Introduction
Bladder cancer (BC) is the most common urinary tract
malignancy and the fifth most common malignancy in the developed
countries. Each year, an estimated 105,000 and 71,000 new patients
are diagnosed with BC in Europe and America, and ~28.5 and 20%,
respectively, succumb to this disease (1). The major cause of mortality and
relapse from BC is metastasis. Metastasis is a complex process
comprised of multiple steps. In the metastatic cascade, local
invasion can be considered an initial, essential step in the
malignancy of carcinomas leading to the generation of a distant
metastasis (2).
It has become evident that, in addition to
alterations in protein-encoding genes, abnormalities in non-coding
genes can also contribute to carcinogenesis (3,4). In
particular, microRNAs (miRNAs), short single-stranded RNAs, have
long been known to be important regulators of various cellular
processes in gene expression at the post-transcriptional level
(5,6). Deregulated miRNAs in cancer may
function as either tumor suppressors or oncogenes and play a
critical role in carcinogenesis (4). Furthermore, evidence indicates that
miRNAs can be implicated in both the promotion and suppression of
metastasis (7). For example, Ma
et al found that miR-10b initiates invasion and metastasis
in breast cancer (8). The present
study provided the first evidence of miRNA in tumor metastasis.
Subsequently, several additional miRNAs have been reported to act
at various steps of metastasis. miR-21 stimulates cell invasion and
metastasis in breast, colon cancer and gliomas (9,10);
let-7 family members inhibit cell adhesion, migration and invasion
in lung, gastric and breast cancer (11–13);
and the miR-200 and miR-205 family inhibit cell migration and
invasion by targeting ZEB1 and ZEB2, both of which are EMT-inducing
transcription factors.
Notably, some miRNAs multitask by interacting with
different target genes in various cellular contexts. miR-10b is a
member of these miRNAs, which has been characterized as an oncogene
in several human cancers by targeting critical cancer-related
pathways. Reports have proven that miR-10b promotes breast cancer
growth by Twist1-miR-10b-HOXD10 axis (8) and miR-10b can facilitate human glioma
growth by targeting BCL2L11/Bim, TFAP2C/AP-2, CDKN1A/p21 and
CDKN2A/p16 (14). Recent studies
indicate it can also enhance the migration and invasion of human
esophageal cancer by targeting KLF4 (15). Our previous study also demonstrated
that KLF4 was downregulated and may significantly inhibit cell
migration and invasion through epithelial-mesenchymal transition
(EMT) inhibition in BC cells (16).
The present study provides the first evidence of the
role of miR-10b in BC metastasis and partially elucidates the
molecular mechanism underlying the phenomenon. We identified that
miR-10b had a high expression both in cell lines and metastatic
tissues in BC. Furthermore, we verified the function of miR-10b in
BC cell migration and invasion in vitro and in vivo.
In addition, KLF4 and HOXD10 were identified as direct and
functional targets of miR-10b in BC cells. Of note, ectopic
expression of miR-10b induced the downregulation of invasion
suppressor E-cadherin and improved the expression of MMP14, a
member of the membrane-type matrix metalloproteinase (MT-MMP)
subfamily involved in the breakdown of extracellular matrix in
physiological or pathological processes. We further demonstrated
that downregulation of the E-cadherin by miR-10b, through targeting
KLF4, and upregulation of the MMP14, through targeting HOXD10,
promoted migration and invasion in BC cells, which may contribute
to the pro-metastatic role of miR-10b.
Materials and methods
Cell culture and tissue collection
BC cell lines T24, 5637, J82 and EJ were purchased
from ATCC and maintained in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS) in a humidified atmosphere of 5%
CO2 maintained at 37°C. SV-HUC-1 was also purchased from
ATCC and maintained in DMEM/F-12 medium. SCaBER was purchased from
AllCells, LLC (Shanghai, China) in MEM medium. Paired samples of
primary BC, and lymph node metastatic tissues were obtained from
patients who had undergone radical cystectomy surgery at Tongji
Hospital, Wuhan, China. All samples were clinically and
pathologically shown to be correctly labeled. The present study was
approved by the Hospital’s Protection of Human Subjects Committee,
and informed consent was obtained from all patients.
