cleared by centrifugation, and mixed with 2X SDS sample
buffer (Invitrogen Life Technologies), followed by electrophoresis
in a polyacrylamide gel containing 0.1% (w/v) gelatin. Following
electrophoresis, the gels were incubated in renaturing buffer (2.5%
Triton X-100) with gentle agitation to remove SDS, followed by
incubation in developing buffer (50 mM Tris-HCl, pH 7.4 and 10 mM
CaCl2) overnight at 37°C to allow digestion of the
gelatin. Gels were then stained with SimplyBlue™ SafeStain
(Invitrogen Life Technologies) until clear bands suggestive of
gelatin digestion appeared.
Western blot analysis
Cells were harvested in ice-cold lysis buffer
consisting of 1% Triton X-100, 1% deoxycholate and 0.1% SDS. The
protein content of the cell lysates was then determined using the
Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA).
Proteins in each sample (50 μg of total protein) were resolved by
12% SDS-PAGE, transferred to a polyvinylidene difluoride membrane,
and exposed to the appropriate antibodies. The proteins were
visualized with the Enhanced Chemiluminescence Detection system
(Amersham Biosciences, Piscataway, NJ, USA) using horseradish
peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary
antibodies. Images were acquired using an ImageQuant 350 analyzer
(Amersham Biosciences).
Real-time PCR
Total cellular RNA was isolated using an RNAspin
Mini Isolation kit (GE Healthcare, Buckinghamshire, UK) according
to the manufacturer's instructions. One microgram of total RNA was
reverse-transcribed using Maxime RT PreMix (Intron Biotechnology,
Seongnam, Korea) and anchored oligo-dT15 primers.
Real-time PCR was performed with SYBR®-Green Master Mix
(Applied Biosystems, Foster City, CA, USA) using a Chromo4
instrument (Bio-Rad Laboratories). The relative amount of target
mRNA was determined using the Ct method by normalizing target mRNA
Ct values to those for GAPDH (ΔCt). The real-time PCR cycling
conditions were: 95°C for 5 min, 95°C for 30 sec for 40 cycles,
55°C for 20 sec and 72°C for 30 sec, followed by fluorescence
measurement. The primer sequences used were as follows: MMP-9-sense
(5′-TTCCCTGGAGACCTGAGAACC-3′), MMP-9-antisense
(5′-CGGCAAGTCTTCCGAGTAGTTT-3′), COX-2-sense
(5′-TACAAGCAGTGGCAAAGGC-3′), COX-2-antisense
(5′-AGATCATCTCTGCCTGAGTATCTT-3′), GAPDH-sense
(5′-AGGTGGTCTCCTCTGACTTC-3′) and GAPDH-antisense
(5′-TACCAGGAAATGAGCTTGAC-3′).
Measurement of prostaglandin
E2 concentrations
Cells were incubated with different concentrations
of sanguinarine for 1 h and then with TPA for 23 h. The
prostaglandin E2 levels were quantified in the culture
medium using an enzyme-linked immunosorbent assay (ELISA) kit
(Cayman Chemical, Ann Arbor, MI, USA) according to the
manufacturer's instructions.
Chromatin immunoprecipitation (ChIP)
assay
To detect the in vivo association of nuclear
proteins with the human MMP-9 promoter, ChIP analysis was conducted
as previously described (18), with
some modifications. Briefly, 2×107 cells were incubated
in culture medium containing 1% formaldehyde for 10 min at room
temperature, and the cross-linking reaction was quenched by adding
glycine to a final concentration of 0.125 M. Isolated nuclei were
digested with 200 units of MNase at 37°C for 15 min, followed by
sonication to produce chromatin of primarily mononucleosomal size.
Fragmented chromatin was incubated with antibodies for 3 h at 4°C.
Protein-DNA complexes were recovered using protein A agarose beads,
washed, and then eluted with elution buffer. Cross-links were
reversed at 65°C in 0.25 M NaCl overnight, and DNA was digested
with proteinase K for 2 h at 50°C. DNA was isolated using a DNA
Purification kit (Qiagen). Immunoprecipitated DNA was used for each
PCR. PCR primers for the MMP-9 promoter (373 bp including the NF-κB
cluster; GenBank accession no. AF538844) were as follows: sense
(5′-CACTTCAAAGTGGTAAGA-3′), antisense (5′-GAAAGTGATGGAAGACTCC-3′)
and for the COX-2 promoter (420 bp including NF-κB cluster): sense
(5′-TCCCGACGTGACTTCCTCGA-3′) and antisense (5′-GGAGAG
GAGGGAAAAATTTG-3′).
