Introduction
Therapeutic approaches to human leukemia include
irradiation, hyperthermia and chemotherapy. Overall survival rates
of children currently range from 83 to 94% for acute lymphoblastic
leukemia (ALL) (1) and from 60 to
65% for acute myeloid leukemia (AML) (2). The survival rates have improved
markedly over time, largely due to conventional chemotherapy.
However, the side-effects of cytotoxic chemotherapy are
significant, and drug resistance in cancer remains a challenge when
attempting to cure leukemia. Therefore, the development of
effective antitumor drugs with high efficacy and low toxicity
represents a focus of current research in this area.
Recently, great attention has been given to the
identification of natural substances capable of inhibiting or
retarding the progression of different stages of carcinogenesis.
Anti-neoplastic drugs from natural sources capable of targeted
specific cytotoxicity and induction of apoptosis in cancer cells
with minimal side-effects are the best choice (3). The Securinega alkaloids are a
class of natural products isolated from the plants of the
Euphorbiaceae family. Securinine was initially isolated from
Securinega suffruticosa by Russian scientists in 1956
(4). Its structure was determined
by chemical and spectroscopic studies in 1963 (5) and was verified by X-ray
crystallography in 1965 (6). There
are two optical isomers, L-securinine and D-securinine, with the
pharmacological activity of D-securinine being weaker (by ~10%)
than that of L-securinine (7).
Securinine exhibits interesting biological activities. It has been
reported to be a GABA receptor antagonist (8) and to exert aplastic anemia activity
(9). Recent publications have
reported that securinine exhibits antimalarial (10) and antibacterial (11) activities as well as apoptotic
activity in human colon cancer SW480 cells (12). Thus, the pharmacology and clinical
applications of securinine have recently attracted significant
attention.
The phosphatidylinositol 3-kinase/AKT/mammalian
target of rapamycin (PI3K/AKT/mTOR) signaling pathway plays an
important role in cellular proliferation, development and death
(13). This pathway, which was
first identified in the 1990s (14), is known to be activated during the
early phase of the onset of lung cancer (15), thereby causing cell growth,
proliferation, angiogenesis and synthesis of various proteins
(16,17). PI3K activates the serine/threonine
kinase AKT, which, in turn, results in the phosphorylation and
activation of the serine/threonine kinase mTOR through a cascade of
regulators. The mTOR controls the PI3K/AKT/mTOR signaling pathway
that promotes cell growth (18).
The PI3K/AKT/mTOR pathway is dysregulated in many types of cancer,
including AML (19).
Among the anticancer agents that interfere with
PI3K/AKT/mTOR signaling, inhibitors of mTOR have reached the
furthest stage in clinical development and have demonstrated
efficacy in renal cell carcinomas (20), neuroendocrine tumors (21) and breast cancer (22). The tumor suppressor, PTEN, is a
phosphatase with a variety of substrate specificities that
functions as a negative regulator of the PI3K/AKT/mTOR signaling
pathway (23). Inactivation of PTEN
increases ABCG2 expression and inhibition of PI3K/AKT/mTOR pathway
components, thus representing an attractive therapeutic target in
AML (24).
Materials and methods
Chemicals
The pure sample of L-securinine (Fig. 1) used in the present study was
provided by the Institute of Traditional Chinese Medicine and
Natural Products, Jinan University, China.
Cell culture
The human promyelocytic leukemia cell line HL-60,
purchased from Nanjing KeyGen Biotech Co., Ltd.(Nanjing, China),
was maintained in RPMI-1640 medium (Gibco, Carlsbad, CA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Sijiqing Biological Engineering Materials, Hangzhou, China;
120316), 100 IU/ml penicillin and 100 μg/ml streptomycin, in a
humidified incubator (Sanyo XD-101; Sanyo, Osaka, Japan) with 5%
CO2 at 37°C.
Analysis of cell viability
Exponentially growing HL-60 cells
(5.0×103) were seeded into 96-well plates (3599; Corning
Incorporated). After 24 h, HL-60 cells were fed with RPMI-1640
medium containing 10% FBS and treated (in triplicate) with
L-securinine (200 μl/well) at concentrations ranging from 0 to 400
μmol/l. The plates were then cultured for 24, 48 and 72 h at 37°C.