RNA extraction and real-time RT-PCR
Total RNA, including miRNA, was extracted using the
mirVana miRNA isolation kit (Ambion, Austin, TX, USA) following the
manufacturer’s protocol. The integrity of the RNA was examined with
an RNA 6000 Nano Assay kit and a 2100 Bioanalyzer (Agilent
Technologies, Santa Clara, CA, USA). The RT and PCR primers for
miR-10b and U6 were purchased from RiboBio (Guangzhou, China). The
PCR primers for KLF4 were: 5′-ACC CACACTTGTGATTACGC-3′ and
5′-CCGTGTGTTTACGG TAGTGC-3′. The PCR primers for GAPDH were:
5′-ACCCA CACTTGTGATTACGC-3′ and 5′-GTGTCGCTGTTGAAGT CAGA-3′. The
first-strand cDNA was synthesized using the RT Reagent kit (Takara,
Dalian, China). Real-time PCR was performed using SYBR Premix Ex
Taq II (Takara) and measured in a LightCycler 480 System (Roche,
Basel, Switzerland). Expression of U6 or glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as internal control. All the
reactions were run in triplicate.
Lentivirus infection and oligonucleotide
transfection
The miR-10b lentivirus were purchased from GeneChem
(Shanghai, China). The constructs containing the pre-miR-10b
sequence, and 100 bases of upstream and downstream flanking these
sequences were cloned into the pGCSIL-GFP vector. Target cells
(1×105) were infected with 1×107 lentivirus
transducing units in the presence of 10 μg/ml polybrene. Empty
lentiviral vector was used as negative control. The miR-10b
inhibitor, mimic, negative control for mimic, negative control for
inhibitor, negative control for siRNA and siRNA of KLF4, HOXD10
were designed and synthesized by RiboBio. Target cells were
transfected with miR-10b inhibitor, mimic, siRNA or negative
control using X-tremeGENE siRNA Transfection Reagent (Roche,
Germany) according to the manufacturer’s protocol.
Plasmid construction and dual-luciferase
reporter assay
To construct a luciferase reporter vector, the
wild-type 3′-UTR of KLF4 and HOXD10 were PCR-amplified using
genomic DNA from 293T, GES and GC9811 cells as templates. The
corresponding mutant constructs were created by mutating the seed
regions of the miR-10b-binding sites. Both wild-type and mutant
3′-UTRs were cloned downstream of the luciferase gene in the
psiCheck-2 luciferase vector. The constructs were verified by
sequencing. For luciferase reporter assay, 5637 and EJ cells were
seeded in 24-well plates and transiently transfected with
appropriate reporter plasmid and miRNA using X-tremeGENEsiRNA
Transfection Reagent. After 48 h, the cells were harvested and
lysed, and luciferase activity was measured using the
Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Firefly-luciferase was used for normalization. For each plasmid
construct, the transfection experiments were performed in
triplicate.
Migration and invasion assay
For migration assay, infected or transfected cells
were harvested and in serum-free RPMI-1640 medium, 5637
~1.5×105 cells or EJ ~5×104 cells were placed
into Boyden chambers (Corning, Cambridge, MA, USA) with an 8.0-mm
pore membrane. For invasion assay, cells were placed into chambers
coated with 150 mg of Matrigel (BD Biosciences, Bedford, MD, USA).
The chambers were then inserted into the wells of a 24-well plate
and incubated for 24 h in RPMI-1640 medium with 10% FBS before
examination. The cells remaining on the upper surface of the
membranes were removed, whereas the cells adhering to the lower
surface were fixed, stained in a dye solution containing 0.5%
crystal violet and counted under a microscope (Olympus Corp.,
Tokyo, Japan) to calculate their relative numbers. The results were
averaged among three independent experiments.
