Introduction
Natural killer (NK)/T cell lymphoma usually shows a
highly aggressive clinical course and is much more common in Asian
and Latin American countries than in Western countries (1). The overall prognosis of this disease
is poor due to frequent relapse or resistance to treatment
(2,3). The response of NK/T cell lymphoma to
combined radiotherapy and chemotherapy is usually frustratingly
inadequate (4). What is more, most
patients initially have low International Prognostic Index (IPI)
scores, as they usually present with localized disease involving
the head and neck with good performance status (5,6).
Although the treatment of NK/T cell lymphoma has
improved greatly, there are no standard therapeutic regimens for
this disease. L-asparaginase (L-Asp) and pegaspargase (PEG-Asp) are
important chemotherapeutic drugs for childhood acute lymphoblastic
leukemia (ALL), and clinical trials have also shown that L-Asp
treatment improves the outcome of NK/T cell lymphoma (7). The exact molecular events that cause
cell death following L-Asp treatment remain unknown. However,
suppression of protein synthesis is an obvious potential target and
it has been documented that L-Asp exposure initiates the apoptosis
of cells (8). Although the
chemotherapeutic protocols containing L-Asp have improved the
efficacy of treatment, a number of patients are resistant to L-Asp
or PEG-Asp, leading to treatment failure and poor long-term
prognosis.
The asparagine synthetase (ASNS) gene encodes the
enzyme that catalyzes the biosynthesis of asparagine from
aspartate. This reaction proceeds in an ATP-dependent manner with
glutamine serving as the nitrogen source (9). Most tissues contain sufficient ASNS
activity to maintain asparagine or the enzyme is upregulated in
response to asparagine depletion (10,11).
It was reported that primary ALL cells and many ALL cell lines
exhibit a particularly low level of ASNS expression (12,13),
and therefore, are unusually sensitive to asparagine depletion.
Early studies demonstrated that the elevated expression of ASNS was
correlated with the resistance of leukemic cells to L-Asp (14,15).
Similarly, the expression and the functional significance of ASNS
have been investigated in solid tumors. ASNS was considered as a
causal, predictive biomarker for L-Asp activity in ovarian cancer
cells (16,17). Furthermore, one study also showed
that the enhanced expression of ASNS protected pancreatic cancer
cells from apoptosis induced by glucose deprivation and cisplatin
(18). Recently, some studies found
that ASNS is overexpressed in castration-resistant prostate cancer
(CRPC) and that depletion of asparagine using ASNS inhibitors may
be a novel strategy for targeting CRPC cells (19). Another study showed that the
expression of ASNS was an independent factor affecting the survival
of HCC patients, and low ASNS expression in HCC was correlated with
worse surgical outcomes (20).
However, the expression and the functional roles of
ASNS in lymphomas remain unclear, particularly in NK/T cell
lymphoma. The significance of the expression level of ASNS in the
prognosis of NK/T cell lymphoma patients who undergo chemotherapy
containing L-Asp or PEG-Asp has not been reported. Therefore, it is
meaningful to identify the level of ASNS expression and investigate
its clinical significance in the development of NK/T cell
lymphoma.
Materials and methods
Cell culture
Seven lymphoma cell lines (DOHH2, Hut-78, Jurkat,
Karpas 299, Raji, SNK-6 and YTS) were stored in the Lymphoma
Diagnosis and Treatment Center of Henan Province in the First
Affiliated Hospital. All the cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) at 37°C in an atmosphere of 5% CO2.
Cell proliferation assays
The effect of PEG-Asp on cell growth was determined
by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma) absorbance in living cells. In brief,
1.0×05 cells/well were seeded in 96-well microtiter
plates. Following exposure to the designated doses of PEG-Asp for
the indicated times, MTT solution [20 μl: 2 mg/ml in
phosphate-buffered saline (PBS)] was added to each well of 96-well
plates. The plates were additionally incubated for 4 h at 37°C.
Medium was withdrawn from the plates by pipetting, and 200 μl DMSO
was added to each well to solubilize the formazan crystals. The
absorbance was recorded using a Teacan 96-well spectrophotometer at
a wavelength of 570 nm.
Quantitative real-time PCR
Cell suspensions of DOHH2, Hut-78, Jurkat, Karpas
299, Raji, SNK-6 and YTS cells (the number of cells were
5×106) were collected, and the concentration of PEG-Asp
at IC50 was used to treat each cell line for 48 h. Total
RNAs of the 7 lymphoma cell lines before and after drug treatment
were then extracted respectively, and each RNA sample (3 μg) was
subjected to cDNA synthesis by means of an RNA transcription kit
(Thermo Fisher Scientific). Quantitative real-time PCR was
performed using the Quantitative SYBR-Green PCR kit (Thermo Fisher
Scientific) on the ABI PRISM 7500 Fast system (Applied Biosystems).