Cell viability was examined using the Cell Counting Kit-8 (CCK-8)
(KGA317; Nanjing KeyGen Biotech) assays, which are based on the
principle of CCK-8 (water-soluble tetrazolium salt) cleavage to
generate a formazan-class dye by mitochondrial succinate
tetrazolium reductase in viable cells. Cell counting solution (10
μl) was added to each well and incubated for 3 h prior to detection
of formazan-class dyes by measuring the absorbance at 450 nm using
a spectrophotometer (ELx800; Bio Tek Instruments, Winooski, VT,
USA). The relative inhibition of cell proliferation (IR) was
calculated according to the following formula: IR = (1 - average
A450 of the experimental group/average A450
of the control group) × 100%.
Electron microscopy
Induction of apoptosis in the L-securinine-treated
HL-60 cells was evaluated by ultrastructural analysis of cell
morphology as previously described. HL-60 cells were treated with
or without L-securinine at a concentration of 25 μmol/l for 48 h,
washed three times with PBS, trypsinized and collected by
centrifugation. Cells were then fixed for 2 h in 2.5% ice-cold
glutaraldehyde for 30 min, then post-fixed with 1% OsO4
in cacodylate buffer for 1 h. Areas were chosen for ultra-thin
sectioning and viewed with an electron microscope (JEM-1011
transmission electron microscope; JEOL, Peabody, MA, USA).
Analysis of cell apoptosis
Cells (106) were treated with medium for
4 h, followed by treatment with medium containing L-securinine at
concentrations of 12.5, 25 and 50 μmol/l for 48 h. After
incubation, cells were harvested into 5-ml centrifuge tubes and
centrifuged at 300 × g for 10 min. Using cold PBS, the cells were
washed three times, and a volume of 100 μl binding buffer (Annexin
V-FITC Apoptosis Detection Kit I (KGA105; Nanjing KeyGen Biotech)
was added into the tube. Subsequently, Annexin V-FITC and propidium
iodide (PI) solutions (both 1.25 μl) were added into the tube and
incubated in the dark for 15 min. Then, 1X binding buffer (400 μl)
was added to each tube and gently vortexed before flow cytometric
analysis (FCM) (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). Approximately, 10,000 events were acquired and sorted
accordingly into viable, early apoptotic, late apoptotic and
necrotic cells (25,26).
Cell cycle analysis
Cells (106) were treated with medium for
4 h, followed by treatment with medium containing L-securinine at
concentrations of 12.5, 25 and 50 μmol/l for 48 h. Cells were then
collected and fixed in 70% ethanol at 4°C overnight. Subsequently,
cells were treated with 1% RNase at 37°C and stained with PI
solution (KGA511; Nanjing KeyGen Biotech) for 30 min at 4°C.
PI-stained nuclei were analyzed by flow cytometry (FACSCalibur; BD
Biosciences). The ratios of the cells in the G0/G1, S and G2/M
phases were calculated (27).
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR assays were performed
on HL-60 cells treated with or without L-securinine in order to
evaluate the expression of the following genes: PTEN, PI3K, AKT and
mTOR. For each gene analyzed, total RNA from the cultured cells was
isolated with TRIzol reagent (15596-026; Invitrogen, Carlsbad, CA,
USA) according to the manufacturer’s recommended protocol. A
two-step reverse transcription PCR was performed. First-strand cDNA
was synthesized using 2 μg of RNA with the First-Strand cDNA
Synthesis kit (PC0002; Fermentas, Vilnius, Lithuania) according to
the manufacturer’s protocol. To investigate the expression of genes
at the mRNA level, the expression of CK8 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes was
quantified by RT-PCR, and GAPDH was used as an internal control.