Western blotting
Whole-cell lysates were prepared in NP-40 buffer
(Byotime, Haimen, China), and western blotting was performed as
previously described. The primary antibodies used were KLF4 (Abcam,
Hong Kong, China), E-cadherin, HOXD10 (both from Cell Signaling
Technology), MMP14 and GAPDH (both from Boster, Wuhan, China).
In vivo metastasis assay
For in vivo metastasis assay,
1×106 EJ cells infected with miR-10b lentivirus and
negative control containing GFP label were suspended in 200 μl
phosphate-buffered saline and injected into the tail vein of nude
mice (three in each group). Mice were sacrificed and lungs were
resected 4 weeks after injection. The incidence and volume of
metastases were estimated by the imaging of mice for
bioluminescence using the LivingImage software (Xenogen, Baltimore,
MD, USA). The photon emission level was used to assess the relative
tumor burden in the mouse lungs. All animal studies were conducted
under approved guidelines of the Animal Care and Use Committee of
Tongji Hospital (Wuhan, China).
Statistical analysis
The SPSS 18.0 program (SPSS Inc., Chicago, IL, USA)
was used for the statistical analysis. Experimental data are
expressed as the means ± SE. The differences between groups were
analyzed using the Student’s t-test when comparing only two groups
or they were assessed by one-way analysis of variance when more
than two groups were compared. For comparison of paired tissues, a
paired Student’s t-test was used to determine the statistical
significance. Differences were considered statistically significant
at P<0.05, *P<0.05.
Results
miR-10b is upregulated in BC cell lines
and human BC metastatic tissue samples
miR-10b expression was detected in five human BC
cell lines T24, 5637, J82, EJ, SCaBER and SV-HUC-1, an immortalized
bladder epithelial cell line. Quantitative reverse-transcription
PCR (qRT-PCR) showed that miR-10b expression was significantly
increased in BC cell lines compared with SV-HUC-1 (Fig. 1A). Ma et al (8) found that miR-10b was highly expressed
in metastatic breast cancer cells and positively regulated cell
migration and invasion. In the present study, miR-10b was also
detected in 20 paired primary tumors and their related metastatic
lymph nodes. Result indicated that miR-10b was expressed higher in
metastatic lymph nodes than in primary tumors in 16/20 matched
specimens with a P-value of 0.0001 (Fig. 1B and C).
miR-10b suppresses BC cell migration and
invasion in vitro
Transwell assay was performed to investigate whether
miR-10b enhance BC cell migration and invasion. First, miR-10b
inhibitor was transfected into 5637 and EJ cells to reduce the
endogenous miR-10b expression. Expression of miR-10b was suppressed
in the transfectant. The results showed that the inhibition of
miR-10b expression led to a 40.7 and 44.2% reduction in migration
and invasion compared to the control in 5637 cells; a similar 45.0
and 48.6% reduction in migration and invasion was also found in
another BC cancer cell line EJ (Fig.
2). Second, miR-10b mimics were transfected to upregulate the
level of miR-10b in 5637 and EJ cells, which led to a marked
increase in migration and invasion (Fig. 2). Taken together, these results
indicate that miR-10b promotes BC cell migration and invasion in
vitro.