All experiments were performed as specified according to the
manufacturer’s protocols. The primers used were as follows: ASNS
forward, CTGCACGCCCTCTATGACA and reverse, TAAAAGGCAGCCAATCCTTCT;
GAPDH forward, GAA GGTGAAGGTCGGAGTC and reverse, GAAGATGGTGAT
GGGATTTC. All samples were analyzed using 3 parallel samples and
repeated at least 3 times. The relative expression of the target
gene was calculated using 2−ΔΔCt values with the
Application Relative Quantification Study program.
Patients and follow-up
A total of 50 patients with NK/T cell lymphoma at
the First Affiliated Hospital of Zhengzhou University from 1 July
2009 to 30 June 2010 were enrolled in the study. The patients were
included if they had histologic and immunohistochemical
confirmation of NK/T cell lymphoma according to the 2008 World
Health Organization criteria (21).Tumor staging was performed according
to the Improved Ann Arbor installments. Physical condition scoring
criteria used Zubrod-ECOG-WHO (ZPS, 5-point method). The patients
who were further subjected to survival analysis had not undergone
any previous treatment for lymphoma and received at least one of
the following therapies: chemotherapy containing L-Asp (10,000
U/day for days 1–7 every cycle) or PEG-Asp (4,750 U every cycle)
for at least 2 cycles. Paraffin-embedded tumor tissues were
available for the patients. Tumor responses were assessed for every
two cycles of chemotherapy. Complete response (CR) was defined
according to the Revised Response Criteria for Lymphoma (22). The follow-up period was defined as
the interval from the date of diagnosis to the date of death or the
last follow-up. Patients who died from other causes were treated as
censored cases. All patients were observed until July 2013. Overall
survival was defined as the interval between the date of diagnosis
and death. Progression-free survival (PFS) was defined as the
interval between the date of diagnosis and recurrence or
progression; if recurrence was not diagnosed, patients were
censored at the date of death or the last follow-up. This study was
approved by the Ethics Committee of The First Affiliated Hospital
of Zhengzhou University and informed consent was obtained from all
participants according to the committee’s regulations.
Branched DNA-liquidchip technology
(bDNA-LCT)
bDNA-LCT of SurExam Bio-Tech Co., Ltd. (Guangzhou,
China) was used to detect the ASNS mRNA expression levels in the
NK/T cell lymphoma tissues. The paraffin-embedded tissue samples of
the patients were lysed. The samples then released the RNA, and the
spherical particles in the kit further captured the target RNA. RNA
signals were then amplified, and the Luminex system was used to
detect the expression of the target mRNA level. The original data
obtained by the Multi-Flow Fluorescence Luminex 200™ matrix was
detected and uniformly processed. The result was the netMFI ASNS
mRNA relative expression. Based on the median value, the patients
were divided accrording to ASNS mRNA expression: the ‘high
expression group’ and the ‘low expression group’.
Statistical analysis
All data were processed by SPSS 16.0 software. The
experimental data are expressed as mean ± standard deviation (mean
± SD). Mean values were considered significantly different at
P≤0.05. Pearson’s χ2 test or Fisher’s exact test was
used to analyze the relationship between ASNS expression and the
clinicopathological features. Survival curves were calculated using
the Kaplan-Meier method and compared using the log-rank test. The
Cox proportional-hazard regression model was used for analyses to
explore the effect of the clinicopathological variables and ASNS
expression on survival, and P≤0.05 was considered to indicate a
statistically significant result.
Results
Cell proliferation assays
The MTT assay results showed that the sensitivity of
the different lymphoma cell lines to PEG-Asp (for 48 h) varied
greatly. YTS cells were the most sensitive to the treatment, DOHH2,
Raji and SNK-6 cells were relatively more sensitive and Hut-78,
Jurkat and Karpas 299 cells were naturally resistant to the
treatment. The IC50 values could not be determined for
concentrations >150 U/ml. The IC50 values of 4 cell
lines are shown in Table I and
Fig. 1.
 | Table IIC50 values for the 7
lymphoma cell lines treated with L-Asp and PEG-asp (mean ± SD). |
Table I
IC50 values for the 7
lymphoma cell lines treated with L-Asp and PEG-asp (mean ± SD).
