Introduction
Liver cancer is the fifth most common cancer
worldwide and the third most common cause of cancer mortality
(1,2). Hepatocellular carcinoma (HCC), which
accounts for 80–90% of primary liver tumors, is characterized by a
poor prognosis and high mortality (1,2).
Despite therapeutic advances, the overall survival of patients with
HCC has not significantly improved in the last two decades.
Surgical resection, radiofrequency ablation (RFA) and percutaneous
ethanol injection (PEI) are accompanied by high recurrence rates
(3–5). Transarterial chemoembolization (TACE),
the most commonly used non-surgical alternative, is usually
reserved for palliative treatment (6), and chemotherapy and radiotherapy
generally offer unsatisfactory response rates. Thus, new
therapeutic strategies are urgently required for the treatment of
HCC.
Vitexins are a group of complex polyphenolic
antioxidants found in plants. Vitexin inhibits α-glucosidase,
restrains rotavirus infection and has significant protective
effects against CCl4-induced hepatotoxicity (7–9).
Several vitexins have been shown to induce apoptosis of human
leukemia and colon cancer cells (10,11).
In previous studies, we demonstrated that a mixture of vitexin
lignans, known as EVn-50, exhibits obvious in vitro and
in vivo anticancer activity against hormone-dependent cancer
and non-hormone dependent cancer such as breast, prostate, ovarian,
gastric cancer and choriocarcinoma via a possible mechanism of
promoting apoptosis of cancer cells (12). The purified vitexin compound 1 (VB1,
a neolignan compound), which is the most abundant Vitexin compound
in EVn-50, has potent cytotoxic effects in several cancer cell
lines, including human HCC cells (13,14).
The broad-ranging antitumor effects and cytotoxicity of VB1 may be
mediated by alternation of Bax/Bcl-2 ratio in favor of Bax, by
activation of caspases, and by suppression of the mTOR pathway
(13,14). These studies strongly suggest that
VB1 can be developed as a cancer preventive agent. However, to
date, no studies address the effects of VB1 on the induction of
apoptosis in HCC cells.
The PI3K/AKT and MEK/ERK pathways have profound
effects on proliferative, apoptotic and differentiation pathways.
Dysregulation of components of these cascades can contribute to
resistance to other pathway inhibitors, chemotherapeutic drug
resistance as well as other diseases (15). Overexpression of AKT and ERK have
been reported in several types of human cancer, including HCC, and
cells expressing elevated levels of AKT and ERK are less sensitive
to apoptosis stimuli (16–18). Other well known risk factors for HCC
such as HBV and HCV infection also seem to utilize the AKT and ERK
pathways for the control of hepatocyte survival and viral
replication (19). Furthermore,
activation of AKT and ERK signaling pathways predicts poor
prognosis in HCC (17), supporting
the participation of activated PI3K/AKT and MEK/ERK axes in this
disease.
FOXO3a is a member of the forkhead/winged helix box
class O (FOXO) transcription factors that participates in a variety
of cellular processes such as cell cycle progression, stress,
detoxification, DNA damage repair, glucose metabolism and
differentiation (20). During tumor
development, inhibition of FOXO3a-induced transcriptional activity
promotes cell transformation, tumor progression and angiogenesis
(21–23). A number of kinases have been shown
to suppress FOXO activity. These include phosphoinositide AKT
(24) and extracellular
signal-regulated kinases 1 and 2 (ERK1/2) (25). However, whether VB1 induces
apoptosis in HCC cells by regulating FOXO3a transcription factor
through inhibition of AKT and ERK1/2 kinases has yet to be
confirmed.
In the present study, we demonstrated that the
selective cytotoxicity of VB1 in HCC cells was accompanied by
inhibition of AKT and ERK1/2 kinase phosphorylation, activation of
FOXO3a transcription factor and apoptotic death in HepG2 cells.
Furthermore, knockdown of AKT by siRNA or PD98059 enhanced
VB1-induced FOXO3a transcriptional activity. These data suggest
that VB1 induces apoptosis by activating FOXO3a transcription
factor and that this is most likely induced by inhibition of AKT
and ERK1/2 phosphorylation.
Materials and methods
Reagents
VB1
[6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydro-methyl-7-methoxy-3,4-dihydro-2-naphthaldehyde]
was purified from EVn-50 (a mixture of lignan compounds obtained
from Vitex negundo seed) as previously described (13). 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was
purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Fluorouracil (5-FU) was purchased from Sigma Chemical Co. The cell
apoptosis enzyme-linked immunosorbent assay (ELISA) detection kit
was obtained from Roche Applied Science, Mannheim, Germany. The
Apoptotic DNA Ladder Detection kit was obtained from the Bodataike
Company, Beijing. The Caspase-3 Activity Detection kit, Caspase-8
Colorimetric Activity Assay kit 25, and Caspase-9 Colorimetric
Activity Assay kit were purchased from Millipore, Billerica, MA,
USA. Caspase inhibitors, such as zVADfmk, zDEVD-fmk, zIETD-fmk and
zLEHD-fmk were purchased from R&D Systems (Minneapolis, MN,
USA). Propidium iodide (PI) was purchased from Sigma Chemical Co.