Transient transfection and dual
luciferase assay
To determine the promoter activity, we used a
Dual-Luciferase Reporter Assay system (Promega Corp., Madison, WI,
USA). Cells were transfected with the NF-κB luciferase reporter
plasmid or the AP-1 luciferase reporter plasmid (Agilent
Technologies, Santa Clara, CA, USA) using the FuGENE-6 reagent
according to the manufacturer's instructions. The Renilla
luciferase control plasmid pRL-CMV (Promega Corp.) was
co-transfected as an internal control to determine the transfection
efficiency. Twenty-four hours following transfection, cells were
incubated with the indicated reagents for 1 h and then treated with
TPA for 24 h. The luciferase activity was assayed with a
Dual-Luciferase Assay kit (Promega Corp.) according to the
manufacturer's instructions. The luminescence was measured with a
GloMax® 96 Microplate Luminometer (Promega Corp.).
Transient transfection of siRNA
Transfection of MCF-7 cells with siRNA was performed
using the X-tremeGENE siRNA transfection reagent, according to the
manufacturer's instructions. Commercially available human
HO-1-specific and negative control siRNAs were used for
transfection. Briefly, X-tremeGENE siRNA transfection reagent (10
μl) was added to 100 μl of serum-free medium containing 2 μg of
each siRNA oligo, and the mixture was incubated for 20 min at room
temperature.
Statistical analysis
Each experiment was repeated at least three times
and all results are expressed as the mean ± SE. Statistical
analysis was performed using the SPSS software (version 18.0) to
determine significant differences. We used one-way analysis of
variance (ANOVA) followed by Tukey's post-hoc test for comparisons
between three or more groups. Data with p<0.05 were considered
to indicate statistically significant differences.
Results
Sanguinarine inhibits the activity and
expression of MMP-9 and COX-2 in human breast cancer cells
MMP-9 and COX-2 have important roles in migration
and invasion (5). To explore the
effect of sanguinarine on the activity and expression of MMP-9 and
COX-2, cells were exposed to different doses of a range of
non-toxic concentrations of sanguinarine. First, we performed
gelatin zymography and ELISA to assess the activity of MMP-9 and
COX-2 in cells exposed to sanguinarine. As shown in Fig. 1A and B, sanguinarine significantly
suppressed the TPA-induced MMP-9 enzymatic activity and the
PGE2 production in a dose-dependent manner in MCF-7
breast cancer cells. We further explored the inhibitory activity of
sanguinarine on TPA-induced MMP-9 and COX-2 expression at the mRNA
and protein levels using western blotting and real-time PCR
(Fig. 1A, C and D). The results
indicated that the inhibitory effect of sanguinarine on MMP-9 and
COX-2 expression and activity was not due to a change in cell
viability (Fig. 1E). These results
suggested that the activity and expression of MMP-9 and COX-2 were
inhibited by sanguinarine in TPA-induced human breast cancer
cells.
 | Figure 1Effects of sanguinarine on matrix
metalloproteinase (MMP)-9, MMP-2, COX-1, COX-2, TIMP-1 and TIMP-2
expression in human breast cancer cells. (A) MCF-7 cells were
treated with sanguinarine for 1 h, followed by
12-O-tetradecanoylphorbol-13-acetate (TPA) treatment (50
ng/ml) for 24 h. The MMP-9 enzymatic activity was analyzed by
gelatin zymography (Zym), secretion by western blotting, and
intracellular protein expression by western blotting. The protein
levels of MMP-9, MMP-2, COX-1, COX-2, TIMP-1 and TIMP-2 were
evaluated by western blotting. C.M, conditioned medium. (B)
PGE2 was measured in the culture supernatant using an
enzyme-linked immunosorbent assay (ELISA) kit. (C) MMP-9 and (D)
COX-2 relative mRNA expression (2−ΔCt) was determined by
real-time RT-PCR relative to GAPDH mRNA (by subtracting the Ct
value for GAPDH from the Ct value for MMP-9 and COX-2, i.e., ΔCt =
CtMMP-9/COX-2 - CtGAPDH). mRNA expression
levels of MMP-9 and COX-2 are expressed as the mean ± SE from three
independent experiments in each group. *P<0.05;
**p<0.01 (both vs. the TPA-treated group). (F) Effect
of sanguinarine on cell viability. Cells were treated with the
indicated concentration of sanguinarine in the presence of TPA (50
ng/ml) for 24 h. Cell viability was determined by the MTT assay.