| Cell line | L-asp (U/ml) | PEG-asp (U/ml) | P-value |
|---|
| YTS | 0.00032±0.00013 | 0.00149±0.00029 | 0.00300 |
| SNK-6 | 3.48000±1.33525 | 4.55000±0.82504 | 0.30300 |
| DOHH2 | 12.38466±2.32507 | 8.00033±1.20000 | 0.04400 |
| Raji | 20.15666±2.31971 | 24.0700±1.69717 | 0.07800 |
| Hut-78 | - | - | - |
| Jurkat | - | - | - |
| Karpas 299 | - | - | - |
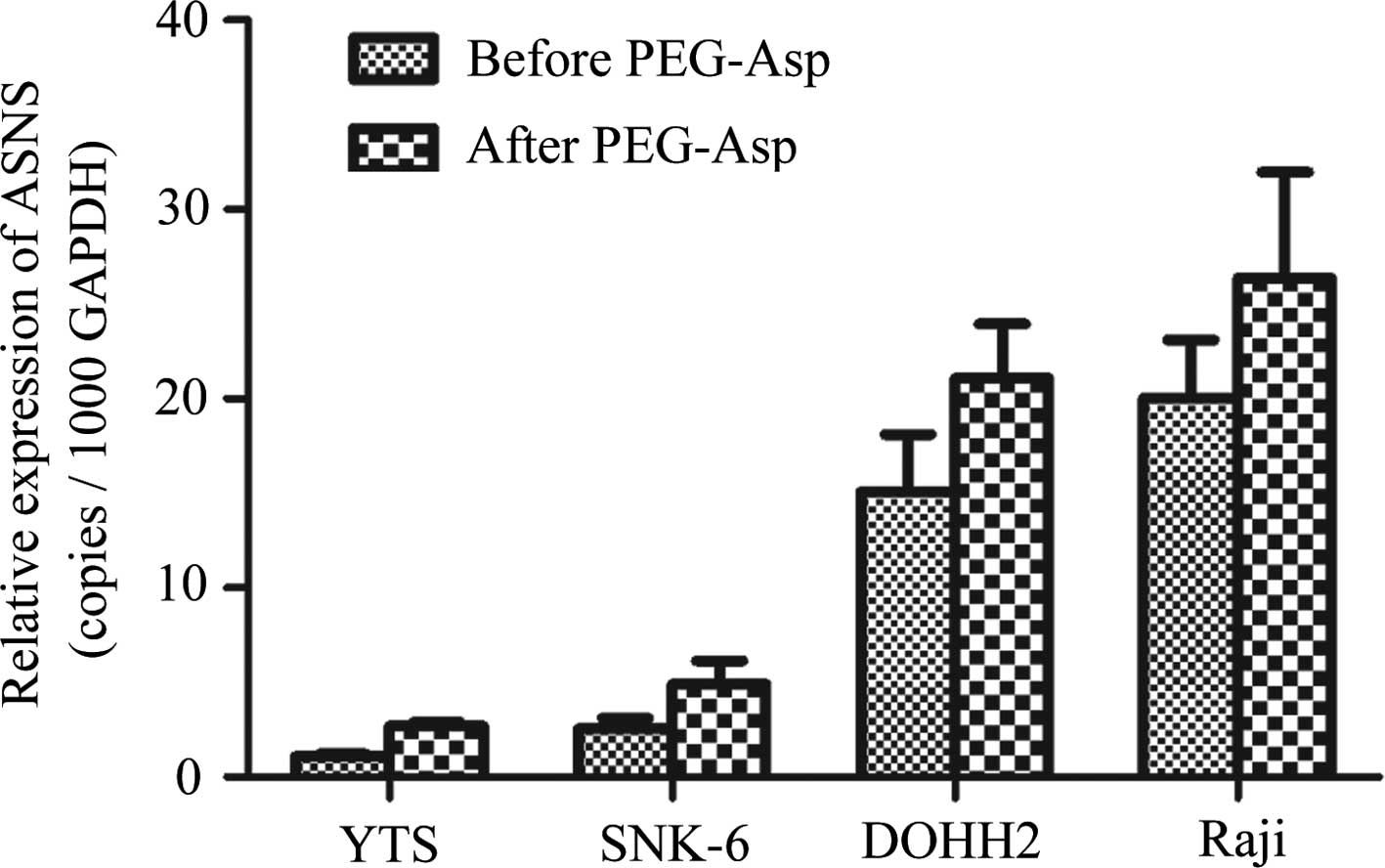
Comparison of ASNS expression in the
lymphoma cell lines before and after treatment with PEG-Asp
Seven cell lines were treated with PEG-Asp with
concentrations at IC50 for 48 h. Hut-78, Jurkat and
Karpas 299 cells were naturally resistant to treatment at a
concentration of 150 U/ml PEG-Asp. The expression levels and the
differences in expression level of ASNS before and after PEG-Asp
treatment are shown in Table II
and Fig. 2. The correlation between
ASNS expression and the IC50 values of PEG-Asp are
presented in Fig. 3. A negative
correlation was noted between ASNS expression levels and the
sensitivity to PEG-Asp (r=−0.953, P=0.47).