Antibodies against phospho-AKT, AKT, phospho-ERK1/2, ERK1/2, Bim,
TRAIL, DR5, DR4, phosphor-FOXO3a, FOXO3a and β-actin were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Enhanced
chemiluminescence (ECL) western blotting detection reagents were
provided by Amersham Life Sciences Inc. (Arlington Heights, IL,
USA).
Cell lines and cell culture
techniques
Human HCC cell lines Hep3B, Huh-7 and HepG2 and
human embryo liver L-02 cells were purchased from the China Centre
for Type Culture Collection (CCTCC, Wuhan, China). The cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; Life
Technologies, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS) (Invitrogen), 100 U/ml penicillin and 100 U/ml
streptomycin, and cultured in a humidified atmosphere at 37°C with
5% CO2.
MTT assay
Cells were seeded in a 96-well plate at a density of
0.5×104 cells/well and maintained in serum-free medium
for 24 h. This was followed by exposure to various concentrations
of experimental agents that were added to each well and cultured
for 24 h, prior to incubation with media containing 5 mg/ml MTT for
4 h. The cell suspension was then centrifuged and 100 μl DMSO was
added to the resulting supernatant. Absorbance at the 570 nm
wavelength (A570) was measured using an enzyme-labeling
instrument (ELX-800 type; BioTek, Shanghai, China). Relative cell
viability inhibition rate was calculated as: (average
A570 of the experimental group/average A570
of the control group) × 100%.
DNA analysis
DNA content was analyzed by flow cytometry. Cancer
cells with or without prior exposure to VB1 were collected by
centrifugation and adjusted to 3×106 cells/ml.
Pre-chilled absolute methanol was added to 0.5 ml of cell
suspension and incubated overnight at 4°C. The methanol was removed
by centrifugation. DNA was stained with PI staining solution (100
μg/ml PI, 0.1% Triton X, 1 mM EDTA in PBS) in the presence of an
equal volume of DNase-free RNase (200 μg/ml). DNA was analyzed
immediately using a Partec CyFlow ML cytometer (FACS 420;
Becton-Dickinson, USA). Debris and clumps were excluded by
monitoring DNA area (FL3-A) and peaks (i.e. signal height, FL3-W).
Fluorescence signals from 10,000 gated cells were collected.
Histone/DNA ELISA detection of
apoptosis
An ELISA kit was used to detect apoptosis in cells
treated with VB1 according to the manufacturer's protocol. Briefly,
cells were seeded in a 96-well plate at a density of
1×104 cells/well for 24 h. The testing agents were then
added to culture medium containing 10% FBS. After 24 h, the
cytoplasm of the control and experimental groups was transferred to
a 96-well plate pre-coated with streptavidin that had been
previously incubated with the biotinylated histone antibody and
peroxidase-tagged mouse anti-human DNA for 2 h at room temperature.
Absorbance was measured at 405 nm, using an EXL-800-type
enzyme-linked immunosorbent apparatus (BioTek).
DNA agarose gel electrophoresis
The cells maintained in serum-free medium for 24 h
were exposed to media containing various concentrations of
experimental agents for 48 h. The cells were washed twice with PBS,
and DNA was extracted using an apoptotic DNA ladder detection kit
according to the manufacturer's instructions. The extracted DNA was
maintained at 4°C overnight. The DNA (8.5 μl) was then mixed with
1.5 μl of 6X buffer solution and electrophoresed on a 20 g/l
agarose gel containing ethidium bromide at 40 V. The results were
observed using a DBT-08 gel image analysis system.
Analysis of caspase-3, −8 and −9
activities
The activity of caspase-3, −8 and −9 was evaluated
using a Caspase-3 Activity Detection kit, Caspase-8 Colorimetric
Activity Assay kit 25 and Caspase-9 Colorimetric Activity Assay
kit, respectively. Briefly, cell lysates were prepared after
exposure to experimental agents. The assays were performed in
96-well plates by incubating 20 μg of cell lysates in 100 μl of
reaction buffer [1% NP-40, 20 mM Tris-HCl (pH 7.5), 137 mM NaCl,
10% glycerol] containing 5 μM of the caspase-3 substrate
Ac-DEVD-pNA, the caspase-8 substrate Ac-IETD-pNA or
the caspase-9 substrate Ac-LEHD-pNA. Lysates were incubated
at 37°C for 2 h. Thereafter, absorbance at 405 nm was measured with
an enzyme-labeling instrument (ELX-800 type). In the caspase
inhibitor assays, cells were exposed to caspase inhibitors (i.e. 20
μM zVAD-fmk, zDEVD-fmk, zIETD-fmk or zLEHD-fmk) for 1 h prior to
the addition of the agents tested.