Each bar represents the mean ± SE from three independent
experiments in each group. *P<0.05;
**p<0.01 (both vs. the TPA-treated group). |
Sanguinarine stimulates TIMP-1 and
TIMP-2
Since the physiological activity of MMP-9 is highly
related to that of its specific endogenous inhibitors TIMP-1 and
TIMP-2 (3,4,19),
western blotting was performed to explore the potential effects of
sanguinarine on TIMP-1 and TIMP-2 expression. As shown in Fig. 1A, sanguinarine increased TIMP-1 and
TIMP-2 protein expression in a dose-dependent manner in the
presence of TPA. In summary, these data indicate the involvement of
TIMP-1 and TIMP-2 in the sanguinarine-induced anti-invasive effect
in TPA-stimulated human breast cancer cells.
Sanguinarine suppresses MMP-9 and COX-2
expression via the NF-κB and AP-1 signaling pathways
MMP-9 and COX-2 expression is regulated by the
transcription factors NF-κB and AP-1. It has been reported that the
binding sites of these transcription factors lie within the MMP-9
and COX-2 promoters (6,7). Therefore, we investigated whether the
inhibitory effect of sanguinarine on MMP-9 and COX-2 expression is
mediated by the NF-κB and AP-1 signaling pathways. As shown in
Fig. 2A, 30 min of TPA stimulation
led to the phosphorylation and nuclear translocation of the NF-κB
subunit p65 in the nuclear extract of MCF-7 cells, and following
treatment with sanguinarine, the phosphorylation and nuclear
translocation of p65 were effectively reduced in a dose-dependent
manner. Similarly, the nuclear translocation level of AP-1 subunits
c-Jun and c-Fos was significantly attenuated by sanguinarine in
comparison to the TPA-induced cells. To further confirm the effect
of sanguinarine on the transactivity of NF-κB and AP-1, we
determined the activity of a luciferase reporter gene containing
the NF-κB and AP-1 binding regions. As shown in Fig. 2B, treatment of the TPA-stimulated
MCF-7 cells with sanguinarine increased the promoter activity of
NF-κB by 3.7-fold and the promoter activity of AP-1 by 4.2-fold;