KLF4 and HOXD10 mRNA are direct targets
of miR-10b
KLF4 and HOXD10, which have putative binding sites
for miR-10b respectively, have been reported to be the direct
targets of miR-10b in other types of cancer. However, these target
effects were not observed in BC. PsiCheck-2-KLF4 3′-UTR,
PsiCheck-2-KLF4-3′-UTR-mut, PsiCheck-2-HOXD10-3′-UTR and
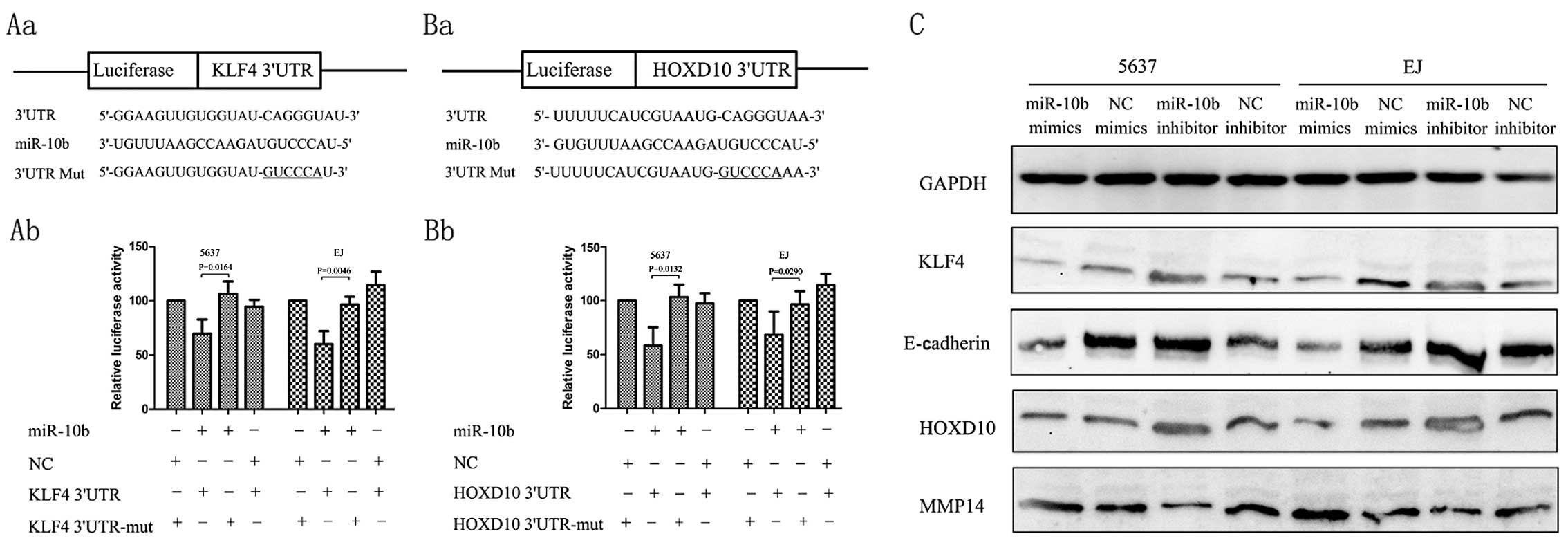
PsiCheck-2-HOXD10-3′-UTR-mut were constructed (Fig. 3Aa and Ba) to examine whether KLF4 and HOXD10 mRNA
are direct targets of miR-10b in renal cancer cells.
Co-transfection of 5637 cells with PsiCheck-2-KLF4 3′-UTR and
miR-10b mimics led to a 30.0% decrease in the luciferase activity
compared with the negative control. Co-transfection of 5637 with
PsiCheck-2-HOXD10-3′-UTR and miR-10b mimics also led to a 42%
decrease in the luciferase activity. This suppression was rescued
by the six-nucleotide substitution in the core binding sites. A
similar effect was also found in EJ cells (Fig. 3). Real-time qPCR of KLF4 mRNA showed
that miR-10b overexpression or inhibition had little effect on KLF4
or HOXD10 mRNA level (data not shown). Collectively, these results
suggested that miR-10b regulates KLF4 and HOXD10 expression in
renal cancer cells by directly targeting those 3′-UTR through
post-transcriptional regulation way.
Immunoblot assay showed that the overexpression of
miR-10b decreased the endogenous expression of protein of KLF4 and
HOXD10, whereas downregulation of miR-10b with an anti-miR-10b
inhibitor increased the expression of KLF4 and HOXD10. E-cadherin
is a central component involved in the conversion between
mesenchymal and epithelial phenotypes, and KLF4 controls the
expression of E-cadherin by reducing the expression of snail
(17), slug (18,19) or
directly binding to the GC-rich/E-box region of the E-cadherin
promoter (20). In the present
study, ectopic overexpression of miR-10b reduced the expression of
E-cadherin in BC cell lines (Fig.