Western blot analysis
Western blotting was performed as previously
described (1). In brief, cells were
lysed in RIPA buffer containing 1X protease inhibitor cocktail, and
protein concentrations were determined using the Bradford assay
(Bio-Rad, Philadelphia, PA, USA). Proteins were separated using
10–12.5% SDS-PAGE and transferred to membranes (Millipore, Bedford,
MA, USA) in a Tris (20 mM), glycine (150 mM) and methanol (20%)
buffer at 55 V for 4 h at 4°C. After blocking in 5% nonfat dry milk
in TBS, the membranes were incubated with primary antibodies at
1:1,000 dilution in TBS overnight at 4°C. They were then washed 3
times with TBS Tween-20, and incubated with secondary antibodies
(conjugated with horseradish peroxidase at 1:5,000 dilution in TBS)
for 1 h at room temperature. The membranes were washed again 3
times in TBS Tween-20 at room temperature. Protein bands were
visualized on X-ray film using an enhanced chemiluminescence
detection system.
DNA transfection
The small interfering RNA (siRNA) duplexes targeting
the sequence 5′-UAAUGUGCCCGUCCU UGUCUU-3′ of the human AKT1 gene,
the sequence 5′-ACUCCGGGUCCAGCUCCAC-3′ of the FOXO3A gene and
control siRNA oligonucleotides were purchased from Dharmacon
Research, Inc. (Lafayette, CO, USA). To silence Akt or FOXO3a,
HepG2 cells were transfected with double stranded siRNA of Akt or
FOXO3a using Signal Silence siRNA kit from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Briefly, 106 cancer
cells were plated in a 60-mm petri dish for 24 h in 3 ml of
transfection medium containing 20 μg Lipofectamine and 100 nM siRNA
for 24 h. Gene silencing in transfected cells was confirmed by
western blotting.
Statistical analysis
Statistical analysis was performed using PRISM
statistical analysis software (GrafPad Software, Inc., San Diego,
CA, USA). Data are presented as means and standard deviations ( ±
SD). Differences between groups were analyzed by one- or two-way
analysis of variance (ANOVA), followed by Bonferoni's multiple
comparison tests. Values of P<0.05 were considered to indicate
statistically significant differences.
Results
Effects of VB1 on the proliferation of
human HCC cells
Since VB1 significantly inhibited the growth of
several cancer cell lines, including HCC cells (13), we first examined the effects of VB1
on cell viability in 3 HCC cell lines HepG2, Hep3B and Huh-7, using
the MTT assay. Fig. 1 shows that
VB1 inhibited cell viability in a concentration-dependent manner.
The HepG2 cell line was the most sensitive, the Hep3B cell line was
moderately sensitive and the Huh-7 cell line was the least
sensitive. VB1 had little effect on the human embryo liver L-02
cell line. These data suggest that VB1 is able to inhibit HCC cell
growth.
Effects of VB1 on apoptosis of HepG2
cells
VB1 has been reported to induce apoptosis in breast
cancer cell lines (13). We,
therefore, investigated apoptosis using flow cytometric analysis to
detect increases in hypodiploid cell populations. Fig. 2A shows that VB1 increased the
percentage of the sub-G1 population of HepG2 cells in a
concentration-dependent manner (P<0.05). We also showed that the
histone/DNA fragment of HepG2 cells increased (P<0.05) in a
dose-dependent manner following exposure to VB1 (Fig. 2B). DNA fragmentation analysis by
agarose gel electrophoresis showed a typical ladder pattern of
inter-nucleosomal DNA fragments in HepG2 cells exposed to VB1 (5 or
10 μmol/l) for 24 h (Fig. 2C).
These results suggest that VB1 inhibits HCC cell growth, in part
through a mechanism involving the induction of apoptosis.
Effects of VB1 on caspase activities of
HepG2 cells
Most chemopreventive agents induce apoptosis through
the mitochondrial pathway. To determine the effectors involved in
VB1-induced apoptotic pathways, we examined whether caspases were
activated during VB1-induced apoptotic death of HepG2 cells.
Fig. 3A,C and E show that exposure
of HepG2 cells to VB1 increased the levels of active caspase-3, −8
and −9 in a concentration-dependent manner (P<0.05).
We next examined the effects of pan-caspase
inhibitor zVAD-fmk, the caspase-3 inhibitor zDEVD-fmk, the
caspase-8 inhibitor zIETD-fmk and the caspase-9 inhibitor zLEHD-fmk
on VB1-induced apoptosis. The results in Fig. 3B,D and F show that zVAD-fmk
abrogated apoptosis induced by VB1, and that zDEVD-fmk zIETD-fmk
and zLEHD-fmk attenuated VB1-induced apoptosis. These data indicate
that VB1-induced apoptosis was dependent on the activation of
caspase-3, −8 and −9.
