Introduction
Ovarian cancer remains the leading cause of death
among gynecologic malignancies and accounts for 6% of all
cancer-related deaths in females in the US (1). Cisplatin, as a first-line agent of
cytotoxic chemotherapy for ovarian cancer is widely used in
clinical practice. However, severe dose-dependent toxicity, such as
nephrotoxicity, neurotoxicity and ototoxicity, often cause patient
intolerance (2,3). Achieving the highest antitumor
efficiency using the lowest possible dose is a challenging problem
in the clinic. A strategy using dual agents rather than a single
agent may have enormous potential for solving this issue.
Claudins (CLDNs) are a family of 17- to 27-kDa
integral membrane proteins forming tight junctions (TJs) (4–6).
Previous studies have revealed that claudin-3 (CLDN3) and claudin-4
(CLDN4) are overexpressed in ovarian cancer (7–11) and
their abnormally high expression enhances tumor cell motility,
invasion and survival (12).
Conversely, in vitro siRNA inhibition of CLDN3 and CLDN4
expression in ovarian cancer cells was found to reduce tumor cell
invasion (12). An in vivo
study carried out in our laboratory showed that silencing of the
CLDN3 gene with short hairpin RNA (shRNA) significantly inhibited
the growth of ovarian tumors (13).
These findings suggest the importance of CLDN3 and CLDN4 as novel
therapeutic targets of ovarian cancer. Furthermore,
chemotherapy-resistant ovarian tumors were reported to express
CLDN3 and CLDN4 genes at significantly higher levels when compared
with chemotherapy-sensitive ovarian tumors (14). Thus, downregulation of CLDN4
expression may increase the chemosensitivity of ovarian cells to
cisplatin (15). As a result, in
the present study, we combined CLDN3 suppression with a low-dose of
cisplatin for the treatment of ovarian cancer, to investigate
whether there is a synergistic effect between CLDN3 suppression and
cisplatin, and to develop a low toxic and novel therapeutic
strategy against ovarian cancer.
The gene delivery system is the crucial factor that
influences the efficiency of CLDN3 suppression. Traditional
cationic polyethyleneimine (PEI) has become one of the most
efficient non-viral gene carriers (16–18).
Yet, it is not biodegradable. Moreover, its transfection efficiency
is strongly correlated to its cytotoxicity, and both efficiency and
cytotoxicity increase when the chain length of PEI increases
(19,20). In order to overcome these issues, we
coupled short PEI chains into longer ones using heparin, resulting
in biodegradable heparin-polyethyleneimine (HPEI) nanogels which
have been proven to be efficient and low toxic in our previous
studies (21–23).
In the present study, we used HPEI nanogels to
deliver the plasmid expressing shRNA targeting CLDN3 (pshCLDN3)
into SKOV3 human ovarian cancer cells to reduce the expression of
CLDN3. Moreover, we evaluated the antitumor effects of the
combination therapy of pshCLDN3/HPEI complexes and low-dose
cisplatin in the treatment of ovarian cancer. Our results showed
that the combination therapy exhibited an enhanced antitumor
efficacy, compared with either agent alone, without obvious
systemic toxicity.
Materials and methods
Plasmid vector construction
As described in our previous study (13), shRNA primers targeting CLDN3 (sense,
5′-TCCCGCAACATCATCACGTCGCATTCAAGACGTGC GACGTGATGTGTTGCTTTTTTG-3′
and antisense, 5′-AGC TCAAAAAAGCAACATCATCACGTCGCACGTCTTGAA
TGCGACGTGATGATGTTGC-3′) were designed and synthesized. We used the
HK sequence which has no homology with any of the known mammalian
gene sequences as the negative control. The above sequences were
then transferred into the pGenesil-2.1 vector (Genesil, Wuhan,
China), which contains a kanamycin-resistance gene. The resulting
recombinant plasmids were named pshCLDN3 or pshHK, respectively.
Both of the two constructs were validated by DNA sequencing. The
plasmids were purified using the EndoFree Plasmid Giga kit (Qiagen,
Chatsworth, CA, USA) from DH5α Escherichia coli
transformants.
Cell culture
The human ovarian serous cystadenocarcinoma cell
line SKOV3 (ATCC, Manassas, VA, USA) was cultured in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) and antibiotics (100 units/ml penicillin and 100 μg/ml
streptomycin) in a humidified atmosphere containing 5%
CO2 at 37°C.
Plasmid transfection
Plasmid transfection was carried out using HPEI
nanogels synthesized at the State Key Laboratory of Biotherapy and
Cancer Center as previously described (21). Briefly, SKOV3 cells
(2×105/well) were seeded in 6-well plates one day prior
to transfection to achieve 80% confluence at the time of
transfection. Plasmid (pshCLDN3 or pshHK)/HPEI complexes (1 μg
plasmid/10 μg HPEI) were prepared in 1 ml DMEM without serum and
antibiotics. An equal volume of normal saline (NS) prepared in 1 ml
DMEM was used as a control agent. Cells were incubated with NS,
HPEI nanogels, pshHK/HPEI complexes or pshCLDN3/HPEI complexes for
6 h, and then the medium was replaced with 2 ml complete medium
(DMEM containing 10% FBS) and further incubated for 72 h.