 | Table IIASNS expression levels in 7 lymphoma
cell lines before and after treatment with PEG-Asp for 48 h (mean ±
SD, copies/1,000 GAPDH mRNA). |
Table II
ASNS expression levels in 7 lymphoma
cell lines before and after treatment with PEG-Asp for 48 h (mean ±
SD, copies/1,000 GAPDH mRNA).
| Cell lines | Before treatment | After treatment | t-value | P-value |
|---|
| YTS | 1.077±0.139 | 2.732±0.192 | −25.450 | 0.000 |
| SNK-6 | 2.546±0.570 | 4.902±1.245 | −4.894 | 0.001 |
| DOHH2 | 15.067±3.047 | 21.067±2.888 | −3.788 | 0.005 |
| Raji | 20.055±3.071 | 26.371±5.605 | −3.309 | 0.011 |
| Hut-78 | 30.593±8.429 | 41.833±14.509 | −2.363 | 0.046 |
| Jurkat | 80.938±15.372 | 106.723±40.533 | −2.208 | 0.058 |
| Karpas 299 | 220.932±47.810 |
808.725±145.675 | −10.702 | 0.000 |
ASNS was weakly expressed in the NK/T
cell lymphoma specimens
bDNA-LCT was performed to analyze ASNS mRNA
expression in paraffin-embedded tissue samples derived from 50
patients with NK/T cell lymphoma and 12 nasal polyps and chronic
rhinitis tissues. The level of ASNS mRNA varied greatly between the
tumor tissues and the polyps and rhinitis tissues (0.480±0.307 and
0.739±0.267; P=0.009) (Fig. 4).
Association between ASNS expression and
clinicopathological features
We next examined the relationship between the ASNS
expression levels in tumor tissues and the clinicopathological
characteristics of 50 patients (Table
III). Pearson’s χ2 test indicated that the
expression of ASNS was associated with III-IV tumor stages
(P=0.041) and high International Prognostic Index (IPI) (P=0.018)
in patients with NK/T cell lymphoma. Other clinical characteristics
were not closely correlated with the expression of ASNS, including
age, gender, ECOG performance status, B symptoms, EBER-positivity,
serum LDH level, efficacy after primary therapy containing L-Asp or
PEG-Asp chemotherapy.
 | Table IIICorrelation of the
clinicopathological features of 50 NK/T cell lymphoma cases and
ASNS expression. |
Table III
Correlation of the
clinicopathological features of 50 NK/T cell lymphoma cases and
ASNS expression.
| ASNS
expression | | |
|---|
|
| | |
|---|
| Features | Low | High | P-value | χ2 |
|---|
| Gender | | | 0.208 | 1.587 |
| Male | 20 | 16 | | |
| Female | 5 | 9 | | |
| Age (years) | | | 0.713 | 0.136 |
| <60 | 21 | 20 | | |
| ≥60 | 4 | 5 | | |
| ECOG PS | | | 0.773 | 0.117 |
| <2 | 20 | 19 | | |
| ≥2 | 5 | 6 | | |
| B symptoms | | | 0.254 | 1.299 |
| Yes | 9 | 13 | | |
| No | 16 | 12 | | |
| LDH | | | 0.747 | 0.104 |
| Normal | 18 | 19 | | |
| Elevated | 7 | 6 | | |
| IPI score | | | 0.018a | 5.556 |
| 0–2 | 20 | 12 | | |
| 3–4 | 5 | 13 | | |
| EBER | | | 0.082 | 0.774 |
| Positive | 15 | 14 | | |
| Negative | 10 | 11 | | |
| Efficacy after
primary therapy | | | 0.393 | 0.728 |
| CR | 17 | 11 | | |
| Non-CR | 8 | 14 | | |
| Ann Arbor
stagea | | | 0.041a | 4.160 |
| I–II | 19 | 12 | | |
| III–IV | 6 | 13 | | |
Lack of correlation between ASNS
expression level and CR rate
The tumor responses to treatment were assessed for
all of the patients who used the chemotherapy regimens containing
L-Asp or PEG-Asp by imaging studies based on the International
Standard Response criteria for non-Hodgkin’s lymphoma. Comparing
patients in the high and low ASNS groups, no significant difference
was found in the response rate (P=0.393).
Association of the ASNS expression level
with prognosis
The Kaplan-Meier survival curves comparing patients
with high ASNS expression and patients with low expression in a
group of 36 NK/T cell lymphoma patients who used chemotherapy
regimens containing L-Asp and PEG-Asp are shown in Figs. 5 and 6. The analysis showed that the mean
progression-free survival times in the low ASNS expression group
and in the high ASNS expression group were 26.6 months (95% CI,
21.9–31.3) and 21.2 months (95% CI, 17.1–25.4), respectively
(P=0.031) (Fig. 5). The median
overall survival (OS) time was 30.1 months (95% CI, 25.6–34.5) in
the low ASNS expression group, whereas it was 24.3 months (95% CI,
19.6–28.9) in the high ASNS expression group (P=0.033; Fig. 6).