treatment with 1 μμM sanguinarine decreased the
TPA-stimulated promoter activity of NF-κB by 1.6-fold and that of
AP-1 by 1.4-fold. Next, the binding activity of NF-κB to the MMP-9
and COX-2 promoters was investigated using a ChIP-PCR assay. A low
level of NF-κB binding activity to the MMP-9 and COX-2 gene
promoters was observed in the unstimulated cells, whereas an
important fraction of the NF-κB binding activity was induced by TPA
treatment. The increased NF-κB binding activity was dramatically
inhibited by sanguinarine (Fig. 2C and
D). Overall, these results show that sanguinarine suppresses
MMP-9 and COX-2 expression through the suppression of NF-κB and
AP-1 activities.
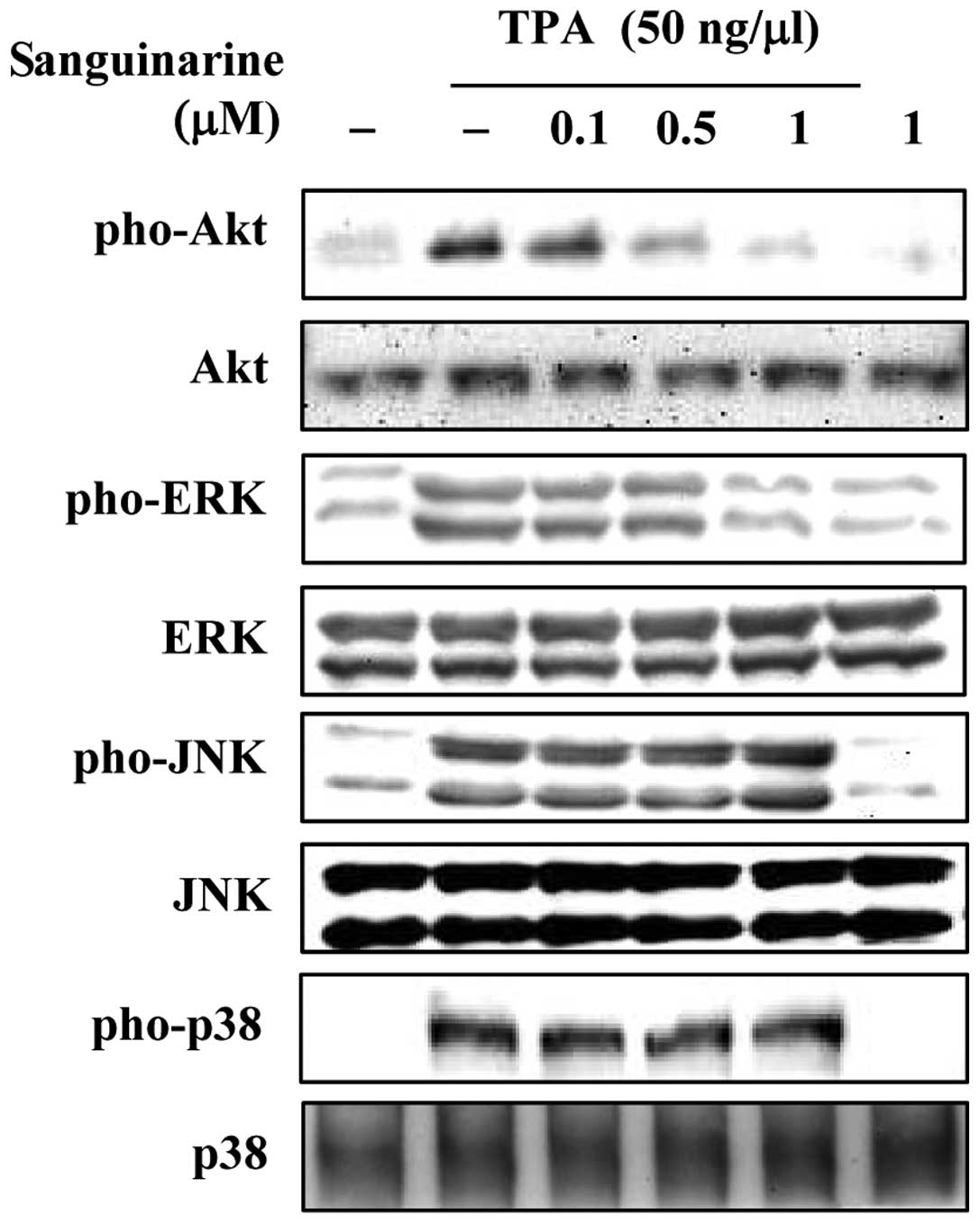
Sanguinarine inhibits Akt and ERK
activation in TPA-stimulated breast cancer cells
Although sanguinarine has been shown to inhibit
MMP-9 and COX-2 in TPA-stimulated breast cancer cells, the
underlying mechanisms are poorly understood. Therefore, we examined
the effects of sanguinarine on TPA-induced MAPK phosphorylation
using western blot analysis. As shown in Fig. 3, sanguinarine inhibited TPA-induced
Akt and ERK activation. However, treatment with up to 1 μM
sanguinarine had no effect on TPA-induced JNK and p38
phosphorylation. Importantly, treatment with sanguinarine or TPA
did not affect the total levels of Akt or ERK. These results
indicate that sanguinarine inhibited TPA-induced MMP-9 and COX-2,
most likely by inhibiting Akt and ERK activation in breast cancer
cells.
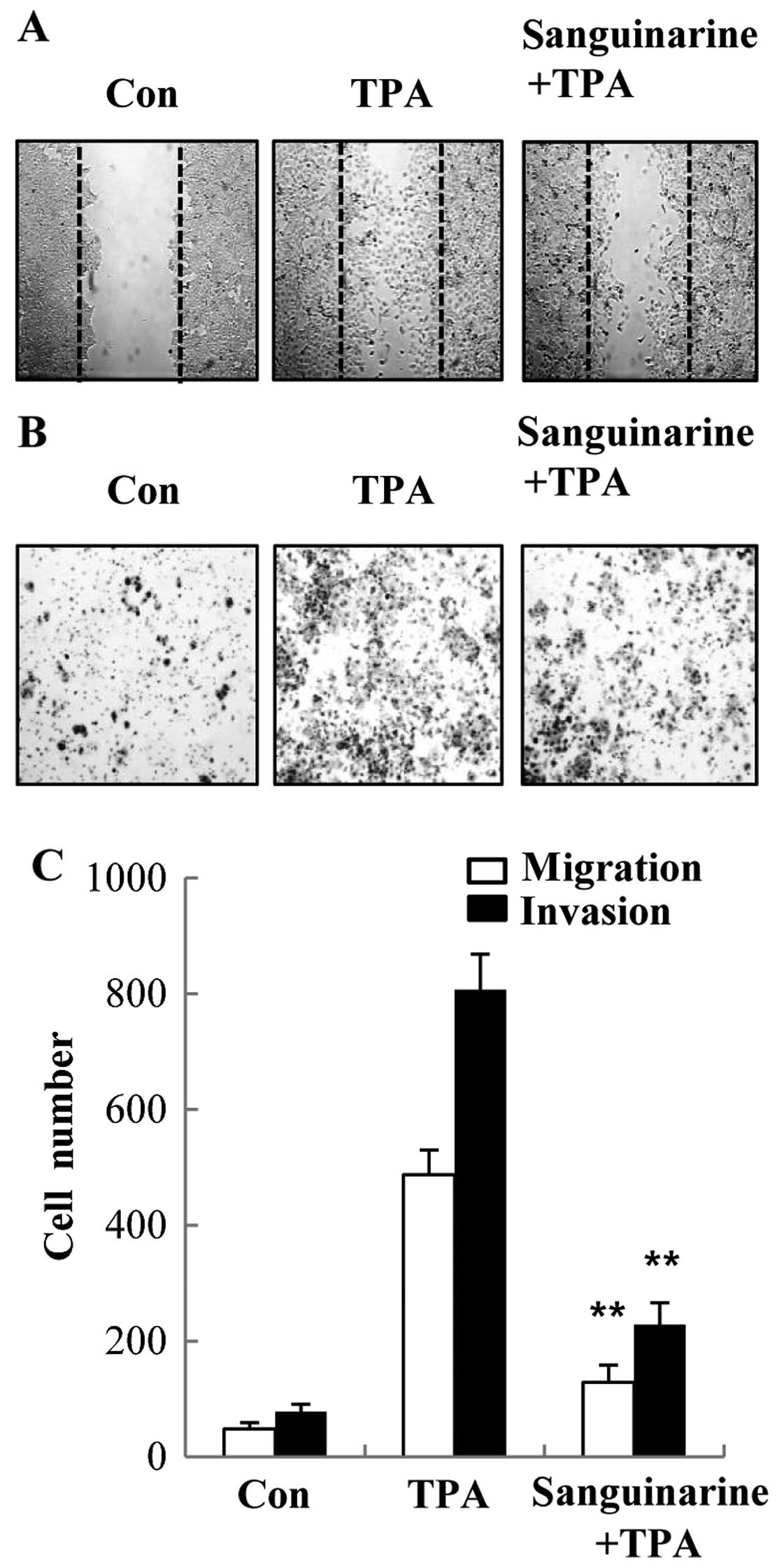
Sanguinarine inhibits TPA-induced
migration and invasion in human breast cancer cells
Metastasis is a complex and multi-step process and
is considered the leading cause of cancer-related mortality
(20). Since proteolytic digestion
of ECM and migration of cancer cells across the blood vessel-lining
endothelial monolayers play crucial roles in the metastatic
process, we investigated the effects of sanguinarine on the
invasive property of breast cancer cells. The inhibitory effect of