Effects of VB1 on the phosphorylated
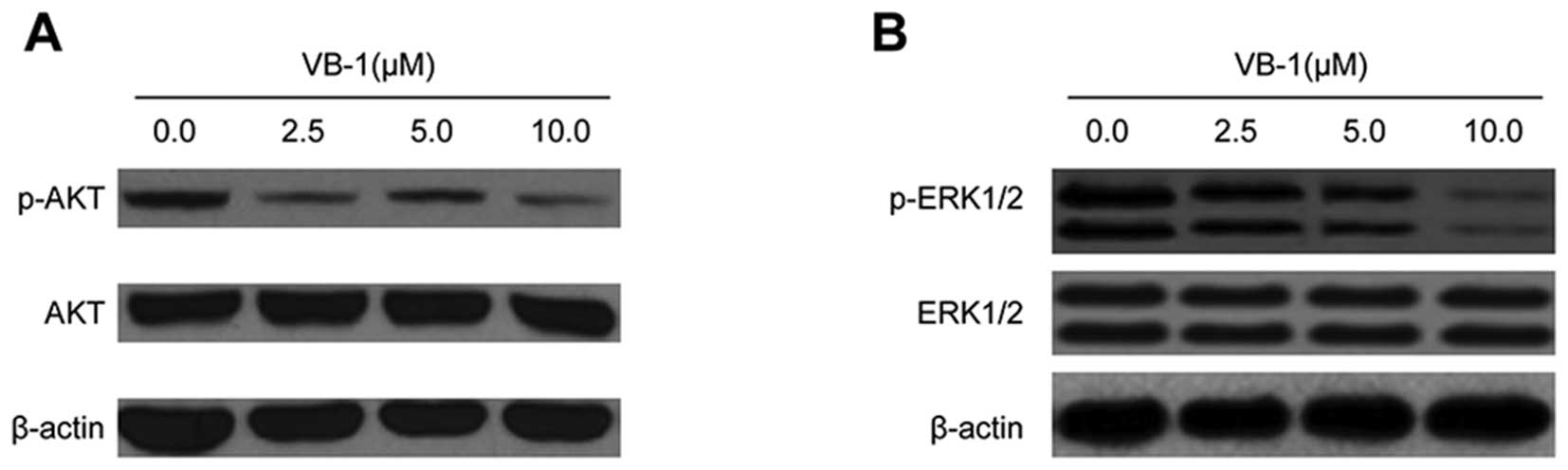
levels of AKT and ERK1/2 proteins in the HCC cell line HepG2
AKT is constitutively active in most cancer cells
and promotes cell survival and apoptosis resistance (24). We, therefore, examined the effects
of VB1 on AKT phosphorylation. HepG2 cells were exposed to VB1, and
phosphorylation of AKT was examined by western blot analysis. VB1
significantly inhibited AKT phosphorylation in HepG2 cells, but had
no effect on total expression of AKT (Fig. 4A). These data suggest that VB1 can
inhibit the phosphorylation of PI3K/AKT proteins, which may play a
major role in mediating its anti-apoptotic effects.
ERK1/2 kinase regulates cellular activities ranging
from gene expression to mitosis, movement, metabolism and apoptosis
(25). Hence, we examined the
effects of VB1 on the activation of ERK1/2 kinases. Fig. 4B shows that exposure of HepG2 cells
to VB1 resulted in a decrease in ERK phosphorylated protein. These
results suggest that VB1 may be involved in the regulation of
ERK1/2 kinase activity.
Inhibition of AKT and ERK1/2 kinases
enhances VB1-induced apoptosis
We used a siRNA that specifically silences AKT to
investigate the ability of AKT to induce apoptosis by VB1.
Expression of siRNAs has previously been shown to silence gene
expression resulting in functional inactivation of the targeted
gene (26,27). Western blot analysis showed that AKT
was downregulated after transfection with specific siRNA targeting
AKT in HepG2 cells (Fig. 5A). The
results in Fig. 5B show that
inhibition of AKT by siRNA increased VB1-induced apoptosis in HepG2
cells. These results suggest that VB1-induced AKT inhibition may
contribute to apoptosis of HepG2 cells.