Univariate analysis and multivariate Cox regression
were used to analyze whether ASNS expression and other clinical
parameters could be independent prognostic factors for NK/T-cell
lymphoma. A total of 10 parameters were analyzed with unvariate
analysis. No significant correlations between OS and a number of
clinicopathological parameters, including gender (P=0.715), age
(P=0.128), ECOG PS (P=0.316), B symptoms (P=0.186), Ann Arbor stage
(P=0.241), EBER (P=0.163) were noted, while 4 factors, ASNS
expression level (P=0.033), LDH (P=0.009), IPI score (P=0.043) and
CR after primary treatment (P<0.001) displayed a correlation
with OS at varying degrees. These four individual parameters were
further subjected to multivariate Cox proportional hazards model,
which demonstrated that LDH (P=0.002), efficacy of primary therapy
(P<0.001) and ASNS expression level (P=0.023) were also
identified as independent prognostic factors for OS (Table IV).
 | Table IVUnvariate and multivariate analysis
for OS. |
Table IV
Unvariate and multivariate analysis
for OS.
| Unvariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
|
Characteristics | HR | CI (95%) | P-value | HR | CI (95%) | P-value |
|---|
| ASNS
expression | 2.426 | 1.031–5.704 | 0.042a | 2.901 | 1.155–7.283 | 0.023a |
| Gender | 1.174 | 0.496–2.774 | 0.715 | | | |
| Age | 2.366 | 0.718–7.167 | 0.128 | | | |
| ECOG PS | 1.668 | 0.614–4.531 | 0.316 | | | |
| B symptoms | 0.571 | 0.249–1.311 | 0.186 | | | |
| LDH | 3.361 | 1.357–8.323 | 0.009a | 5.067 | 1.796–14.291 | 0.002a |
| Ann Arbor
stage | 1.667 | 0.710–3.914 | 0.241 | | | |
| IPI score | 2.687 | 1.003–6.991 | 0.043a | 0.303 | 0.617–4.742 | 0.303 |
| EBER | 1.901 | 0.770–4.692 | 0.163 | | | |
| CR rate | 5.545 | 2.114–14.548 | 0.000a | 6213 | 2.250–17.154 | 0.000a |
Discussion
In the present study, we demonstrated for the first
time the expression patterns and prognostic value of ASNS mRNA in
NK/T cell lymphoma, including cell lines and clinical samples. We
also demonstrated the downregulation of ASNS at the mRNA level in
the NK/T cell lymphoma tissues when compared with the levels in
chronic rhinitis and nasal polyps using bDNA-LCT. Our findings
showed that expression of ASNS was linked strongly with the
sensitivity to L-Asp and PEG-Asp treatment and to prognostic
factors, including III-IV stage, Ann Arbor stage, high IPI scores,
but not with efficacy after primary therapy containing PEG-Asp or
L-Asp. The Kaplan-Meier analysis showed that patients with NK/T
cell lymphoma who had low ASNS expression in general had a better
prognosis than those with high ASNS expression. Multivariate
analysis revealed that the ASNS expression level might be a
significant prognostic indicator affecting survival after
chemotherapy containing PEG-Asp or L-Asp.
The cells that expressed low ASNS protein levels
were more sensitive to L-Asp treatment probably because they
produce less asparagine and are, therefore, more dependent on
extracellular asparagine to meet metabolic demands. Yet, the mRNA
expression level of ASNS does not necessarily correlate with
protein level and enzyme activity of ASNS. However, Hutson et
al (23) confirmed that the
mRNA level corresponded to protein and activity levels. In a
previous study, it was reported that most types of tumors,
particularly leukemia and lymphoma, lack activity of ASNS (24). Although ASNS mRNA analysis led to
variable conclusions regarding the relationship between ASNS mRNA
levels in primary ALL cells and L-Asp sensitivity, in the present
study, we confirmed that there is some relationship between ASNS
mRNA expression and the sensitivity to PEG-Asp in vitro.
Moreover, recent research suggests that the expression level of
ASNS in cell lines can better reflect the sensitivity to L-Asp,
while in clinical specimens the results have been questionable
(25). Previous studies on
pediatric ALL showed that ASNS mRNA expression was linked with
L-Asp resistance only in TEL-AML1-negative but not
TEL-AML1-positive ALL. TEL-AML1-positive ALL patients were more
sensitive to L-Asp compared with TEL-AML1-negative patients,
although the former had a relative high level of ASNS (26), and also high expression of ASNS was
associated with poor prognosis compared with low expression in
TEL-AML1-negative but not in TEL-AML1-positive B-lineage ALL
(27). Therefore, the ASNS level
alone does not determine sensitivity to L-Asp at least in some
subtypes of hematological malignancies. Thus, it is generally
accepted that the role of ASNS in leukaemic cells with regard to
L-Asp resistance may vary among genetic subtypes (26–28).