Western blot analysis
Cells or tumor tissue samples were lysed in RIPA
lysis buffer containing proteinase inhibitor (1 mM cocktail plus 1
mM PMSF). The supernatant was collected, and the protein
concentration was quantified using Pierce BCA protein assay kit
(Thermo Scientific, Rockford, IL, USA). Equal amounts of protein
(20 μg) were loaded onto 12% SDS-PAGE gel for electrophoresis and
blotted onto a PVDF membrane (Millipore, Bedford, MA, USA).
Subsequently, the membrane was blocked in 5% skimmed milk for 2 h,
and then incubated with rabbit anti-human polyclonal antibody
against CLDN3 (1:200; Invitrogen) at 4°C overnight, followed by
horseradish peroxidase-conjugated secondary antibody. The
immunoreactive bands were visualized by chemiluminescence
detection. GAPDH served as the protein loading control.
Intraperitoneal carcinomatosis model
The following procedures for the animal experiments
were approved by the Institutional Animal Care and Use Committee of
Sichuan University. Pathogen-free female athymic BALB/c nude mice,
6–8 weeks of age, were used to establish the intraperitoneal
carcinomatosis model as previously described (24).
Briefly, SKOV3 cells (5×106) in 0.1 ml
serum-free DMEM were injected s.c. into the right flank of 5 mice.
Tumors were collected and minced into small particles (diameter ≤1
mm), when the tumor diameter reached ~1 cm. The small tumor
particles were then resuspended in serum-free DMEM to reach a final
volume of 15 ml. Thirty nude mice were inoculated i.p. with 0.5 ml
of the above tumor particle suspension, respectively.
Therapy studies in vivo
To explore the therapeutic efficacy of pshCLDN3/HPEI
plus cisplatin, we treated the mice 7 days after inoculation. The
mice were randomly divided into 6 groups (5/group), and received
the following intraperitoneal (i.p.) administration: (i) 100 μl
normal saline (NS); (ii) 50 μg HPEI nanogels in 100 μl NS, every
two days for 12 times; (iii) 5 μg pshHK/50 μg HPEI complexes in 100
μl NS every two days for 12 times; (iv) 5 μg pshCLDN3/50 μg HPEI
complexes in 100 μl NS, every two days for 12 times; (v) 100 μl of
cisplatin, weekly for 4 times (3 mg/kg; Qinu Pharmacy Corporation,
China); (vi) 5 μg pshCLDN3/50 μg HPEI complexes in 100 μl NS for 12
times and 100 μl of cisplatin (3 mg/kg) for 4 times. The dose of
cisplatin used in this study was 3 mg/kg, since it was previously
reported that treatment with 3 mg/kg cisplatin was well tolerated,
and did not lead to a complete response but significantly inhibited
tumor growth in an ovarian cancer model (25,26).
Two days after the last intraperitoneal injection,
mice were sacrificed, and the intraperitoneal tumors were collected
and weighed. At the same time following sacrifice, the location of
the macroscopic tumors of each mouse was carefully observed and
recorded. Tumors were divided into two parts. One was fixed in 10%
formalin (pH 7.0) and embedded in paraffin. The other was stored at
−80°C for protein extraction.
Histological analysis
Paraffin-embedded intraperitoneal tumors were cut
into 3- to 5-μm sections. Apoptosis was evaluated by terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) assay according to the manufacturer’s instructions
(Promega, Madison, WI, USA). Cell nuclei presenting dark green
fluorescence were considered to be TUNEL-positive nuclei. The
apoptosis index was determined by calculating the average
percentage of TUNEL-positive cells in 5 random fields from 3
different sections at a magnification of ×400.
CLDN3 and Ki-67 immunostaining were carried out with
rabbit anti-human CLDN3 antibody (1:100; Invitrogen) and rabbit
anti-human Ki-67 antibody (1:100; Thermo Scientific), respectively.
Briefly, tumor sections were first deparaffinized, rehydrated and
incubated with 3% H2O2 for 10 min. Then,
antigen retrieval was conducted using 10 mM citrate buffer (pH
8.0). After blocking for 15 min with normal rabbit serum, sections
were incubated with the primary antibody, followed by biotinylated
secondary antibody and the streptavidin-biotin complex,
successively. Detection was performed using diaminobenzidine (DAB)
peroxide solution, and then the cellular nuclei were counterstained
with hematoxylin. The proliferation index was determined by
calculating the average percentage of Ki-67-positive cells with
brown-staining nuclei in 5 random fields from 3 different sections
at a magnification of ×400.
Assessment of toxicity
Health correlated indices such as weight loss,
anorexia, diarrhea, cachexia, skin ulcerations or toxic death were
evaluated every 4 days to evaluate the possible side-effects.