Quantitative real-time RT-PCR was conducted using 2 μl of the
primer mixture (forward and reverse; 10 μmol), added to 10 μl
SYBR-Green and then diluted with 7 μl DEPC water. A final volume of
19 μl was dispensed into each well, and 1 μl of diluted cDNA was
added. Each sample was tested in triplicate for each gene, and PCR
reactions were performed using real-time fluorescence quantitative
PCR (DA7600; Zhongshandaan, China). The thermal profile consisted
of 95°C for 5 min, followed by 40 cycles of 94°C for 15 sec, 60°C
for 20 sec, and 72°C for 40 sec. The experiment was repeated three
times. The efficiency of cDNA synthesis for each sample was
estimated by PCR with GAPDH-specific primers. The sequences of the
primers used were as follows: GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′ and reverse, 5′-CTCCACGACGTACTCAGCG-3′;
PTEN forward, 5′-CAAGATGATGTTTGAAACTATTCCAATG-3′ and reverse,
5′-CCTTTAGCTGGCAGACCACAA-3′; PI3K forward,
5′-GGGGATGATTTACGGCAAGATA-3′ and reverse,
5′-CACCACCTCAATAAGTCCCACA-3′; AKT1 forward,
5′-GCAGCACGTGTACGAGAAGA-3′ and reverse, 5′-GGTGTCAGTCTCCGACGTG-3′;
mTOR forward, 5′-ATT TGATCAGGTGTGCCAGT-3′ and reverse, 5′-GCTTAGGA
CATGGTTCATGG-3′.
Data analysis was performed using the Sequence
Detector System software. The relative quantification was
calculated by the 2−ΔΔCt method with GAPDH as the
housekeeping gene and the control cells as the baseline, and the
results are expressed as fold-changes.
Statistical analysis
The data are expressed as means ± SD. Statistically
significant differences between two groups were analyzed using the
Student’s t-test, and multiple comparisons were performed by
one-way analysis of variance (ANOVA). All statistical analyses were
performed using the SPSS 13.0 software. Statistical significance
was accepted at a level of P<0.05.
Results
L-securinine treatment inhibits HL-60
cell growth in vitro
The CCK-8 assay was used to determine the effects of
L-securinine on the proliferation of HL-60 cells. L-securinine
significantly inhibited the growth of HL-60 cells in a dose- and
time-dependent manner (Fig. 2),
with IC50 values of 47.88, 23.85 and 18.87 μmol/l at 24,
48 and 72 h post-treatment, respectively.
L-securinine treatment induces apoptosis
of HL-60 cells in vitro
The induction of apoptosis in HL-60 cells by
L-securinine treatment was determined by electron microscopic
analysis. The formation of apoptotic bodies, which are suggestive
of active apoptosis, was observed in HL-60 cells treated with 25
μmol/l L-securinine for 48 h, whereas none were observed in HL-60
cells in the control groups (Figs.
3 and 4).
L-securinine treatment inhibits HL-60
cell cycle phase progression in vitro
Cell cycle analysis of HL-60 cells following
treatment with L-securinine (0, 12.5, 25 and 50 μmol/l) for 48 h
was performed using flow cytometric techniques. A dose-dependent
increase in apoptosis was observed in the sub-G1 population of
HL-60 cells treated with L-securinine (Fig. 5). Furthermore, the percentage of
HL-60 cells in the G1 phase was observed to be 51.14, 59.82 and
64.02% following treatment with 12.5, 25 and 50 μmol/l
L-securinine, respectively (Fig. 5;
Table I).
 | Table ICell cycle distribution of HL-60 cells
treated with L-securinine at different concentrations for 48 h by
flow cytometry. |
Table I
Cell cycle distribution of HL-60 cells
treated with L-securinine at different concentrations for 48 h by
flow cytometry.