3C). Tumor invasive factors MMP14 have been reported to be
directly regulated by HOXD10 (21).
Then, we examined the levels of MMP14 after transfection with
miR-10b inhibitors and the results showed that the protein was
downregulated by the miR-10b inhibitors, whereas transfection with
the miR-10b mimics may upregulate MMP14 expression levels (Fig. 3C).
Silencing KLF4 and HOXD10 expression
efficiently blocks the effect of miR-10b downregulation on BC cell
migration and invasion
We then tested whether miR-10b inhibits BC cell
migration and invasion through targeting KLF4 and HOXD10. Small
interfering RNA target KLF4 and HOXD10 were used to knock down
endogenous KLF4 and HOXD10. Using Transwell assay, the inhibition
effect of migration and invasion by miR-10b inhibitor in 5637 cells
may be partially prevented by KLF4 or HOXD10 knockdown with
siRNA-KLF4 or siRNA-HOXD10 to varying degrees (Fig. 4). The same effect was also
identified in EJ cells.
miR-10b suppresses BC cell metastasis in
vivo
To further determine whether miR-10b promotes
metastatic behaviors in vivo, EJ cells stably transfected by
Lenti-miR-10b were delivered into nude mice through tail vein
injection. Bioluminescence imaging taken 28 days later revealed
that the fluorescence signal in the Lenti-miR-10b group was
significantly stronger than in the Lenti-NC group, indicating that
more metastasis formed in the lung after miR-10b overexpression
(Fig. 5). This assay in vivo
suggested that miR-10b has a potential to promote metastasis in BC
cells.
Discussion
MicroRNAs (miRNAs) have emerged as important
regulators of gene expression at the post-transcriptional level and
regulate a wide range of physiological and developmental processes.
Over the past few years, it has become clear that alterations in
the expression of miRNAs contribute to the pathogenesis of most
human cancers, where they act as either oncogenes or tumor
suppressors (22).
Aberrant miR-10b expression is correlated with
carcinogenesis. miR-10b plays an oncogenic effect in breast cancer,
neurofibromatosis type 1, oral, colorectal, gastric, pancreatic,
human glioma cancer, human nasopharyngeal carcinoma and human
esophageal cancer (14,15,23–29).
Ma et al first discovered that miR-10b initiates breast
cancer invasion and metastasis (8).
Nishida et al showed that transfection with miR-10b confers
resistance to the chemotherapeutic agent 5-fluorouracil in
colorectal cancer cells (28).
miR-10b also reduces glioma cell growth by cell cycle arrest and
apoptosis (14). In the present
study, we investigated the role and the functional targets of
miR-10b in human bladder cancer (BC). miR-10b expression was
detected in several BC cell lines, an immortal urothelial cell line
SV-HUC-1 and bladder tissue by RT-PCR. miR-10b expression was
significantly increased in all five BC cell lines. Furthermore,
miR-10b expression in primary bladder tumor tissue was lower than
that in the metastatic tissues. Then, upregulated miR-10b
expression may significantly promote BC cell migration and invasion
in vitro and in vivo. However, there was no obvious
evidence that miR-10b promotes BC cell proliferation or confers
resistance to the chemotherapeutic agent 5-fluorouracil or
cisplatin (data not shown), which had been reported in other types
of cancer.
KLF4 and HOXD10, which have putative binding sites
for miR-10b have been reported to be direct targets of miR-10b in
other types of cancer. However, these target effects were not
observed in BC.
KLF4 is a zinc-finger transcription factor that
regulates a multitude of processes in normal tissue including
proliferation, differentiation, apoptosis, tissue homeostasis and
self-renewal (30–34). Inactivation or silencing of KLF4 has
been observed in a number of human cancers including colorectal,
gastric, pancreas, esophageal, lung, prostate and hepatocellular
cancer (35–42), which suggests KLF4 may function as a
tumor suppressor and a potential prognostic marker for overall
survival time and lymph node metastasis (35,40).