Additionally, at the termination of the animal studies, the main
organs (heart, liver, spleen, lung and kidney) were fixed in 10%
formalin (pH 7.0), embedded in paraffin, and then cut into 3- to
5-μm sections for H&E staining.
Statistical analysis
Values are expressed as means ± SD. ANOVA and the
Student-Newman-Keuls test were used for comparisons. P<0.05 was
considered to indicate a statistically significant result.
Results
Suppression of CLDN3 expression by
pshCLDN3/HPEI complexes in SKOV3 ovarian cancer cells in vitro
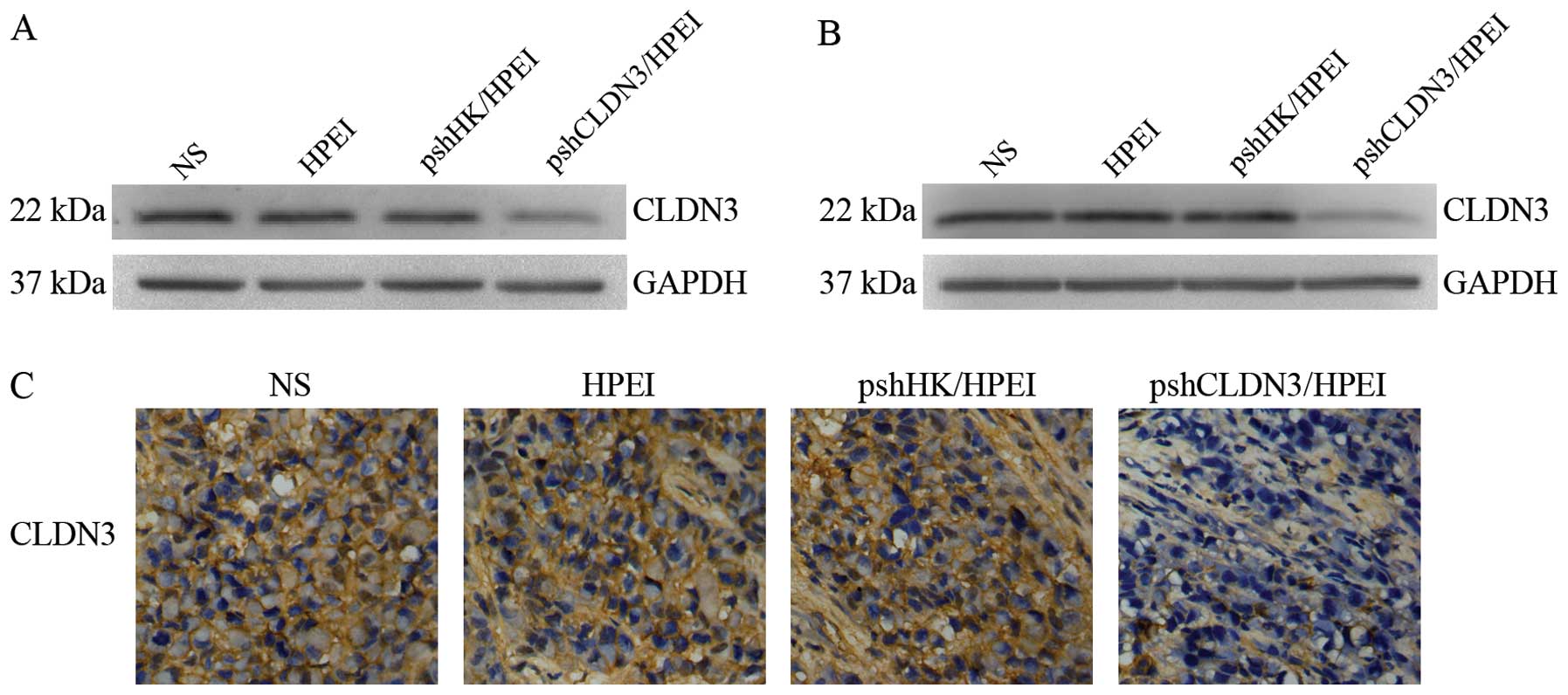
Evaluation of CLDN3 expression in the SKOV3 cells
was performed by western blot analysis 72 h after transfection. As
shown in Fig. 1A, a marked
reduction in CLDN3 expression was noted in the cells transfected
with the pshCLDN3/HPEI complexes, whereas no apparent alteration in
CLDN3 expression was observed in cells transfected with the
pshHK/HPEI complexes or the HPEI nanogels, when compared with that
in the blank control (NS).
Suppression of CLDN3 expression by
pshCLDN3/HPEI complexes in vivo
We investigated whether pshCLDN3/HPEI complexes
reduce the expression of CLDN3 in vivo. The intraperitoneal
tumors were harvested for western blot analysis and
immunohistochemistry at the termination of the animal experiments.