 | Figure 5Effects of the knockdown of AKT1 and
by siRNA and MEK1/2 inhibitor (PD98059) on apoptosis in HepG2 cells
in the presence or absence of VB1. (A) HepG2 cells were transfected
with 100 nM of siRNA control or the siRNA duplexes against AKT
mRNA. Twenty-four hours after the transfection, the cells were
exposed to 5 μM VB1 for 24 h. Western blotting of p-AKT, AKT was
performed to confirm the downregulation of AKT by siRNA
transfection. β-actin was used as the loading control. (B) HepG2
cells were transfected with 100 nM of siRNA control or the siRNA
duplexes against AKT mRNA. Twenty-four hours after the
transfection, the cells were exposed to the indicated concentration
of VB1 for 24 h. Histone/DNA fragment of HepG2 cells were measured
by the cell apoptosis ELISA detection kit. Data shown are means ±
SD. (n=3). *P<0.05, **P<0.01,
***P<0.001 vs. 0.1% DMSO or 0 h.
#P<0.05 vs. the same concentration of VB1 in
combination with siRNA control transfection. (C) HepG2 cells were
exposed to 5 μM VB1 for 24 h in the presence or absence of 20 μM
PD98059. Western blotting of p-ERK1/2, total-ERK1/2 and the loading
control β-actin was undertaken. (D) HepG2 cells were exposed to the
indicated concentrations of VB1 for 24 h in the presence or absence
of 20 μM PD98059. Histone/DNA fragment of HepG2 cells was measured
using a cell apoptosis ELISA detection kit. Data shown are means ±
SD. (n=3). *P<0.05, **P<0.01,
***P<0.001 vs. 0.1% DMSO or 0 h.
#P<0.05 vs. the same concentration of VB1 alone. VB1,
purified vitexin compound 1; siRNA, small interfering RNA. |
We next examined whether VB1 induces apoptosis
through inhibition of ERK1/2. HepG2 cells were exposed to 5 μM VB1
for 24 h in the presence or absence of 20 μM PD98059. The MEK1/2
inhibitor (PD98059) inhibited ERK1/2 phosphorylation and enhanced
VB1-induced apoptosis in HepG2 cells (Fig. 5C and D). These data suggest that
VB1-induced apoptosis may be mediated by the MEK/ERK pathway, and
that inhibition of the MEK/ERK pathway enhances VB1-induced
apoptosis.
Inhibition of AKT and ERK1/2 kinases
enhances VB1-induced FOXO3a transcriptional activity
We next examined whether inhibition of AKT and
ERK1/2 kinases regulates FOXO3a activity in the presence or absence
of VB1. Knockdown of AKT by siRNA and MEK1/2 inhibitor (PD98059)
inhibited the phosphorylation of FOXO3a in the presence or absence
of VB1. Furthermore, knockdown of AKT by siRNA or PD98059 enhanced
VB1-induced FOXO3a transcriptional activity (Fig. 6A and B). These data suggest that
inhibition of AKT or ERK1/2 kinases acts synergistically with VB1
to induce FOXO3a transcriptional activity in the HCC cell line
HepG2.
 | Figure 6Effects of inhibition of AKT and
ERK1/2 kinases on VB1-induced FOXO3a transcriptional activation.
(A) HepG2 cells were transfected with 100 nM of siRNA control or
the siRNA duplexes against AKT mRNA. Twenty-four hours after
transfection, the cells were treated with 5 μM VB1 for 24 h.
Western blotting of AKT, p-FOXO3a and FOXO3a was performed to
confirm the effects of the downregulation of AKT by siRNA
transfection. (B) HepG2 cells were pretreated with 20 μmol/l
PD98059. Two hours after incubation, the cells were treated with 5
μM VB1 for 24 h. Western blotting of p-ERK1/2, ERK1/2, p-FOXO3a and
FOXO3a was performed to confirm the effects of the inhibition of
ERK1/2. (C) HepG2 cells were transfected with 100 nM of siRNA
control or the siRNA duplexes against FOXO3a mRNA. Twenty-four
hours after transfection, the cells were treated with 5 μM VB1 for
24 h. Western blotting of FOXO3a, p-AKT, AKT, p-ERK1/2 and ERK1/2
was carried out to confirm the effects of the downregulation of
FOXO3a by siRNA transfection. VB1, purified vitexin compound 1;
siRNA, small interfering RNA. |
We next sought to examine the effects of FOXO3a
transcription factor on the regulation of AKT and ERK1/2 kinases.
We used a specific siRNA targeting FOXO3a in HepG2 cells.
Downregulation of FOXO3a by siRNA has no effect on the expression
of p-AKT, AKT, p-ERK1/2 and ERK1/2. Effects of VB1 on the
phosphorylated levels of AKT and ERK1/2 proteins in the HCC cell
line HepG2 were not reversed by the inhibition of FOXO3a
transcriptional activity (Fig. 6C).
These data suggest that the FOXO3a protein is a downstream mediator
of AKT and ERK1/2 kinases.
Effects of FOXO3a transcription factor on
the regulation of pro-apoptotic effects of VB1
AKT and ERK1/2 kinase have been shown to regulate
the phosphorylation of FOXO3a protein (24,25).
We measured the phosphorylation of FOXO3a protein using western
blot analysis. As shown in Fig. 6A,
VB1 inhibited the phosphorylation of FOXO3a protein. However, VB1
had no effect on the expression of total FOXO3a protein (Fig. 7A).
Western blot analysis showed that FOXO3a was
downregulated after transfection with specific siRNA targeting
FOXO3a in HepG2 cells (Fig. 7B).
The results also indicated that inhibition of FOXO3a expression by
siRNA inhibited VB1-induced apoptosis (Fig. 7C). These data suggest that
VB1-induced apoptosis may be mediated by regulation of FOXO3a, and
that inhibition of FOXO3a inhibits VB1-induced apoptosis.
Effects of VB1 on the expression of
FOXO3a downstream apoptosis-associated target genes
It has been reported that activation of FOXO3a
results in upregulation of the apoptosis-associated target gene
products Bim, TRAIL, DR4 and DR5 proteins (20). We, therefore, examined the effect of
VB1 on the expression of Bim, TRAIL, DR4 and DR5 proteins. These
genes are direct targets of FOXO3a transcription factor. The
results indicated that VB1 induced the expression of Bim, TRAIL,
DR4 and DR5 proteins (Fig. 8A–D).