| Concentration of
L-securinine | G1 (%)a | S (%)a | G2 (%) |
|---|
| 0 (μmol/l) | 42.13 | 40.54 | 17.33 |
| 12.5 | 51.14 | 37.78 | 11.08 |
| 25 | 59.82 | 32.55 | 7.63 |
| 50 | 64.02 | 31.79 | 4.20 |
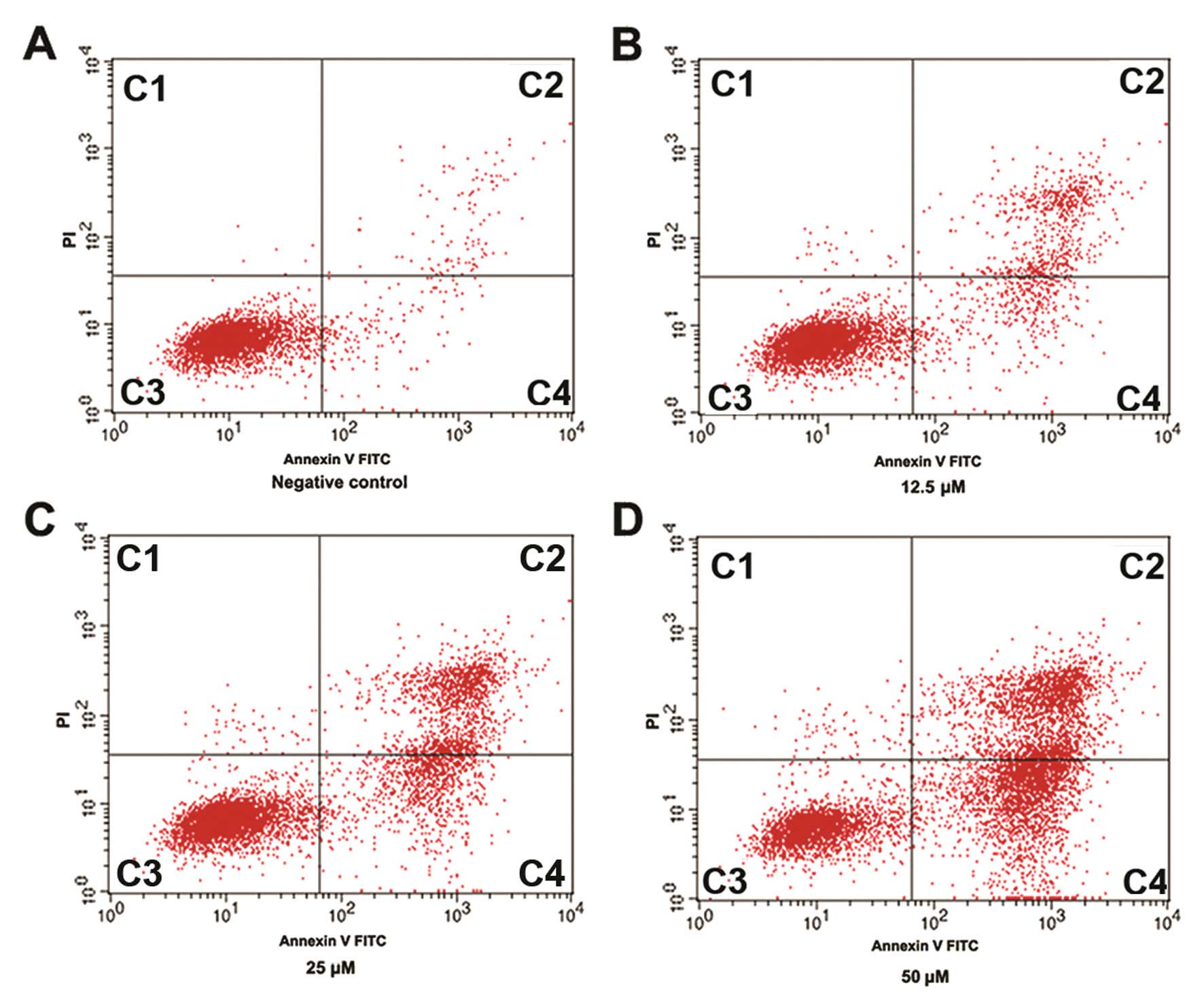
L-securinine treatment induces apoptosis
in HL-60 cells in vitro
Apoptosis rates in HL-60 cells treated with
L-securinine were determined by flow cytometric analysis of
FITC-Annexin V and PI staining. The percentages of cells in each
quadrant in Fig. 6 are
representative of: (C1) necrosis, (C2) late apoptosis, (C3) live
cells and (C4) early apoptosis. A marked dose-dependent increase in
both the early and late stages of apoptosis was obvious in the
HL-60 cells after L-securinine treatment compared with the control
group. The percentages of apoptotic cells treated with 12.5, 25.0
and 50.0 μmol/l L-securinine for 48 h were 20.42, 37.14 and 66.36%,
respectively (Fig. 6; Table II).