Similar to the in vitro culture, the pshCLDN3/HPEI complexes
markedly reduced the expression of CLDN3 (Fig. 1B), whereas the pshHK/HPEI complexes
or HPEI nanogels had no discernible effect on CLDN3 expression.
Similar results were observed with immunohistochemistry (Fig. 1C).
Enhanced antitumor efficacy of the
combination regimen of the pshCLDN3/HPEI complexes and low-dose
cisplatin
At the termination of the animal studies, the mice
were sacrificed, and the location of the macroscopic tumors was
carefully observed. In the NS, HPEI and pshHK/HPEI groups, each
mouse developed intraperitoneally macroscopic tumor nodules
scattered on various viscera. A number of tumors were observed not
only deposited on the surface of the liver, but also invading the
parenchyma. In the other 3 groups, the intraperitoneal tumor
nodules were localized. Tumor invasion in tissues and organs was
not obvious in these groups.
Next, the intraperitoneal tumors were harvested and
weighed. As showed in Fig. 2, the
mean tumor weight was 1.35±0.21, 1.29±0.18, 1.16±0.14, 0.45±0.13,
0.40±0.11 and 0.17±0.07 g in the NS, HPEI nanogel, pshHK/HPEI
complex, pshCLDN3/HPEI complex, low-dose cisplatin and
pshCLDN3/HPEI complex plus low-dose cisplatin group, respectively.
The data showed that the pshCLDN3/HPEI complexes or low-dose
cisplatin alone significantly inhibited tumor growth, compared with
the control therapies (P<0.05). Furthermore, the combination of
pshCLDN3/HPEI and cisplatin had a superior antitumor effect,
compared with pshCLDN3/HPEI or cisplatin alone (P<0.05). No
significant difference in tumor weight was found among the control
groups (P>0.05).
 | Figure 2Tumor weights in the xenograft model
of human ovarian cancer in nude mice. Nude mice bearing
intraperitoneal ovarian carcinomas were divided into 6 groups
(5/group), and received i.p. administration of (a) NS, (b) HPEI
nanogels, (c) pshHK/HPEI complexes, (d) pshCLDN3/HPEI complexes,
(e) low-dose cisplatin or (f) pshCLDN3/HPEI plus low-dose
cisplatin, respectively. The results indicated that both
pshCLDN3/HPEI complexes and low-dose cisplatin alone significantly
inhibited the growth of ovarian carcinomas. The combination of
pshCLDN3/HPEI and cisplatin displayed enhanced antitumor activity,