These data further suggest that VB1-induced apoptosis in HepG2
cells is mediated through mechanisms that involve the activation of
FOXO3a transcription factor.
Discussion
In the present study, we demonstrated that
VB1-induced apoptosis in HCC cancer cells appears to be mediated
through inhibition of AKT and ERK1/2 kinases and activation of
FOXO3a transcription factor. Our results showed that inhibition of
AKT and ERK1/2 kinases increased VB1-induced apoptosis, and that
inhibition of FOXO3a transcription factor by siRNA blocked
VB1-induced apoptosis. We also showed that VB1 upregulated Bim,
TRAIL, DR4 and DR5. These findings indicate that VB1 induces
apoptosis by inhibiting AKT and ERK1/2 kinases which results in
activation of FOXO3a transcription factor in HCC cells. Apoptotic
cells share a number of common characteristics. Caspase-3
activation, an enhanced sub-G1 cell population, histone/DNA
fragmentation and DNA ladders are regarded as specific indicators
of apoptosis. In the present study, we demonstrated activation of
caspase-3, an increase in the sub-G1 population, histone/DNA
fragments and the presence of a DNA ladder in VB1-treated HepG2
cells, thus demonstrating that VB1 induces HCC cell apoptosis.
Overall, these properties of VB1 strongly suggest that it may be
used as a cancer chemopreventive agent.
Caspase activation is known to play an important
role in apoptosis triggered by various pro-apoptotic signals
(28,29). It is generally recognized that there
are two major apoptotic pathways: one involves the transduction of
death signals through death receptors, and the other involves
mitochondrial signaling (28,30).
Both pathways require ordered activation of a set of caspases,
which cleave cellular substrates resulting in the morphological and
biochemical changes that characterize apoptosis. Activation of
caspase-8 and −9, have respectively been shown to play a central
role in mediating apoptosis signaled by death receptors and by
mitochondria. In the present study, we demonstrated that VB1 was
able to activate caspase-3 −8 and −9 activity. The presence of the
caspase inhibitor attenuated apoptosis induced by VB1, indicating
that VB1-induced apoptosis was essentially dependent on the
activation of caspase-3, −8 and −9.
Forkhead O transcription factors (FOXO) factors have
been shown to be dysregulated in a variety of tumor types,
including HCC, owing to the constitutive activation of AKT and
ERK1/2 kinases (31,32). FOXOs are regulated by synthesis,
phosphorylation, acetylation and ubiquitination at three different
levels involving transcriptional activity, subcellular localization
and stability (33). AKT and ERK
kinases are thought to phosphorylate FOXO3a at different sites in
response to growth factor and insulin stimulation. Phosphorylation
of FOXO3a by these oncogenic kinases results in its translocation
from the nucleus to cytoplasm where it is subsequently degraded
(34,35). Previous studies have shown that
FOXO3a is at least one of the missing links connecting the two
pathways. Inhibition of the PI3K/Akt/mTOR and MEK/ERK pathways has
been shown to result in loss of phosphorylation at Akt and ERK
phosphorylation sites, accompanied by nuclear accumulation and
increased transcriptional activity of FOXO3a (35). In the present study, we demonstrated
that inhibition of AKT and ERK1/2 kinases acts synergistically to
regulate apoptotic effects of VB1 through activation of FOXO3a
transcription factor. VB1 inhibited the accumulation of
phosphorylated FOXO3a protein.
FOXO factors are also critical for the regulation of
cell cycle arrest, cell death and DNA damage repair. Consequently,
inactivation of FOXO proteins has been shown to be associated with
tumorigenesis in breast and prostate cancer, glioblastoma, leukemia
and hepatocellular carcinoma (20,36).
The FOXO subfamily contains 4 members (FOXO1, FOXO3, FOXO4 and
FOXO6), which activate or repress multiple genes such as Bim,
TRAIL, DR4, DR5 and survivin that are involved in apoptosis
(37–41). Our findings demonstrate that VB1
regulates the expression of FOXO3a apoptosis-associated target
genes. We also showed that inhibition of FOXO3a by siRNA blocked
the apoptotic effects of VB1. These results suggest that FOXO3a
transcription factor mediated the anti-proliferative and
pro-apoptotic effects of VB1.
Previous studies indicated that the effects of
anticancer agents may be regulated through activation of FOXO
transcription factors (41–43). In addition to their effects on cell
cycle, apoptosis, differentiation and reactive oxygen species, FOXO
proteins have also been implicated in the negative regulation of
angiogenesis. For example, resveratrol and ECGG inhibit
angiogenesis through activation of FOXO (44,45).
Collectively, these findings demonstrate that dephosphorylation and
activation of FOXO by inhibition of AKT and ERK kinases may have
significant implications for the treatment and prevention of, among
others, diabetic complications, cardiovascular diseases and
malignant neoplastic disease.
In summary, our results suggest that VB1 induces
apoptosis through regulation of FOXO3a transcription factor in
human HCC cells. Pharmacological and genetic inhibition of AKT and
ERK1/2 kinases may act synergistically with VB1 to induce FOXO3a
transcriptional activity which are mediated by dephosphorylation.