In the present study, we also came to the same conclusion that no
correlation was found between the ASNS expression level and CR
rate. This could also be due to the relatively small number of
patients and the varied chemotherapy regimens except L-Asp. What is
more, for NK/T cell lymphoma, we did not examine the genetic
aberrations, and whether there is a potential relationship between
gene fusion or other subtypes of aberrations and ASNS
expression.
Pancreatic and ovarian tumors and HCC with low ASNS
expression levels have also been proposed for L-Asp therapy
(17,20,29).
In addition, one study revealed that this drug has an
antiproliferative effect in β-catenin-mutated HepG2 cells (30). This result extends the findings of
those from previous studies on pancreatic and ovarian tumors and
HCC indicating that ASNS may be a potential therapeutic target in
the treatment of NK/T cell lymphoma. Based on the severe
side-effects of this drug, such as pancreatitis and allergic
reactions, it is necessary to stratify patients based on the ASNS
expression level in the tumor. Low expression of ASNS identified by
bDNA-LCT might be an early warning sign that patients should be
closely monitored and receive chemotherapy containing L-Asp or
PEG-Asp. It has been proposed that NK/T cell lymphoma patients with
low ASNS expression levels may benefit more from treatment with
L-Asp, which can also alleviate the adverse effect and economic
burden of patients with high ASNS expression.
How the ASNS gene affects prognosis is an important
issue. One reason is that the expression level of ASNS has a
certain relationship with the sensitivity to L-Asp. A high
expression level of ASNS indicates that these patients are more
likely to be resistant to chemotherapy regimens containing L-Asp or
PEG-Asp. Therefore, they may not easily achieve complete remission
which will affect long-term prognosis. On the other hand, Greco
et al (31) and Gong and
Basilico (32) determined that ASNS
could complement temperature-sensitive hamster BHK ts11 cells,
which are specifically blocked in progressing through the G1 phase
of the cell cycle when grown at a non-permissive temperature. This
loss in ASNS activity leads to cell cycle arrest, as a consequence
of a depletion of cellular asparagine, and a corresponding increase
in ASNS mRNA due to regulatory mechanisms. Thus, we conclude that
the ASNS gene plays a critical role in the regulation of the cell
cycle. A high level of ASNS results in cell cycle dysregulation in
tumors. Thus, it may have specific impact on the malignant
phenotype of tumors. Accordingly it may have an effect on the
prognosis of NK/T cell lymphoma patients. Activation of the
GCN2-eIF2-ATF4 signaling pathway, leading to increased ASNS
expression, appears to be a component of solid tumor adaptation to
nutrient deprivation and/or hypoxia, so that ASNS function extends
beyond asparagine biosynthesis (24). There is also evidence suggesting
that ASNS is important in certain metastatic mechanisms. When
cancer spreads from the primary tumor to distant sites, cells enter
into the bloodstream and exist in suspension until they reach a
metastatic site. Patrikainen et al (33) and Ameri et al (34) observed that PC-3 prostate cancer
cells and human MDA-MB-231 breast cancer cells which have a strong
potential for distant metastasis and an increased capacity for
colony formation exhibit an elevated expression of ASNS. Its
increased abundance in metastasizing cells suggests that ASNS
activity is beneficial for cancer cell survival once they detach
from the primary tumor and enter the bloodstream. Patients with
high ASNS expression are more susceptible to tumors than patients
with low ASNS expression and have a tendency to develop distant
metastasis thus are rendered with a malignant phenotype.
In conclusion, the present study explored the
effects of ASNS on the drug sensitivity and prognosis in NK/T cell
lymphoma patients for the first time. High ASNS expression is
associated with a poorer performance at diagnosis and a less
favorable long-term outcome. Based on these results, low ASNS
expression may be used to select patients for treatment with L-Asp
or PEG-Asp-containing chemotherapy regimens. In addition, an
alternative therapeutic strategy for patients with low ASNS
expression was also addressed.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81172118).
References
|
1
|
Aozasa K, Ohsawa M, Tajima K, Sasaki R,
Maeda H, Matsunaga T and Friedmann I: Nation-wide study of lethal
mid-line granuloma in Japan: frequencies of wegener’s granulo
matosis, polymorphic reticulosis, malignant lymphoma and other
related conditions. Int J Cancer. 44:63–66. 1989.PubMed/NCBI
|
|
2
|
Kim GE, Cho JH, Yang WI, Chung EJ, Suh CO,
Park KR, Hong WP, Park IY, Hahn JS, Roh JK and Kim BS: Angiocentric
lymphoma of the head and neck: patterns of systemic failure after
radiation treatment. J Clin Oncol. 18:54–63. 2000.PubMed/NCBI
|
|
3
|
Lee J, Park YH, Kim WS, Lee SS, Ryoo BY,
Yang SH, Park KW, Kang JH, Park JO, Lee SH, Kim K, Jung CW, Park
YS, Im YH, Kang WK, Lee MH, Ko YH, Ahn YC and Park K: Extranodal
nasal type NK/T-cell lymphoma: elucidating clinical prognostic
factors for risk-based stratification of therapy. Eur J Cancer.