when compared with pshCLDN3/HPEI or cisplatin alone. Data are
expressed as means ± SD. *P<0.05 vs. the NS group;
+P<0.05 vs. the pshCLDN3/HPEI group;
#P<0.05 vs. the cisplatin group. NS, normal saline;
HPEI, heparin-polyethyleneimine; CLDN3, claudin-3. |
Inhibition of proliferation in vivo
Ki-67 immunostaining was used to evaluate the tumor
cell proliferation in each group. As shown in Fig. 3A and B, both the pshCLDN3/HPEI
complexes and low-dose cisplatin monotherapy group exhibited weak
staining for Ki-67, when compared with the control groups
(P<0.05). No significant difference in Ki-67 staining was
visible in the two groups (P>0.05). Moreover, the percentage of
Ki-67-positive cells was significantly reduced in the combination
therapy group, when compared with the percentage in the
pshCLDN3/HPEI or low-dose cisplatin monotherapy group
(P<0.05).
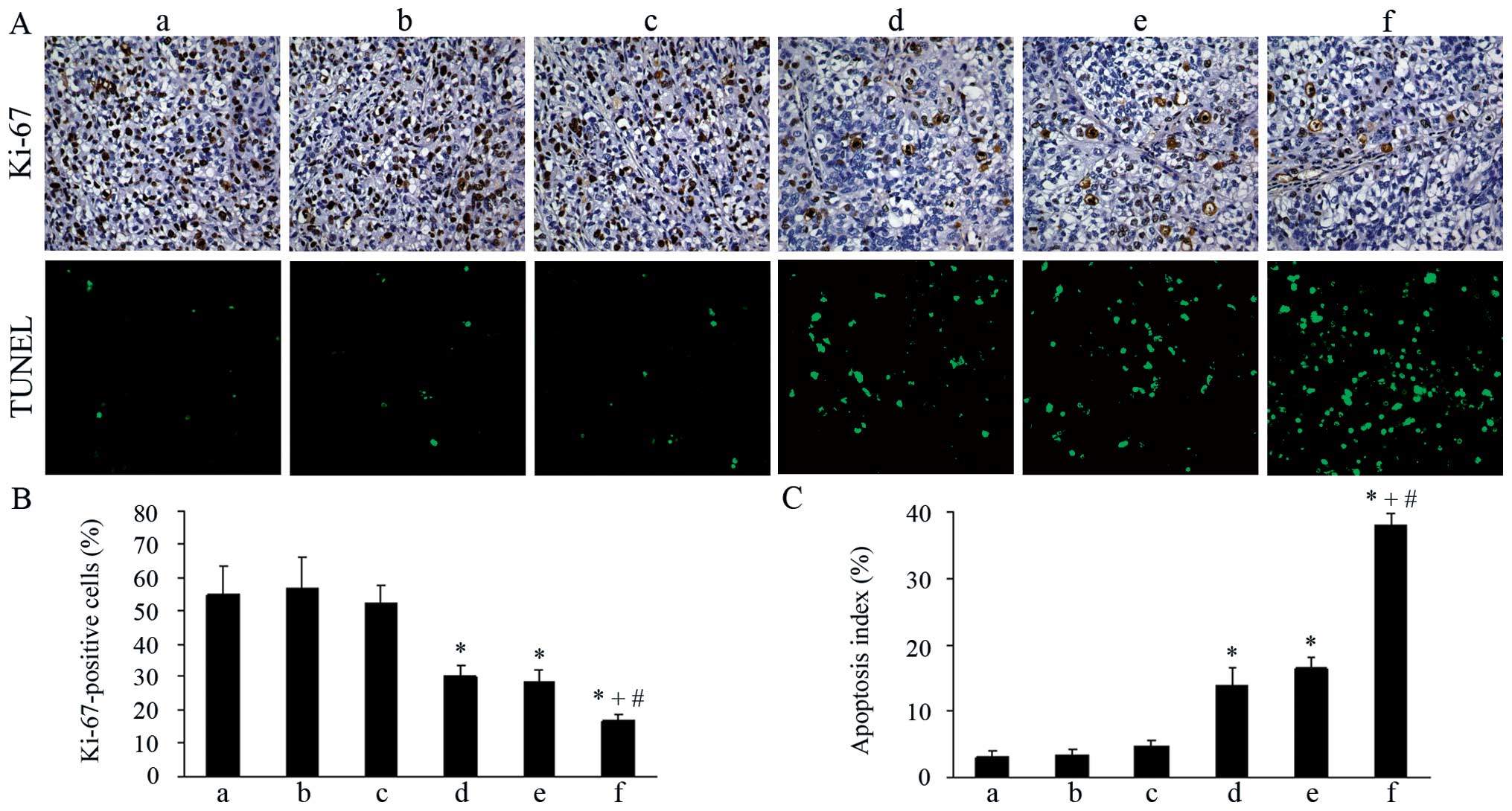 | Figure 3Ki-67 immunostaining and TUNEL assay
of tumor tissues in the different groups. (A) Top panel, tumor
sections immunostained with rabbit anti-human Ki-67 antibody
(×200). Bottom panel, tumor sections stained for TUNEL (×200).
(a-f) NS, HPEI, pshHK/HPEI, pshCLDN3/HPEI, low-dose cisplatin and
pshCLDN3/HPEI plus low-dose cisplatin group, respectively. (B)
Quantification of Ki-67-positive cells. (C) Quantification of TUNEL
staining (apoptotic index). Data are presented as means ± SD.
*P<0.05 vs. the NS group; +P<0.05 vs.
the pshCLDN3/HPEI group; #P<0.05 vs. the cisplatin
group. NS, normal saline; HPEI, heparin-polyethyleneimine; CLDN3,
claudin-3. |
Induction of apoptosis in vivo
We used TUNEL assay to detect apoptotic cells in the
tumor tissues of each group. The results showed that both the
pshCLDN3/HPEI and cisplatin monotherapy resulted in a significant
increase in apoptotic tumor cells when compared with that following
treatment with the control agents (P<0.05). Tumors in the
combination therapy group showed an increased number of positive
nuclei, when compared with the number in the pshCLDN3/HPEI or
low-dose cisplatin monotherapy group (P<0.05). However, positive
nuclei were rare in the control groups (Fig. 3A and C).
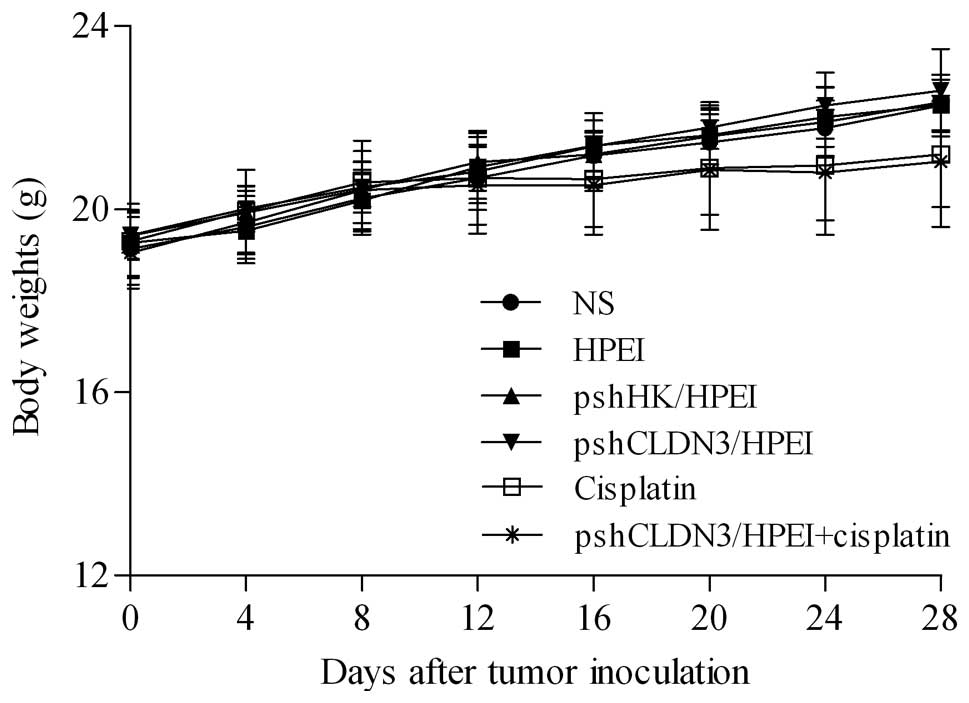
Observation of toxicity
In the present study, no gross abnormalities were
observed in any group. As shown in Fig.