 | Table IIComparison of HL-60 cell apoptosis
induced by L-securinine at different concentrations at 48 h as
assayed by Annexin V-FITC method. |
Table II
Comparison of HL-60 cell apoptosis
induced by L-securinine at different concentrations at 48 h as
assayed by Annexin V-FITC method.
| Concentration of
L-securinine | Apoptosis (%)a | LL (%)a |
|---|
| 0 (μmol/l) | 6.24 | 93.65 |
| 12.5 | 20.42 | 79.11 |
| 25 | 37.14 | 62.19 |
| 50 | 66.36 | 32.72 |
L-securinine treatment influences the
PI3K/AKT/mTOR signaling pathway gene expression in HL-60 cells
The PI3K/AKT/mTOR signaling pathway, which is vital
in promoting cell growth and proliferation (13), is implicated in the mechanism
underlying L-securinine-induced apoptosis in HL-60 cells.
PI3K/AKT/mTOR pathway gene expression was measured by quantitative
real-time RT-PCR. L-securinine treatment induced downregulation of
PI3K, AKT and mTOR gene expression and upregulation of PTEN gene
expression in HL-60 cells in a dose-dependent manner (Fig. 7; Table
III).
 | Table IIIqPCR analysis of PTEN, PI3K, Akt and
mTOR mRNA in HL-60 cells treated with L-securinine at 6.25, 12.5
and 25.0 μmol/l for 48 h. |
Table III
qPCR analysis of PTEN, PI3K, Akt and
mTOR mRNA in HL-60 cells treated with L-securinine at 6.25, 12.5
and 25.0 μmol/l for 48 h.
| Concentration of
L-securinine | mTOR/GAPDH | PI3K/GAPDH | AKT/GAPDH | PTEN/GAPDH |
|---|
| 0 (μmol/l) |
1.00±0.03a |
1.00±0.03a |
1.00±0.02a |
1.00±0.02a |
| 12.5 |
0.72±0.02b |
0.80±0.03b |
0.72±0.02b |
2.14±0.04b |
| 25 |
0.45±0.01c |
0.53±0.01c |
0.47±0.03c |
5.62±0.12c |
| 50 |
0.12±0.02d |
0.25±0.02d |
0.26±0.01d |
10.24±0.43d |
Discussion
Identification of novel natural compounds that
mediate cancer cell cytotoxicity with high specificity and low
non-specific toxicity is an important area in cancer research. In
the present study, we showed that L-securinine inhibits HL-60 cell
growth, induces apoptosis and enhances the expression of genes
involved in the PI3K/AKT/mTOR signaling pathway in a dose-dependent
manner. Our studies revealed that the IC50 values for
L-securinine in HL-60 cells at 24, 48 and 72 h post-treatment were
47.88, 23.85 and 18.87 μmol/l, respectively. According to The US
National Cancer Institute NCI Plant Screening Program, in
vitro cytotoxicity activity of a crude extract is demonstrated
by IC50 values of <20 μg/ml (919 μmol/l) following
incubation between 48 and 72 h (28). Thus, our data demonstrated that
L-securinine exhibits in vitro cytotoxic activity in HL-60
cells.
Anti-neoplastic drugs act by interfering with cell
proliferation or, in most cases, by inducing programmed cell death,
known as apoptosis (29). In the
present study, numerous apoptotic bodies were observed by electron
microscopy in HL-60 cells following treatment with 25 μmol/l
L-securinine for 48 h. Furthermore, FCM analysis revealed that the
rate of apoptosis in L-securinine-treated HL60 cells was increased
in a dose-dependent manner over the range of 12.5, 25 and 50 μmol/l
and that this effect correlated with an increase in the number of
cells arrested in the G1 phase of the cell cycle. Apoptosis
provides a number of clues with respect to effective anticancer
therapy, and many anti-neoplastic agents exert their antitumor
effects in cancer cells by inducing apoptosis. These data provide
strong evidence that L-securinine has the potential to be developed
as an antineoplastic agent for clinical use.