The present study reported that KLF4 was downregulated partially by
promoter methylation and has a function as a tumor suppressor gene
in renal clear cell cancer and bladder urothelial cancer. However,
KLF4 in BC has never been reported to be connected with miR-10b. In
the present study, we used luciferase reporter assay to identify
that KLF4 was also the direct target of miR-10b in BC cells; we not
only found the mediate role in the promotion of migration and
invasion of miR-10b, but we also showed another cause of KLF4
downregulation besides promoter methylation (16). HOXD10, a member of type I class
homeobox (Hox) genes, plays an important role in suppressing
angiogenesis and maintaining a quiescent, differentiated phenotype
in endothelial cells (21).
Specifically, HOXD10 suppresses expression of genes that directly
affect remodeling of the extracellular matrix and cell migration
during angiogenesis such as a3 integrin, matrix metalloproteinase
14 (MMP14), and urokinase-type plasminogen activator receptor
(uPAR). To date, there is no report on the function of HOXD10 in
BC. In the present study, we found that the siRNA for HOXD10 can
promote the migration and invasion of BC cells. Taken together,
these results established a functional connection between miR-10b,
KLF4 and HOXD10, and confirmed that miR-10b functions as a
pro-metastatic miRNA in BC cells by targeting KLF4 and HOXD10.
Metastasis arises through a multistep process that
begins when cancer cells within tumors in situ detach from
neighboring cells and invade the basement membrane (43). The best characterized alteration
mainly involved the loss of E-cadherin, a key cell-to-cell adhesion
molecule (44). Expression of
E-cadherin is controlled by several transcriptional repressors,
including Twist, Snail1, Snail2/Slug, E47, ZEB1/TCF8 and ZEB2/SIP1,
which bind to E-boxes in the E-cadherin promoter. Yori et al
(20) found that KLF4 silencing
increased Snail expression in breast cancer. However, Liu et
al (19) proved that KLF4
reverses epithelial-mesenchymal transition (EMT) through
downregulation of Slug not snail in prostate cancer. Yori et
al also found that E-cadherin is a novel transcriptional target
of KLF4. Our previous study also reported that KLF4 overexpression
inhibits urothelial cancer cell EMT, a process by which epithelial
cells lose their cell polarity and cell-cell adhesion, and gain
migratory and invasive properties to become mesenchymal cells, and
promote the expression of cell-cell adhesion markers such as
E-cadherin (16). In the present
study, miR-10b inhibitor transfection in BC was sufficient to
promote E-cadherin expression. Ibrahim et al found that
miR-10b can downregulate E-cadherin by miR-10b-syndecan-1 axis
(45). Our report suggested that
promotion of E-cadherin by miR-10b, through targeting KLF4, can
promote BC cell metastasis in the early stage.
In summary, miR-10b was significantly upregulated in
highly metastatic cells and tissues. miR-10b overexpression can
enhance the migration, invasion and metastasis ability of BC cells
in vitro and in vivo. Additionally, KLF4 and HOXD10
were found to be direct and functional targets of miR-10b, and
mediated the effect in BC cell migration and invasion.
Acknowledgements
We thank all the donors who participated in this
program, all our coworkers who contributed to the construction of
the urologic tumor tissue bank sponsored by the Department of
Urology, Tongji Hospital, Tongji Medical College, Huazhong
University of Science and Technology. The present study was
supported by the National Natural Science Foundation of China (nos.
31072238, 31172441, 31372562 and 81170650), and the National Major
Scientific and Technological Special Project for Significant New
Drugs Development (2012ZX09303018). The sponsors had no role in the
study design, data collection and analysis, decision to publish, or
preparation of the manuscript.