4, although the mean body weights in the two cisplatin-treated
groups were less than that in the other four groups after the
treatment course, the differences did not reach statistical
significance (P>0.05). Furthermore, no pathologic change in the
heart, liver, spleen, lung or kidney was noted by microscopic
examination after the administration of pshCLDN3/HPEI or
pshCLDN3/HPEI plus cisplatin.
Discussion
In the present study, we demonstrated that
pshCLDN3/HPEI complexes effectively inhibited ovarian tumor growth,
reduced tumor cell proliferation and increased tumor cell
apoptosis. In addition, from the differences in the tumor location
among the groups, we inferred that pshCLDN3/HPEI complexes also
inhibited ovarian tumor invasion. These findings are consistent
with a previous study carried out in our laboratory, in which
pshCLDN3 was delivered by polyp(lactic-co-glycolic acid) (PLGA)
(13), and another study carried
out by Huang et al (27).
Moreover, in the present study, the combination therapy of
pshCLDN3/HPEI and low-dose cisplatin exhibited enhanced antitumor
activity, compared with either agent alone, as evidenced by
analysis of the mean tumor weight, Ki-67 immunostaining analysis
and TUNEL assay, indicating that there may be a synergistic effect
between CLDN3 suppression and cisplatin in the treatment of ovarian
cancer. The combination therapy holds much promise as an effective
strategy against ovarian cancer.
In addition to efficacy, the safety of therapy is an
important factor in considering its utility for clinical
application. It has been reported that CLDN3 is also expressed in
several other normal organs and tissues (28,29).
Systemic administration of pshCLDN3/HPEI may interfere with the
expression of CLDN3 in these tissues, thus, causing various
unpredictable side-effects. Therefore, in the present study, we
utilized the i.p. route for pshCLDN3/HPEI administration, which may
reduce the adverse effects of silencing CLDN3 in normal tissues
that reside outside the peritoneum. Moreover, considering the
severe side-effects owing to a high dose of cisplatin, we used a
low-dose of cisplatin (3 mg/kg) in this study. Although the final
mean body weights in the two cisplatin-treated groups were less
than that in the other four groups, the differences did not reach
statistical significance (P>0.05). Overall, the i.p.
administration of pshCLDN3/HPEI combined with low-dose cisplatin
was well tolerated, with no obvious toxicity throughout the course
of treatment, and macroscopic examination of vital organs was
normal at sacrifice. These results indicate that the combination
therapy of pshCLDN3/HPEI and low-dose cisplatin has low toxicity
and has potential clinical application.
However, to date, the exact mechanisms of how the
combination therapy exerts its effect remain unclear. Two possible
mechanisms may be involved. First, the enhanced antitumor effect
may result from the enhanced activity of induced apoptosis. In the
present study, we observed a higher number of apoptotic cells in
the tumors treated with pshCLDN3/HPEI plus cisplatin, compared with
the pshCLDN3/HPEI or cisplatin alone group (Fig. 3A and C). Cisplatin is a well-known
DNA damaging agent. It has the capability to form platinum-DNA
adducts which activate several cellular processes, ultimately
leading to cell cycle arrest, transcription inhibition and cell
apoptosis (30–32). Suppression of CLDN3 in ovarian
cancer cells has also been found to induce cell apoptosis (13,27).
Thus, there may be a common synergistic apoptotic pathway between
pshCLDN3/HPEI and cisplatin. Second, the enhanced antitumor effect
may result from the increased penetration of cisplatin into tumor
tissues. Tight junctions (TJs) act as a barrier and regulator of
the passage of molecules and ions between cells. CLDNs, as the
major component of TJs, influence barrier functions. It has been
suggested that CLDN suppression in tumor cells increases TJ
permeability, thus, increasing the penetration of chemotherapeutic
agents into tumor tissues, resulting in greater effectiveness of
the chemotherapeutic agents (15,33).