41:1402–1408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim TM, Park YH, Lee SY, Kim JH, Kim DW,
Im SA, Kim TY, Kim CW, Heo DS, Bang YJ, Chang KH and Kim NK: Local
tumor invasiveness is more predictive of survival than
International Prognostic Index in stage I(E)/II(E) extranodal
NK/T-cell lymphoma, nasal type. Blood. 106:3785–3790. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee
JH, Lee DH, Huh J, Oh SY, Kwon HC, Kim HJ, Lee SI, Kim JH, Park J,
Oh SJ, Kim K, Jung C, Park K and Kim WS: Extranodal natural killer
T-cell lymphoma, nasal-type: a prognostic model from a
retrospective multicenter study. J Clin Oncol. 24:612–618. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi M: Current and future management
of NK/T-cell lymphoma based on clinical trials. Int J Hematol.
96:562–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueno T, Ohtawa K, Mitsui K, Kodera Y,
Hiroto M, Matsushima A, Inada Y and Nishimura H: Cell cycle arrest
and apoptosis of leukemia cells induced by L-asparaginase.
Leukemia. 11:1858–1861. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richards NG and Schuster SM: Mechanistic
issues in asparagine synthetase catalysis. Adv Enzymol Relat Areas
Mol Biol. 72:145–198. 1998.PubMed/NCBI
|
|
10
|
Chen H, Pan YX, Dudenhausen EE and Kilberg
MS: Amino acid deprivation induces the transcription rate of the
human asparagine synthetase gene through a timed program of
expression and promoter binding of nutrient-responsive bZIP
transcription factors as well as localized histone acetylation. J
Biol Chem. 279:50829–50839. 2004. View Article : Google Scholar
|
|
11
|
Kilberg MS, Pan YX, Chen H and
Leung-Pineda V: Nutritional control of gene expression: how
mammalian cells respond to amino acid limitation. Annu Rev Nutr.
25:59–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li BS, Gu LJ, Luo CY, Li WS, Jiang LM,
Shen SH, Jiang H, Shen SH, Zhang B, Chen J, Xue HL and Tang JY: The
downregulation of asparagine synthetase expression can increase the
sensitivity of cells resistant to L-asparaginase. Leukemia.
20:2199–2201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashimoto K, Suzuki F and Sakagami H:
Declined asparagine synthetase mRNA expression and enhanced
sensitivity to asparaginase in HL-60 cells committed to monocytic
differentiation. Anticancer Res. 29:1303–1308. 2009.PubMed/NCBI
|
|
14
|
Aslanian AM, Fletcher BS and Kilberg MS:
Asparagine synthetase expression alone is sufficient to induce
L-asparaginase resistance in MOLT-4 human leukaemia cells. Biochem
J. 357:321–328. 2001. View Article : Google Scholar
|
|
15
|
Zwaan CM, Kaspers GJ, Pieters R, Hahlen K,
Janka-Schaub GE, van Zantwijk CH, Huismans DR, de Vries E, Rots MG,
Peters GJ, Jansen G, Creutzig U and Veerman AJ: Different drug
sensitivity profiles of acute myeloid and lymphoblastic leukemia
and normal peripheral blood mononuclear cells in children with and
without Down syndrome. Blood. 99:245–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lorenzi PL, Llamas J, Gunsior M, Ozbun L,
Reinhold WC, Varma S, Ji H, Kim H, Hutchinson AA, Kohn EC,
Goldsmith PK, Birrer MJ and Weinstein JN: Asparagine synthetase is
a predictive biomarker of L-asparaginase activity in ovarian cancer
cell lines. Mol Cancer Ther. 7:3123–3128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lorenzi PL, Reinhold WC, Rudelius M,
Gunsior M, Shankavaram U, Bussey KJ, Scherf U, Eichler GS, Martin
SE, Chin K, Gray JW, Kohn EC, Horak ID, Von Hoff DD, Raffeld M,
Goldsmith PK, Caplen NJ and Weinstein JN: Asparagine synthetase as
a causal, predictive biomarker for L-asparaginase activity in
ovarian cancer cells. Mol Cancer Ther. 5:2613–2623. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui H, Darmanin S, Natsuisaka M, Kondo T,
Asaka M, Shindoh M, Higashino F, Hamuro J, Okada F, Kobayashi M,
Nakagawa K and Koide H: Enhanced expression of asparagine
synthetase under glucose-deprived conditions protects pancreatic
cancer cells from apoptosis induced by glucose deprivation and
cisplatin. Cancer Res. 67:3345–3355. 2007. View Article : Google Scholar
|
|
19
|
Sircar K, Huang H, Hu L, Cogdell D,
Dhillon J, Tzelepi V, Efstathiou E, Koumakpayi IH, Saad F, Luo D,
Bismar TA, Aparicio A, Troncoso P, Navone N and Zhang W:
Integrative molecular profiling reveals asparagine synthetase is a
target in castration-resistant prostate cancer. Am J Pathol.