References
|
1
|
Zaravinos A, Radojicic J, Lambrou GI, et
al: Expression of miRNAs involved in angiogenesis, tumor cell
proliferation, tumor suppressor inhibition, epithelial-mesenchymal
transition and activation of metastasis in bladder cancer. J Urol.
188:615–623. 2012. View Article : Google Scholar
|
|
2
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zamore PD and Haley B: Ribo-gnome: the big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
White NM, Fatoohi E, Metias M, Jung K,
Stephan C and Yousef GM: Metastamirs: a stepping stone towards
improved cancer management. Nat Rev Clin Oncol. 8:75–84. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asangani IA, Rasheed SA, Nikolova DA, et
al: MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor
suppressor Pdcd4 and stimulates invasion, intravasation and
metastasis in colorectal cancer. Oncogene. 27:2128–2136. 2008.
View Article : Google Scholar
|
|
11
|
Johnson SM, Grosshans H, Shingara J, et
al: RAS is regulated by the let-7 microRNA family.
Cell. 120:635–647. 2005. View Article : Google Scholar
|
|
12
|
Liang S, He L, Zhao X, et al: MicroRNA
let-7f inhibits tumor invasion and metastasis by targeting MYH9 in
human gastric cancer. PLoS One. 6:e184092011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sampson VB, Rong NH, Han J, et al:
MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth
in Burkitt lymphoma cells. Cancer Res. 67:9762–9770. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gabriely G, Yi M, Narayan RS, et al: Human
glioma growth is controlled by microRNA-10b. Cancer Res.
71:3563–3572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian Y, Luo A, Cai Y, et al: MicroRNA-10b
promotes migration and invasion through KLF4 in human esophageal
cancer cell lines. J Biol Chem. 285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Wang J, Xiao W, et al: Epigenetic
inactivation of KLF4 is associated with urothelial cancer
progression and early recurrence. J Urol. 191:493–501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yori JL, Seachrist DD, Johnson E, et al:
Krüppel-like factor 4 inhibits tumorigenic progression and
metastasis in a mouse model of breast cancer. Neoplasia.
13:601–610. 2011.
|
|
18
|
Lin ZS, Chu HC, Yen YC, Lewis BC and Chen
YW: Krüppel-like factor 4, a tumor suppressor in hepatocellular
carcinoma cells reverts epithelial mesenchymal transition by
suppressing slug expression. PLoS One. 7:e435932012.
|
|
19
|
Liu YN, Abou-Kheir W, Yin JJ, et al:
Critical and reciprocal regulation of KLF4 and SLUG in transforming
growth factor β-initiated prostate cancer epithelial-mesenchymal
transition. Mol Cell Biol. 32:941–953. 2012.PubMed/NCBI
|
|
20
|
Yori JL, Johnson E, Zhou G, Jain MK and
Keri RA: Krüppel-like factor 4 inhibits epithelial-to-mesenchymal
transition through regulation of E-cadherin gene expression. J Biol
Chem. 285:16854–16863. 2010.
|
|
21
|
Myers C, Charboneau A, Cheung I, Hanks D
and Boudreau N: Sustained expression of homeobox D10 inhibits
angiogenesis. Am J Pathol. 161:2099–2109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iorio MV and Croce CM: Causes and
consequences of microRNA dysregulation. Cancer J. 18:215–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun L, Yan W, Wang Y, et al: MicroRNA-10b
induces glioma cell invasion by modulating MMP-14 and uPAR
expression via HOXD10. Brain Res. 1389:9–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
miR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|
|
25
|
Nakata K, Ohuchida K, Mizumoto K, et al:
MicroRNA-10b is overexpressed in pancreatic cancer, promotes its
invasiveness, and correlates with a poor prognosis. Surgery.
150:916–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Wu Z, Peng Y, et al: MicroRNA-10b
induced by Epstein-Barr virus-encoded latent membrane protein-1
promotes the metastasis of human nasopharyngeal carcinoma cells.
Cancer Lett. 299:29–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chai G, Liu N, Ma J, et al: MicroRNA-10b
regulates tumorigenesis in neurofibromatosis type 1. Cancer Sci.