Therefore, we infer that pshCLDN3/HPEI increases the penetration of
cisplatin into ovarian tumor tissues, and consequently enhances the
antitumor effect of cisplatin. Moreover, CLDNs are transmembrane
proteins and are associated with the membrane permeability of
molecules. A recent study using fluorescence-labeled cisplatin
showed that downregulation of CLDN4 expression in ovarian cancer
cells in vitro resulted in an increased cellular
accumulation of fluorescence-labeled cisplatin, indicating that
CLDN suppression may also affect the transmembrane transportation
of cisplatin, and consequently enhance its antitumor effect
(15). However, the precise
mechanisms by which pshCLDN3/HPEI plus cisplatin exerts its effect
require further investigation.
The application of gene therapy in cancer treatment
depends on a safe and efficient gene delivery system. Although
viral carriers have high transfection efficiency, they consistently
cause severe side-effects (34). In
contrast, non-viral gene carriers such as cationic lipids and
cationic polymers have many advantages, including the ease of
production, low immunogenicity, and feasibility of delivering
larger DNA molecules (35,36). However, toxicity also hinders their
applications (37). In our previous
studies, we developed a novel non-viral gene delivery system using
HPEI nanogels. Different from traditional PEI, HPEI nanogels are
biodegradable. Moreover, they are less toxic, and have a better
blood compatibility (21,22). Based on these advantages, we used
HPEI nanogels as the gene carrier in our present study. Our data
showed that pshCLDN3/HPEI complexes significantly reduced the
expression of CLDN3 in vitro and in vivo, indicating
that HPEI nanogels efficiently deliver pshCLDN3 into SKOV3 human
ovarian cancer cells. No apparent cytotoxicity and systemic toxic
effects of HPEI nanogels were found in this study. pshCLDN3
delivered by HPEI nanogels showed an excellent tolerance throughout
the treatment process.
In conclusion, our data showed that pshCLDN3/HPEI
complexes obviously inhibited the growth of ovarian cancer. The
combination therapy of pshCLDN3/HPEI and low-dose cisplatin
exhibited enhanced antitumor activity, when compared with either
agent alone, without obvious toxicity. The HPEI nanogel as a new
non-viral gene carrier exhibited high efficiency and low toxicity.
Our study offers a novel and promising therapeutic strategy for
human ovarian cancer.
Acknowledgements
This study was supported by the National 973 Program
of China (2010CB529905, 2011CB910703), the National Natural Science
Foundation of China (NSFC81071861), the Specialized Research Fund
for the Docoral Program of Higher Education of China
(20120181110029), and the National Science and Technology Major
Project (2009zx09503-020).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
McKeage MJ: Comparative adverse effect
profiles of platinum drugs. Drug Saf. 13:228–244. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piccart MJ, Lamb H and Vermorken JB:
Current and future potential roles of the platinum drugs in the
treatment of ovarian cancer. Ann Oncol. 12:1195–1203. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morita K, Furuse M, Fujimoto K and Tsukita
S: Claudin multigene family encoding four-transmembrane domain
protein components of tight junction strands. Proc Natl Acad Sci
USA. 96:511–516. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukita S and Furuse M: Pores in the wall:
claudins constitute tight junction strands containing aqueous
pores. J Cell Biol. 149:13–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hough CD, Sherman-Baust CA, Pizer ES, et
al: Large-scale serial analysis of gene expression reveals genes
differentially expressed in ovarian cancer. Cancer Res.
60:6281–6287. 2000.PubMed/NCBI
|
|
8
|
Rangel LB, Agarwal R, D’Souza T, et al:
Tight junction proteins claudin-3 and claudin-4 are frequently
overexpressed in ovarian cancer but not in ovarian cystadenomas.
Clin Cancer Res. 9:2567–2575. 2003.PubMed/NCBI
|
|
9
|
Hibbs K, Skubitz KM, Pambuccian SE, et al:
Differential gene expression in ovarian carcinoma: identification
of potential biomarkers. Am J Pathol. 165:397–414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu KH, Patterson AP, Wang L, et al:
Selection of potential markers for epithelial ovarian cancer with
gene expression arrays and recursive descent partition analysis.
Clin Cancer Res. 10:3291–3300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santin AD, Zhan F, Bellone S, et al: Gene
expression profiles in primary ovarian serous papillary tumors and
normal ovarian epithelium: identification of candidate molecular
markers for ovarian cancer diagnosis and therapy. Int J Cancer.
112:14–25. 2004. View Article : Google Scholar
|
|
12
|
Agarwal R, D’Souza T and Morin PJ:
Claudin-3 and claudin-4 expression in ovarian epithelial cells
enhances invasion and is associated with increased matrix
metalloproteinase-2 activity. Cancer Res. 65:7378–7385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun C, Yi T, Song X, et al: Efficient
inhibition of ovarian cancer by short hairpin RNA targeting
claudin-3. Oncol Rep. 26:193–200. 2011.PubMed/NCBI
|
|
14
|
Santin AD, Cané S, Bellone S, et al:
Treatment of chemotherapy-resistant human ovarian cancer xenografts
in C.B-17/SCID mice by intraperitoneal administration of
Clostridium perfringens enterotoxin. Cancer Res.