180:895–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Dong LW, Tan YX, Zhang J, Pan YF,
Yang C, Li MH, Ding ZW, Liu LJ, Jiang TY, Yang JH and Wang HY:
Asparagine synthetase is an independent predictor of surgical
survival and a potential therapeutic target in hepatocellular
carcinoma. Br J Cancer. 109:14–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JWL: WHO Classification
of Tumours of Haematopoietic and Lymphoid Tissue (IARC WHO
Classification of Tumours). IARC Press; Lyon: 2008
|
|
22
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al; International Harmonization Project on Lymphoma. Revised
response criteria for malignant lymphoma. J Clin Oncol. 25:579–586.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hutson RG, Kitoh T, Moraga Amador DA,
Cosic S, Schuster SM and Kilberg MS: Amino acid control of
asparagine synthetase: relation to asparagi-nase resistance in
human leukemia cells. Am J Physiol. 272:1691–1699. 1997.PubMed/NCBI
|
|
24
|
Balasubramanian MN, Butterworth EA and
Kilberg MS: Asparagine synthetase: regulation by cell stress and
involvement in tumor biology. Am J Physiol Endocrinol Metab.
304:789–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fine BM, Kaspers GJ, Ho M, Loonen AH and
Boxer LM: A genome-wide view of the in vitro response to
L-asparaginase in acute lymphoblastic leukemia. Cancer Res.
65:291–299. 2005.PubMed/NCBI
|
|
26
|
Stams WA, den Boer ML, Beverloo HB,
Meijerink JP, Stigter RL, van Wering ER, Janka-Schaub GE, Slater R
and Pieters R: Sensitivity to L-asparaginase is not associated with
expression levels of asparagine synthetase in t(12; 21)+
pediatric ALL. Blood. 101:2743–2747. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stams WA, den Boer ML, Holleman A, Appel
IM, Beverloo HB, van Wering ER, Janka-Schaub GE, Evans WE and
Pieters R: Asparagine synthetase expression is linked with
L-asparaginase resistance in TEL-AML1-negative but not
TEL-AML1-positive pediatric acute lymphoblastic leukemia. Blood.
105:4223–4225. 2005. View Article : Google Scholar
|
|
28
|
Den Boer ML, Evans WE and Pieters R:
TEL-AML1-positive ALL: a discordant genotype. Cell Cycle.
4:997–998. 2005.PubMed/NCBI
|
|
29
|
Dufour E, Gay F, Aguera K, Scoazec JY,
Horand F, Lorenzi PL and Godfrin Y: Pancreatic tumor sensitivity to
plasma L-asparagine starvation. Pancreas. 41:940–948. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tardito S, Chiu M, Uggeri J, Zerbini A, Da
Ros F, Dall’Asta V, Missale G and Bussolati O: L-Asparaginase and
inhibitors of glutamine synthetase disclose glutamine addiction of
beta-catenin-mutated human hepatocellular carcinoma cells. Curr
Cancer Drug Targets. 11:929–943. 2011. View Article : Google Scholar
|
|
31
|
Greco A, Gong SS, Ittmann M and Basilico
C: Organization and expression of the cell cycle gene, ts11, that
encodes asparagine synthetase. Mol Cell Biol. 9:2350–2359.
1989.PubMed/NCBI
|
|
32
|
Gong SS and Basilico C: A mammalian
temperature-sensitive mutation affecting G1 progression results
from a single amino acid substitution in asparagine synthetase.
Nucleic Acids Res. 18:3509–3513. 1990. View Article : Google Scholar
|
|
33
|
Patrikainen L, Porvari K, Kurkela R,
Hirvikoski P, Soini Y and Vihko P: Expression profiling of PC-3
cell line variants and comparison of MIC-1 transcript levels in
benign and malignant prostate. Eur J Clin Invest. 37:126–133. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ameri K, Luong R, Zhang H, Powell AA,
Montgomery KD, Espinosa I, Bouley DM, Harris AL and Jeffrey SS:
Circulating tumour cells demonstrate an altered response to hypoxia
and an aggressive phenotype. Br J Cancer. 102:561–569. 2010.
View Article : Google Scholar : PubMed/NCBI
|