sanguinarine on migration of breast cancer cells was determined by
a wound-healing assay. The confluent (~90% of the maximum cell
density) monolayer was first scraped and then scratched using a
micropipette. Following incubation with sanguinarine, cells were
stimulated with TPA. The data presented in Fig. 4A indicate that sanguinarine
suppressed TPA-induced cell migration to the denuded area. To
further determine the effect of sanguinarine on the invasive
activity of human breast cancer cells, the Matrigel Transwell
invasion assay was used. The results showed that the number of
cells invading the lower chamber was dramatically reduced following
sanguinarine treatment when compared to the TPA-treated group in
MCF-7 cells (Fig. 4B). Thus, we
demonstrate that sanguinarine might be an effective agent in
preventing cell migration and invasion of MCF-7 cells.
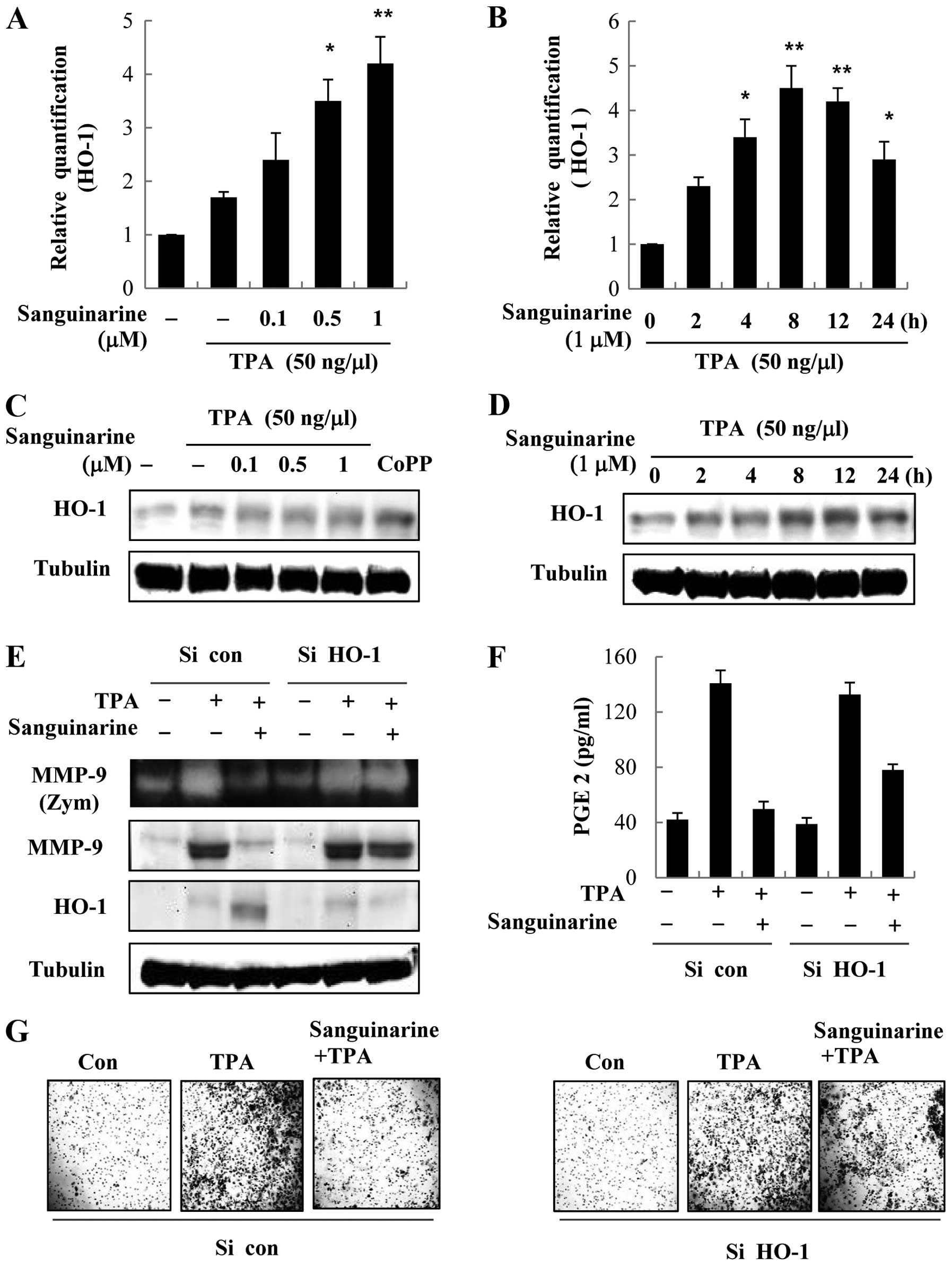
HO-1 knockdown abolishes the inhibitory
effect of sanguinarine on TPA-induced MMP-9 and COX-2 expression
and activity
We investigated whether sanguinarine suppresses
TPA-induced MMP-9 and COX-2 expression in breast cancer cells
through HO-1 expression. First, we examined the mRNA and protein
expression of HO-1 in the presence of sanguinarine in MCF-7 breast
cancer cells. As shown in Fig. 5A and
B, the expression of HO-1 mRNA and protein increased with
sanguinarine in a concentration-dependent manner in the presence of
TPA, and peaked at 8 h. To investigate the effects of HO-1
silencing, breast cancer cells were transiently transfected with
HO-1-siRNA, and the effects of sanguinarine on TPA-induced MMP-9
and COX-2 expression and activity were evaluated. As shown in
Fig. 5E and F, HO-1 silencing
abrogated HO-1 expression. HO-1 silencing blocked
sanguinarine-mediated suppression of TPA-induced MMP-9 and COX-2
expression and activity compared to control siRNA.
Sanguinarine-mediated inhibition of invasive activity was also
suppressed by knockdown of endogenous HO-1 in breast cancer cells
(Fig. 5G). In summary, these