65:4334–4342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida H, Sumi T, Zhi X, Yasui T, Honda K
and Ishiko O: Claudin-4: a potential therapeutic target in
chemotherapy-resistant ovarian cancer. Anticancer Res.
31:1271–1277. 2011.PubMed/NCBI
|
|
16
|
Boussif O, Lezoualc’h F, Zanta MA, et al:
A versatile vector for gene and oligonucleotide transfer into cells
in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA.
92:7297–7301. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lungwitz U, Breunig M, Blunk T and
Göpferich A: Polyethylenimine-based non-viral gene delivery
systems. Eur J Pharm Biopharm. 60:247–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neu M, Fischer D and Kissel T: Recent
advances in rational gene transfer vector design based on
poly(ethylene imine) and its derivatives. J Gene Med. 7:992–1009.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Godbey WT, Wu KK and Mikos AG: Size
matters: molecular weight affects the efficiency of
poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res.
45:268–275. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kunath K, von Harpe A, Fischer D, et al:
Low-molecular-weight polyethylenimine as a non-viral vector for DNA
delivery: comparison of physicochemical properties, transfection
efficiency and in vivo distribution with high-molecular-weight
polyethylenimine. J Control Release. 89:113–125. 2003. View Article : Google Scholar
|
|
21
|
Gou M, Men K, Zhang J, et al: Efficient
inhibition of C-26 colon carcinoma by VSVMP gene delivered by
biodegradable cationic nanogel derived from polyethyleneimine. ACS
Nano. 4:5573–5584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie C, Gou ML, Yi T, et al: Efficient
inhibition of ovarian cancer by truncation mutant of FILIP1L gene
delivered by novel biodegradable cationic heparin-polyethyleneimine
nanogels. Hum Gene Ther. 22:1413–1422. 2011. View Article : Google Scholar
|
|
23
|
Liu P, Gou M, Yi T, et al: The enhanced
antitumor effects of biodegradable cationic
heparin-polyethyleneimine nanogels delivering HSulf-1 gene combined
with cisplatin on ovarian cancer. Int J Oncol. 41:1504–1512.
2012.
|
|
24
|
Lin XJ, Chen XC, Wang L, et al: Dynamic
progression of an intraperitoneal xenograft model of human ovarian
cancer and its potential for preclinical trials. J Exp Clin Cancer
Res. 26:467–474. 2007.PubMed/NCBI
|
|
25
|
Mabuchi S, Altomare DA, Cheung M, et al:
RAD001 inhibits human ovarian cancer cell proliferation, enhances
cisplatin-induced apoptosis, and prolongs survival in an ovarian
cancer model. Clin Cancer Res. 13:4261–4270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mabuchi S, Terai Y, Morishige K, et al:
Maintenance treatment with bevacizumab prolongs survival in an in
vivo ovarian cancer model. Clin Cancer Res. 14:7781–7789. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang YH, Bao Y, Peng W, et al: Claudin-3
gene silencing with siRNA suppresses ovarian tumor growth and
metastasis. Proc Natl Acad Sci USA. 106:3426–3430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiuchi-Saishin Y, Gotoh S, Furuse M,
Takasuga A, Tano Y and Tsukita S: Differential expression patterns
of claudins, tight junction membrane proteins, in mouse nephron
segments. J Am Soc Nephrol. 13:875–886. 2002.
|
|
29
|
Hewitt KJ, Agarwal R and Morin PJ: The
claudin gene family: expression in normal and neoplastic tissues.
BMC Cancer. 6:1862006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vaisman A, Varchenko M, Said I and Chaney
SG: Cell cycle changes associated with formation of Pt-DNA adducts
in human ovarian carcinoma cells with different cisplatin
sensitivity. Cytometry. 27:54–64. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohen SM and Lippard SJ: Cisplatin: from
DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol.
67:93–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kominsky SL: Claudins: emerging targets
for cancer therapy. Expert Rev Mol Med. 8:1–11. 2006. View Article : Google Scholar
|
|
34
|
Relph K, Harrington K and Pandha H: Recent
developments and current status of gene therapy using viral vectors
in the United Kingdom. BMJ. 329:839–842. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Glover DJ, Lipps HJ and Jans DA: Towards
safe, non-viral therapeutic gene expression in humans. Nat Rev
Genet. 6:299–310. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferber D: Gene therapy. Safer and
virus-free? Science. 294:1638–1642. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv H, Zhang S, Wang B, Cui S and Yan J:
Toxicity of cationic lipids and cationic polymers in gene delivery.
J Control Release. 114:100–109. 2006. View Article : Google Scholar : PubMed/NCBI
|