Introduction
Glioblastoma (GBM) is the most common primary brain
tumor in adults. It is also the most aggressive and resistant to
therapy and has the worst prognosis. To date, there is no effective
treatment for patients suffering from this disease (1). After excision of the tumor, its
relapse is inevitable, and the mean survival time of patients
rarely exceeds several months (2).
The molecular pathology of GBM is diverse, and a number of
chromosomal aberrations are known to be hallmarks of glioblastoma
carcinogenesis. Among them are the loss of heterozygosity on 10q,
EGFR amplification, CDKN2A deletion and mutations of
the PTEN and TP53 genes (3). Ineffective treatment, as well as the
very high mortality rate and particularly complex molecular
background of GBM constitute a strong rationale for research aiming
to elucidate the processes underlying the carcinogenesis of neural
cells.
The WWOX gene is localized at a common
fragile site FRA16D (4). It is
known to behave as a tumor suppressor. Unlike most tumor-suppressor
genes, the loss of functionality of only one of its alleles is
enough to predispose a patient to cancerogenesis - the
haploinsufficiency phenomenon (5).
Growing evidence indicates that WWOX is not a classical
tumor suppressor. Its action is clearly not limited only to cell
cycle control or genome integrity maintenance. Although
interactions with several transcription factors and signal
transduction proteins are well documented (6–9), it
seems that only a tiny piece is known of the physiological cellular
role of WWOX and its implications for cancerogenesis. Our
previous findings on glioblastoma samples showed that the
WWOX expression level is correlated with genes important to
tumor formation and progression, such as ERBB4, Ki67
and Bcl-2 (10). The present
study was conducted on the glioblastoma cell line T98G and aimed to
assess the influence of WWOX upregulation on the
transcriptome and phenotype of these cells.
Materials and methods
Cell line and culture conditions
T98G cells, derived from a human glioblastoma, were
obtained from the European Collection of Cell Cultures (ECACC). The
cells were grown according to the manufacturer’s protocol in
Minimum Essential Medium (MEM; Gibco) supplemented with 2 mM
L-glutamine (Gibco), 0.1 mM NEAA (Gibco), 10% heat inactivated FBS
(Gibco), 0.05 mg/ml penicillin (Gibco), 0.05 mg/ml streptomycin
(Gibco) and 0.1 mg/ml neomycin (Gibco) in a humidified atmosphere
containing 5% CO2 at 37°C.
Stable retroviral transfection
The WWOX gene cDNA was introduced into T98G
glioblastoma cells by retroviral transfection. The pLNCX2
retroviral vector with cloned WWOX was produced in the PT67
packaging line. Target cells were grown to 30% confluency and
infected with the viruses (~106 colony-forming units/ml)
suspended in culture medium with Polybrene as vehicle (8 μg/ml,
Sigma-Aldrich). After 24 h, the medium was replaced, and stable
transfectants were selected with 400 μg/ml G418 (Sigma-Aldrich) for
3 weeks. A pool of stable transfectants was used for the microarray
study of global gene expression and biological experiments.
Transfection efficiency was confirmed by real-time RT-PCR and
western blot analysis.
Real-time RT-PCR
The real-time RT-PCR procedure was conducted to
assess the efficiency of the retroviral transfection and to
validate the microarray experiment. Total RNA was isolated using
TRIzol reagent (Life Technologies). The cDNA synthesis was
performed using 10 μg of total RNA at a volume of 100 μl using
ImProm RT-II™ reverse transcriptase (Promega). Reverse
transcription was carried out under the following conditions:
incubation at 25°C for 5 min and 42°C for 60 min and heating at
70°C for 15 min. cDN A samples were diluted with sterile deionized
water to a total volume of 150 μl, and 2 μl was added to the PCR
reaction. Real-time RT-PCR was performed using Light Cycler 480
(Roche). PCR products were detected using SYBR® Green I
and qPCR Core kit for SYBR® Green I (Eurogentec). All
reactions were performed in duplicate. The relative expression
levels of the WWOX, BIRC5 and ID3 genes were
assessed. The expression levels of the investigated genes were
normalized to 3 reference housekeeping genes (RPS17,
H3F3A, RPLP0). The relative gene expression was
calculated based on the Pfaffl method (11). Universal Human Reference RNA
(Stratagene) was used as a calibrator. The primer sequences, PCR
reaction conditions and lengths of the obtained products are
available upon request.
Western blot analysis
Cells were lysed on ice with RIPA protein extraction
buffer containing protease inhibitor cocktail (Sigma-Aldrich).