results indicate that sanguinarine-dependent HO-1 expression plays
a crucial role in downregulation of MMP-9 and COX-2 expression and
activation.
Discussion
Recently, considerable attention has been given to
the use of natural products as novel effective anticancer agents in
humans (1,2). Sanguinarine is a benzophenanthridine
alkaloid with a wide range of preclinical antitumor activities in
hepatocellular carcinoma, prostate cancer, osteosarcoma cancer and
bladder cancer (13,15,16).
Although sanguinarine has been shown to exert antitumor effects in
various models, only a few studies have reported the antitumor
properties of sanguinarine and the mechanism by which sanguinarine
interacts with human breast cancer cells. Therefore, in the present
study, we examined the potential role of sanguinarine as a
chemotherapeutic agent, in particular during metastasis, using
MCF-7 human breast cancer cells.
Metastatic spread of cancer is accountable for ~90%
of cancer-related mortality in humans and remains a serious
obstruction to cancer treatment. Thus, inhibiting the metastatic
ability of cancer cells has become an important aspect in the
development of successful anticancer agents (3, 20). In
the present study, we aimed to explore the inhibitory effect of
sanguinarine on cell migration and invasion in TPA-induced human
breast cancer cells. TPA is a well-known tumor promoter that
activates most of the protein kinase C (PKC) isozymes by direct
binding. This activation results in a dramatic PKC-mediated
induction of tumor cell invasion (5). When human breast cancer cells were
treated with sanguinarine at non-toxic concentrations, cell
migration and invasion were suppressed. These data implied that the
suppressive activity of sanguinarine on cell migration and invasion
is not due to its cytotoxic effect. In the present study, we
demonstrated that sanguinarine exerts its inhibitory effect on
TPA-induced cell migration and invasion in association with the
inhibition of MMP-9 expression via the NF-κB and AP-1 signaling
pathways.