Although a variety of clinical and in vivo studies are
required to explore the therapeutic potential of VB1, our present
study, together with our previous studies, indicates that VB1 may
be a potential agent for further development in the prevention and
treatment of HCC as well as other types of cancer.
Acknowledgements
The authors thank Dr Jian-Guo Cao (Medical College,
Hunan Normal University, Changsha, Hunan, China) for the critical
input into the manuscript. The present study was supported by Major
State Science and Technology Special Purpose of China
(2009ZX09102-109), the National Science and Technology Major
Projects for ‘Major New Drugs Innovation and Development’
(2012ZX09303014001), the International Science and Technology
Cooperation Program of China (2011DFA30620), and the Hunan Province
Science and Technology Project (2009FG3142).
References
|
1
|
Zhu AX: Molecularly targeted therapy for
advanced hepatocellular carcinoma in 2012: current status and
future perspectives. Semin Oncol. 39:493–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
3
|
Padma S, Martinie JB and Iannitti DA:
Liver tumor ablation: percutaneous and open approaches. J Surg
Oncol. 100:619–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ichida T, Van Thiel DH and Hassanein T:
The medical management of hepatocellular carcinoma (HCC) in Japan:
a review with implications for HCC seen in the west.
Hepatogastroenterology. 43:1575–1583. 1996.PubMed/NCBI
|
|
5
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases.
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
6
|
Llovet JM, Real MI, Montaña X, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: a
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar
|
|
7
|
Choo CY, Sulong NY, Man F and Wong TW:
Vitexin and isovitexin from the leaves of Ficus deltoidea
with in-vivo α-glucosidase inhibition. J Ethnopharmacol.
142:776–781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knipping K, Garssen J and van't Land B: An
evaluation of the inhibitory effects against rotavirus infection of
edible plant extracts. Virol J. 9:1372012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sahreen S, Khan MR and Khan RA:
Hepatoprotective effects of methanol extract of Carissa
opaca leaves on CCl4-induced damage in rat. BMC
Complement Altern Med. 11:482011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee CY, Chien YS, Chiu TH, et al:
Apoptosis triggered by vitexin in U937 human leukemia cells via a
mitochondrial signaling pathway. Oncol Rep. 28:1883–1888.
2012.PubMed/NCBI
|
|
11
|
Papi A, Farabegoli F, Iori R, et al:
Vitexin-2-O-xyloside, raphasatin and (−)-epigallocatechin-3-gallate
synergistically affect cell growth and apoptosis of colon cancer
cells. Food Chem. 138:1521–1530. 2013.PubMed/NCBI
|
|
12
|
Xin H, Kong Y, Wang Y, et al: Lignans
extracted from Vitex negundo possess cytotoxic activity by
G2/M phase cell cycle arrest and apoptosis induction.
Phytomedicine. 20:640–647. 2013.
|
|
13
|
Zhou Y, Liu YE, Cao J, et al: Vitexins,
nature-derived lignan compounds, induce apoptosis and suppress
tumor growth. Clin Cancer Res. 15:5161–5169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan Z and Zhang Y, Deng J, Zeng G and
Zhang Y: Purified vitexin compound 1 suppresses tumor growth and
induces cell apoptosis in a mouse model of human choriocarcinoma.
Int J Gynecol Cancer. 22:360–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCubrey JA, Steelman LS, Chappell WH, et
al: Mutations and deregulation of Ras/Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR cascades which alter therapy response.
Oncotarget. 3:954–987. 2012.PubMed/NCBI
|
|
16
|
Muntané J, De la Rosa AJ, Docobo F,
Garcia-Carbonero R and Padillo FJ: Targeting tyrosine kinase
receptors in hepatocellular carcinoma. Curr Cancer Drug Targets.
13:300–312. 2013.PubMed/NCBI
|
|
17
|
Cervello M, McCubrey JA, Cusimano A,
Lampiasi N, Azzolina A and Montalto G: Targeted therapy for
hepatocellular carcinoma: novel agents on the horizon. Oncotarget.
3:236–260. 2012.PubMed/NCBI
|
|
18
|
Cai C, Teng L, Vu D, et al: The heme
oxygenase 1 inducer (CoPP) protects human cardiac stem cells
against apoptosis through activation of the extracellular
signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine
release. J Biol Chem. 287:33720–33732. 2012. View Article : Google Scholar
|
|
19
|
Xu X, Fan Z, Kang L, et al: Hepatitis B
virus X protein represses miRNA-148a to enhance tumorigenesis. J
Clin Invest. 123:630–645. 2013.PubMed/NCBI
|
|
20
|
Yang JY and Hung MC: Deciphering the role
of forkhead transcription factors in cancer therapy. Curr Drug
Targets. 12:1284–1290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arden KC: Multiple roles of FOXO
transcription factors in mammalian cells point to multiple roles in
cancer. Exp Gerontol. 41:709–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu MC, Lee DF, Xia W, et al: IκB kinase
promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell.