101:1997–2004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishida N, Yamashita S, Mimori K, et al:
MicroRNA-10b is a prognostic indicator in colorectal cancer and
confers resistance to the chemotherapeutic agent 5-fluorouracil in
colorectal cancer cells. Ann Surg Oncol. 19:3065–3071. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu YC, Chen YJ, Wang HM, et al: Oncogenic
function and early detection potential of miRNA-10b in oral cancer
as identified by microRNA profiling. Cancer Prev Res. 5:665–674.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shields JM, Christy RJ and Yang VW:
Identification and characterization of a gene encoding a
gut-enriched Krüppel-like factor expressed during growth arrest. J
Biol Chem. 271:20009–20017. 1996.PubMed/NCBI
|
|
31
|
Garrett-Sinha LA, Eberspaecher H, Seldin
MF and de Crombrugghe B: A gene for a novel zinc-finger protein
expressed in differentiated epithelial cells and transiently in
certain mesenchymal cells. J Biol Chem. 271:31384–31390. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Segre JA, Bauer C and Fuchs E: Klf4 is a
transcription factor required for establishing the barrier function
of the skin. Nat Genet. 22:356–360. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghaleb AM, McConnell BB, Nandan MO, Katz
JP, Kaestner KH and Yang VW: Haploinsufficiency of Krüppel-like
factor 4 promotes adenomatous polyposis coli dependent intestinal
tumorigenesis. Cancer Res. 67:7147–7154. 2007.
|
|
34
|
Jiang J, Chan YS, Loh YH, et al: A core
Klf circuitry regulates self-renewal of embryonic stem cells. Nat
Cell Biol. 10:353–360. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei D, Gong W, Kanai M, et al: Drastic
down-regulation of Krüppel-like factor 4 expression is critical in
human gastric cancer development and progression. Cancer Res.
65:2746–2754. 2005.PubMed/NCBI
|
|
36
|
Zhao W, Hisamuddin IM, Nandan MO, Babbin
BA, Lamb NE and Yang VW: Identification of Krüppel-like factor
4 as a potential tumor suppressor gene in colorectal cancer.
Oncogene. 23:395–402. 2004.
|
|
37
|
Wei D, Kanai M, Jia Z, Le X and Xie K:
Krüppel-like factor 4 induces p27Kip1
expression in and suppresses the growth and metastasis of human
pancreatic cancer cells. Cancer Res. 68:4631–4639. 2008.
|
|
38
|
Yang Y, Goldstein BG, Chao HH and Katz JP:
KLF4 and KLF5 regulate proliferation, apoptosis and invasion in
esophageal cancer cells. Cancer Biol Ther. 4:1216–1221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu W, Hofstetter WL, Li H, et al: Putative
tumor-suppressive function of Krüppel-like factor 4 in primary lung
carcinoma. Clin Cancer Res. 15:5688–5695. 2009.
|
|
40
|
Wang J, Place RF, Huang V, et al:
Prognostic value and function of KLF4 in prostate cancer: RNAa and
vector-mediated overexpression identify KLF4 as an inhibitor of
tumor cell growth and migration. Cancer Res. 70:10182–10191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kanai M, Wei D, Li Q, et al: Loss of
Krüppel-like factor 4 expression contributes to Sp1 overexpression
and human gastric cancer development and progression. Clin Cancer
Res. 12:6395–6402. 2006.
|
|
42
|
Li Q, Gao Y, Jia Z, et al: Dysregulated
Krüppel-like factor 4 and vitamin D receptor signaling contribute
to progression of hepatocellular carcinoma. Gastroenterology.
143:799–810. 2012.
|
|
43
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
44
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ibrahim SA, Yip GW, Stock C, et al:
Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer
cell motility and invasiveness via a Rho-GTPase- and
E-cadherin-dependent mechanism. Int J Cancer. 131:E884–E896. 2012.
View Article : Google Scholar : PubMed/NCBI
|