The induction of MMP-9 and COX-2 expression is
closely associated with tumor angiogenesis, invasion and
metastasis, and the suppression of MMP-9 and COX-2 expression plays
a crucial role in cancer therapy, since MMPs catalyze the
degradation of the ECM and trigger tumor invasion. MMP-9 and COX-2
are overexpressed in aggressive breast cancer and are relevant to
the clinical outcome (5,8,21). To
further investigate the mechanism of sanguinarine-induced
inhibition of cell migration and invasion, we conducted
experiments, including gelatin zymography and western blotting, to
determine the enzymatic activity and protein levels of MMP-9 and
COX-2, respectively. The results showed that the activity and
expression of MMP-9 and COX-2 were significantly reduced by
treatment with sanguinarine, whereas the expression of MMP-2 and
COX-1 were not affected. Accordingly, we suggest that the
suppressive effect of sanguinarine on cell migration and invasion
is related to the inhibition of enzyme-catalyzed degradation steps
in the tumor metastatic process.
Many transcription factors, including NF-κB and
AP-1, are involved in regulating the expression of MMP-9 and COX-2.
These transcription factors play pivotal roles in metastasis due to
their ability to induce the transcription of metastasis-related
genes, including MMP-9 and COX-2 (6,7). The
results of the present study also revealed that the anti-invasive
effects of sanguinarine are associated with the prevention of NF-κB
and AP-1 activation. Sanguinarine inhibits the nuclear
translocation and activation of NF-κB and AP-1. We also
investigated the upstream signaling pathways regulating TPA-induced
MMP-9 and COX-2 expression and activity. Akt and MAPKs are involved
in the expression of MMP-9 and COX-2. We examined whether
sanguinarine regulates the activity of MAPKs and found that
sanguinarine significantly inhibited Akt and ERK phosphorylation,
while having no influence on JNK and p38. These results suggest
that sanguinarine suppresses MMP-9 and COX-2 expression and
activity through the inhibition of NF-κB, AP-1, Akt and ERK in
breast cancer cells.
Growing evidence has demonstrated that HO-1 exhibits
anti-metastatic activity by inhibiting the expression of
pro-metastatic genes, thereby suggesting a potential therapeutic
strategy for treating breast cancer metastasis. Several studies
have shown an inverse correlation between HO-1 and pro-metastatic
genes (10,22). These results support a role for HO-1
expression as a negative regulator of pro-metastatic genes such as
MMP-9 and COX-2. In agreement with these reports, the results of
the present study demonstrated that sanguinarine induces the
expression of HO-1, providing a potential explanation for its
anti-invasive properties. We examined whether HO-1 expression
correlates with the inhibition of TPA-induced MMP-9 and COX-2
activities. Knockdown of HO-1 expression using siRNA markedly
reversed the inhibitory effects of sanguinarine on MMP-9 and COX-2
activity in TPA-induced breast cancer cells. Moreover, knockdown of
endogenous HO-1 in cells suppressed TPA-induced invasion compared
to the control siRNA knockdown. These results suggest that the
inhibition of breast cancer cell invasion by sanguinarine is
consistent with the inhibition of MMP-9 and COX-2 activity via HO-1
expression. Therefore, the anti-invasive effect of sanguinarine
might be related to the expression of HO-1 in breast cancer
cells.
In conclusion, we demonstrated that sanguinarine
inhibits MMP-9 and COX-2 expression and activity in TPA-stimulated
MCF-7 breast cancer cells. These effects are not only mediated by
the inhibition of the activities of NF-κB, AP-1, Akt and ERK, but
also by the induction of HO-1 expression. This is the first study
to demonstrate that sanguinarine suppresses MMP-9 and COX-2
activity by inducing HO-1 expression. The present study provides
new insights into the anti-invasive mechanisms of sanguinarine in
human breast cancer cells and presents evidence that sanguinarine
is a promising candidate for the prevention and treatment of human
breast cancer.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(2012R1A1A3010601).
References
|
1
|
Love RR and Koroltchouk V: Tamoxifen
therapy in breast cancer control worldwide. Bull World Health
Organ. 71:795–803. 1993.PubMed/NCBI
|
|
2
|
Agarwal R, Agarwal C, Ichikawa H, et al:
Anticancer potential of silymarin: From bench to bed side.