The present study also revealed that L-securinine
influences the expression of genes involved in the PI3K/AKT/mTOR
signaling pathway, which promotes cell survival, proliferation and
progression in cancer cells. Specifically PI3K, AKT and mTOR gene
expression was downregulated in a dose-dependent manner in response
to L-securinine treatment, while PTEN gene expression was
upregulated. These observations indicate that targeting the
PI3K/AKT/mTOR signaling pathway may lead to the development of
novel therapeutic approaches for human cancers (30). Taken together, these data illustrate
a new and imperative role for PI3K/AKT/mTOR signaling in the
mechanism by which L-securinine induces apoptosis in HL-60
cells.
The aim of the present study was to investigate the
potential of natural compounds, such as L-securinine, to
exclusively target cancer cells. Our results demonstrated that
L-securinine induces apoptosis and inhibition of cell cycle
progression in HL-60 cells via a mechanism that involves modulation
of PI3K/AKT/mTOR pathway gene expression. Although further studies
are required to investigate the effects of L-securinine in
vitro in normal cell lines, in vivo in animal models and
finally, in humans through clinical trials, these observations
indicate the potential of L-securinine for development as an
antitumor agent.
Acknowledgements
The present study was supported by the fund of the
National Natural Science Foundation of China (81241102). We would
particularly like to thank the Institute of Traditional Chinese
Medicine and Natural Products, Jinan University for providing the
pure sample of L-securinine.
References
|
1
|
Pui CH: Recent research advances in
childhood acute lymphoblastic leukemia. J Formos Med Assoc.
109:777–787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaspers GJ and Creutzig U: Pediatric acute
myeloid leukemia: international progress and future directions.
Leukemia. 19:2025–2029. 2005. View Article : Google Scholar
|
|
3
|
Kim KC, Kim JS, Son JK and Kim IG:
Enhanced induction of mitochondrial damage and apoptosis in human
leukemia HL-60 cells by the Ganoderma lucidum and
Duchesnea chrysantha extracts. Cancer Lett. 246:210–217.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muraveva V and Bankovskii A: Chemical
study of alkaloids of Securinega suffruticosa. Doklady Akad
Nauk SSSR. 110:998–1000. 1956.
|
|
5
|
Saito S, Kotera K, Shigematsu N, Ide A,
Sugimoto N, Horii Z, Hanaoka M, Yamawaki Y and Tamura Y: Structure
of securinine. Tetrahedron. 19:2085–2099. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imado S, Shiro M and Horii Z: The crystal
structure of securinine hydrobromide dihydrate and the molecular
structure of securinine. Chem Pharm Bull. 13:643–651. 1965.
View Article : Google Scholar
|
|
7
|
Peng JZ: Securinine pharmacological and
clinical progress. J Ningxia Med Coll. 16:21994.
|
|
8
|
Beutler JA, Karbon EW, Brubaker AN, Malik
R, Curtis DR and Enna SJ: Securinine alkaloids: a new class of GABA
receptor antagonist. Brain Res. 330:135–140. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
CL Y, LQ L and LS S: The treatment of
aplastic anemia. Tianjin Med J. 9:662–665. 1981.
|
|
10
|
Weenen H, Nkunya MH, Bray DH, Mwasumbi LB,
Kinabo LS, Kilimali VA and Wijnberg JB: Antimalarial compounds
containing an α, β-unsaturated carbonyl moiety from Tanzanian
medicinal plants. Planta Med. 56:371–373. 1990.
|
|
11
|
Mensah JL, Lagarde I, Ceschin C, Michel G,
Gleye J and Fouraste I: Antibacterial activity of the leaves of
Phyllanthus discoideus. J Ethnopharmacol. 28:129–133. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CR, Xia YH, Yao SY, Zhang Q, Wang Y
and Ji ZN: Virosecurinine induces apoptosis by affecting Bcl-2 and
Bax expression in human colon cancer SW480 cells. Pharmazie.