117:225–237. 2004.
|
|
23
|
Potente M, Urbich C, Sasaki K, et al:
Involvement of Foxo transcription factors in angiogenesis and
postnatal neovascularization. J Clin Invest. 115:2382–2392. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang JY, Zong CS, Xia W, et al: ERK
promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated
degradation. Nat Cell Biol. 10:138–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SH, Jeong JH, Kim TI, Kim SW and Bull
DA: VEGF siRNA delivery system using arginine-grafted bioreducible
poly(disulfide amine). Mol Pharm. 6:718–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du J, Gao S, Luo J, et al: Effective
inhibition of foot-and-mouth disease virus (FMDV) replication in
vitro by vector-delivered microRNAs targeting the 3D gene. Virol J.
8:2922011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guerrero AD, Schmitz I, Chen M and Wang J:
Promotion of caspase activation by caspase-9-mediated feedback
amplification of mitochondrial damage. J Clin Cell Immunol. 3:pii:
1000126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ranjan K, Surolia A and Pathak C:
Apoptotic potential of Fas-associated death domain on regulation of
cell death regulatory protein cFLIP and death receptor mediated
apoptosis in HEK 293T cells. J Cell Commun Signal. 6:155–168. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamasaki Y, Yamasaki M, Tachibana H and
Yamada K: Important role of β1-integrin in fucoidan-induced
apoptosis via caspase-8 activation. Biosci Biotechnol Biochem.
76:1163–1168. 2012.
|
|
31
|
Kornblau SM, Singh N, Qiu Y, Chen W, Zhang
N and Coombes KR: Highly phosphorylated FOXO3A is an adverse
prognostic factor in acute myeloid leukemia. Clin Cancer Res.
16:1865–1874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vadlakonda L, Pasupuleti M and Pallu R:
Role of PI3K-AKT-mTOR and Wnt signaling pathways in transition of
G1-S phase of cell cycle in cancer cells. Front Oncol. 3:852013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pardo PS, Lopez MA and Boriek AM: FOXO
transcription factors are mechanosensitive and their regulation is
altered with aging in the respiratory pump. Am J Physiol Cell
Physiol. 294:C1056–C1066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nho RS, Hergert P, Kahm J, Jessurun J and
Henke C: Pathological alteration of FoxO3a activity promotes
idiopathic pulmonary fibrosis fibroblast proliferation on type I
collagen matrix. Am J Pathol. 179:2420–2430. 2011. View Article : Google Scholar
|
|
35
|
Wang X, Chen WR and Xing D: A pathway from
JNK through decreased ERK and Akt activities for FOXO3a nuclear
translocation in response to UV irradiation. J Cell Physiol.
227:1168–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie C, Song LB, Wu JH, et al: Upregulator
of cell proliferation predicts poor prognosis in hepatocellular
carcinoma and contributes to hepatocarcinogenesis by downregulating
FOXO3a. PLoS One. 7:e406072012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghosh AP, Klocke BJ, Ballestas ME and Roth
KA: CHOP potentially co-operates with FOXO3a in neuronal cells to
regulate PUMA and BIM expression in response to ER stress. PLoS
One. 7:e395862012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao XC, Cao XC, Liu F, Quan MF, Ren KQ
and Cao JG: Regulation of the FOXO3a/Bim signaling pathway by
5,7-dihydroxy-8-nitrochrysin in MDA-MB-453 breast cancer cells.
Oncol Lett. 5:929–934. 2013.PubMed/NCBI
|
|
39
|
Cunha DA, Igoillo-Esteve M, Gurzov EN, et
al: Death protein 5 and p53-upregulated modulator of apoptosis
mediate the endoplasmic reticulum stress-mitochondrial dialog
triggering lipotoxic rodent and human β-cell apoptosis. Diabetes.
61:2763–2775. 2012.PubMed/NCBI
|
|
40
|
He L, Yang X, Cao X, Liu F, Quan M and Cao
J: Casticin induces growth suppression and cell cycle arrest
through activation of FOXO3a in hepatocellular carcinoma. Oncol
Rep. 29:103–108. 2013.PubMed/NCBI
|
|
41
|
Chen Q, Ganapathy S, Singh KP, Shankar S
and Srivastava RK: Resveratrol induces growth arrest and apoptosis
through activation of FOXO transcription factors in prostate cancer
cells. PLoS One. 5:e152882010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boreddy SR, Pramanik KC and Srivastava SK:
Pancreatic tumor suppression by benzyl isothiocyanate is associated
with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res.
17:1784–1795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shankar S, Marsh L and Srivastava RK: EGCG
inhibits growth of human pancreatic tumors orthotopically implanted
in Balb C nude mice through modulation of FKHRL1/FOXO3a and
neuropilin. Mol Cell Biochem. 372:83–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Srivastava RK, Unterman TG and Shankar S:
FOXO transcription factors and VEGF neutralizing antibody enhance
antiangiogenic effects of resveratrol. Mol Cell Biochem.
337:201–212. 2010. View Article : Google Scholar : PubMed/NCBI
|