Anticancer Res. 26:4457–4498. 2006.PubMed/NCBI
|
|
3
|
Gillard JA, Reed MW, Buttle D, et al:
Matrix metalloproteinase activity and immunohistochemical profile
of matrix metalloproteinase-2 and −9 and tissue inhibitor of
metalloproteinase-1 during human dermal wound healing. Wound Repair
Regen. 12:295–304. 2004.
|
|
4
|
Jinga DC, Blidaru A, Condrea I, et al:
MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in
breast cancer: correlations with prognostic factors. J Cell Mol
Med. 10:499–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim S, Kim SH, Hur SM, et al: Silibinin
prevents TPA-induced MMP-9 expression by down-regulation of COX-2
in human breast cancer cells. J Ethnopharmacol. 126:252–257. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH, Lee KW, Lee MW, et al: Hirsutenone
inhibits phorbol ester-induced upregulation of COX-2 and MMP-9 in
cultured human mammary epithelial cells: NF-κB as a potential
molecular target. FEBS Lett. 580:385–392. 2006.PubMed/NCBI
|
|
7
|
Anand P, Sundaram C, Jhurani S, et al:
Curcumin and cancer: an ‘old-age’ disease with an ‘age-old’
solution. Cancer Lett. 267:133–164. 2008.
|
|
8
|
Simeone AM, Nieves-Alicea R, McMurtry VC,
et al: Cyclooxygenase-2 uses the protein kinase
C/interleukin-8/urokinase-type plasminogen activator pathway to
increase the invasiveness of breast cancer cells. Int J Oncol.
30:785–792. 2007.PubMed/NCBI
|
|
9
|
Otterbein LE and Choi AM: Heme oxygenase:
Colors of defense against cellular stress. Am J Physiol Lung Cell
Mol Physiol. 279:L1029–L1037. 2000.PubMed/NCBI
|
|
10
|
Farombi EO and Surh YJ: Heme oxygenase-1
as a potential therapeutic target for hepatoprotection. J Biochem
Mol Biol. 39:479–491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jozkowicz A, Was H and Dulak J: Heme
oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal.
9:2099–2117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye F, Feng F and Liu W: Alkaloids from
Macleaya cordata. Zhongguo Zhong Yao Za Zhi. 34:1683–1686.
2009.(In Chinese).
|
|
13
|
Pang JX, Ma RQ, Liu LM, et al: Total
alkaloid of Macleaya cordata: In vitro cytotoxic effect on
Hep3B cells and in vivo antitumor effect in mice. Di Yi Jun Yi Da
Xue Xue Bao. 25:325–328. 2005.(In Chinese).
|
|
14
|
Luo XB, Chen B and Yao SZ: Rapid
determination of protopine, allocryptopine, sanguinarine and
chelerythrine in fruits of Macleaya cordata by
microwave-assisted solvent extraction and HPLC-ESI/MS. Phytochem
Anal. 17:431–438. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cornblatt BS, Ye L, Dinkova-Kostova AT, et
al: Preclinical and clinical evaluation of sulforaphane for
chemoprevention in the breast. Carcinogenesis. 28:1485–1490. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jang BC, Park JG, Song DK, et al:
Sanguinarine induces apoptosis in A549 human lung cancer cells
primarily via cellular glutathione depletion. Toxicol In Vitro.
23:281–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim S, Lee TJ, Leem J, Choi KS, et al:
Sanguinarine-induced apoptosis: Generation of ROS, down-regulation
of Bcl-2, c-FLIP, and synergy with TRAIL. J Cell Biochem.
104:895–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson KD and Bresnick EH: Dissecting
long-range transcriptional mechanisms by chromatin
immunoprecipitation. Methods. 26:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simeone AM, McMurtry V, Nieves-Alicea R,
et al: TIMP-2 mediates the anti-invasive effects of the nitric
oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer
Res. 10:R442008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Formenti S, Felix J, Salonga D, et al:
Expression of metastases-associated genes in cervical cancers
resected in the proliferative and secretory phases of the menstrual
cycle. Clin Cancer Res. 6:4653–4657. 2000.PubMed/NCBI
|
|
21
|
Larkins TL, Nowell M, Singh S and Sanford
GL: Inhibition of cyclooxygenase-2 decreases breast cancer cell
motility, invasion and matrix metalloproteinase expression. BMC
Cancer. 6:1812006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill M, Pereira V, Chauveau C, et al: Heme
oxygenase-1 inhibits rat and human breast cancer cell
proliferation: mutual cross inhibition with indoleamine
2,3-dioxygenase. FASEB J. 19:1957–1968. 2005. View Article : Google Scholar : PubMed/NCBI
|