67:351–354. 2012.PubMed/NCBI
|
|
13
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burnett PE, Barrow RK, Cohen NA, Snyder SH
and Sabatini DM: RAFT1 phosphorylation of the translational
regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA.
95:1432–1437. 1998. View Article : Google Scholar
|
|
15
|
West KA, Linnoila IR, Belinsky SA, Harris
CC and Dennis PA: Tobacco carcinogen-induced cellular
transformation increases activation of the phosphatidylinositol
3′-kinase/Akt pathway in vitro and in vivo. Cancer
Res. 64:446–451. 2004.PubMed/NCBI
|
|
16
|
Janku F, Stewart DJ and Kurzrock R:
Targeted therapy in non-small-cell lung cancer - is it becoming a
reality? Nat Rev Clin Oncol. 7:401–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
19
|
Ozpolat B, Akar U, Steiner M,
Zorrilla-Calancha I, Tirado-Gomez M, Colburn N, Danilenko M,
Kornblau S and Berestein GL: Programmed cell death-4 tumor
suppressor protein contributes to retinoic acid-induced terminal
granulocytic differentiation of human myeloid leukemia cells. Mol
Cancer Res. 5:95–108. 2007. View Article : Google Scholar
|
|
20
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E,
O’Toole T, Lustgarten S, Moore L, Motzer RJ and Global AT:
Temsirolimus, interferon alfa, or both for advanced renal-cell
carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar
|
|
21
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D and
Oberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial
(RADIANT-3) Study Group. Everolimus for advanced pancreatic
neuroendocrine tumors. N Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori
L, Xu Z, Mukhopadhyay P, Lebwohl D and Hortobagyi GN: Everolimus in
postmenopausal hormone-receptor-positive advanced breast cancer. N
Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maiuri MC, Tasdemir E, Criollo A, Morselli
E, Vicencio JM, Carnuccio R and Kroemer G: Control of autophagy by
oncogenes and tumor suppressor genes. Cell Death Differ. 16:87–93.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang FF, Wu DS, Zhang L, Yu YH, Yuan XY,
Li WJ, Chen XP, Zhao XL, Chen FP and Zeng H: Inactivation of PTEN
increases ABCG2 expression and the side population through the
PI3K/Akt pathway in adult acute leukemia. Cancer Lett. 336:96–105.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inayat-Hussain SH, Osman AB, Din LB and
Taniguchi N: Altholactone, a novel styryl-lactone induces apoptosis
via oxidative stress in human HL-60 leukemia cells. Toxicol Lett.
131:153–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pelicano H, Feng L, Zhou Y, Carew JS,
Hileman EO, Plunkett W, Keating MJ and Huang P: Inhibition of
mitochondrial respiration: a novel strategy to enhance drug-induced
apoptosis in human leukemia cells by a reactive oxygen
species-mediated mechanism. J Biol Chem. 278:37832–37839. 2003.
View Article : Google Scholar
|
|
27
|
Li L, Pan S, Zhou X, Meng X, Han X, Ren Y,
Yang K and Guan Y: Reduction of in-stent restenosis risk on
nickel-free stainless steel by regulating cell apoptosis and cell
cycle. PLoS One. 8:e621932013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castello-Branco MVS, Tavares JF, Silva MS,
Barbosa Filho JM, Anazetti MC, Frungillo L, Haun M, Melo Diniz MF
and Melo PS: Xylodiol from Xylopia langsdorfiana induces
apoptosis in HL60 cells. Revista Brasileira de Farmacognosia.
21:1035–1042. 2011.
|
|
29
|
Nahata A, Saxena A, Suri N, Saxena AK and
Dixit VK: Sphaeranthus indicus induces apoptosis through
mitochondrial-dependent pathway in HL-60 cells and exerts cytotoxic
potential on several human cancer cell lines. Integr Cancer Ther.
12:236–247. 2013. View Article : Google Scholar
|
|
30
|
Liu S, Wang XJ, Liu Y and Cui YF:
PI3K/AKT/mTOR signaling is involved in
(−)-epigallocatechin-3-gallate-induced apoptosis of human
pancreatic carcinoma cells. Am J Chin Med. 41:629–642. 2013.
|