Proteins (60 μg) were resolved on 10% SDS-PAGE and were transferred
on PVDF membranes. The membranes were blocked for 1 h in 5% non-fat
milk and incubated with a primary antibody for 18 h at 4°C. The
antibodies used were goat polyclonal anti-WWOX (cat. no. sc-20529),
mouse monoclonal anti-ARK1 (cat. no. sc-56881), goat polyclonal
anti-KLF8 (cat. no. sc-69294), rabbit polyclonal anti-JAK1 (cat.
no. sc-277) (all from Santa Cruz Biotechnology). Subsequently, the
membranes were washed with TBST and incubated with the appropriate
secondary antibody conjugated with alkaline phosphatase
(Sigma-Aldrich) for 1 h at room temperature (RT). Next, the
membranes were washed with TBST and developed with
Novex® AP Chromogenic Substrate (Invitrogen). GAPDH was
used as a reference protein. The relative protein amount was
assessed with ImageJ (NIH) based on the integrated density of the
bands.
Microarray transcriptome study
Human OneArray™ (Phalanx Biotech) high-density
microarrays were used in flip dye experiments in 4 replicates for
each cell variant. Each sample was hybridized against Universal
Human Reference RNA (Stratagene) and labelled with ULS™ Labeling
Kit (Kreatech Diagnostics). Preparation of the slide for
hybridization included prewash in ethanol and pre-hybridization
according to the manufacturer’s protocol. Hybridization was
performed in a humidity chamber filled with 2 SSPE buffer at 42°C
for 16–18 h. Post-hybridization washes were performed with the
following buffers: 1 SSPE/0.03% SDS (2 min, 42°C), 1 SSPE (2 min,
RT), 0.1 SSPE (rinsed several times, RT). Slide scanning and
preliminary normalization were performed with ProScanArray
(Perkin-Elmer) and ScanArray Express, respectively.
Further data analysis was performed with the
MultiExperiment Viewer (MeV) from the TM4 package provided by The
Institute for Genomic Research at http://www.tm4.org/site. For the ontological
classification of genes, the PANTHER classification system was
used, which allowed a determination to be made of which pathways
are susceptible to change depending on the WWOX expression
level. The microarray results were validated by real-time RT-PCR
and western blot analysis. The data have been deposited in NCBI’s
Gene Expression Omnibus and are accessible through GEO Series
accession no. GSE51481.
Proliferation, redox potential and
apoptosis assays
The three assays assessing cell proliferation, redox
potential and apoptosis were multiplexed to eliminate population
and culture differences. Proliferation was evaluated with
5-bromo-2′-deoxyuridine (BrdU). BrdU incorporated into DNA during
replication was detected with an anti-BrdU monoclonal antibody
labeled with europium (Perkin-Elmer). The redox potential was
measured with the alamarBlue® cell viability reagent
(Invitrogen), the redox indicator metabolized in mitochondria.
Apoptosis was assessed by TUNEL reaction with the DELFIA DNA
fragmentation assay (Perkin-Elmer). Cells were seeded on a white,
clear bottom 96-well plate at a density of 10,000 cells/well. All
tests were conducted in a starvation medium (without serum).
Adhesion assay
In order to assess the ability of the cells to
integrate into the extracellular matrix, a colorimetric CytoSelect™
48-well adhesion assay (Cell Biolabs, Inc.) was carried out. The
assay evaluates the capability of cells to adhere to five ECM
proteins: fibronectin, collagen I, collagen I V, laminin and
fibrinogen. The cells were seeded on a 48-well plate coated with
selected ECM proteins at a density of 150,000 cells/well in
starvation medium and were allowed to adhere for 90 min at 37°C.
Next, the adherent cells were dyed and their number was analyzed
colorimetrically.
Invasion assay
The invasive potential of the investigated cells was
evaluated using the colorimetric CytoSelect™ 24-well invasion assay
(Cell Biolabs, Inc.). The assay contains a membrane coated with a
layer of basement membrane matrix solution and allows for
discrimination of invasive cells. The cells were seeded on inserts
placed in a 24-well plate at a density of 300,000 cells/well and
left to invade for 48 h. Next, the cells that crossed the membrane
were dyed and their number was analyzed colorimetrically.
3D culture growth
For a three dimensional (3D) culture assay, cells
were seeded on a 96-well plate at a density of 15,000 cells/well on
a solidified 2-mm layer of growth factor-reduced Geltrex™ basement
membrane matrix (Gibco). The cells were grown in an assay buffer
consisting of full culture medium and 2% Geltrex™. The assay buffer
was exchanged every 4 days. The cells were cultured for 12
days.
Statistical analysis of biological
assays
The results are presented as means. Statistical
significance in all biological tests was assessed with the
Student’s t-test. The results were recognized as being
statistically significant at a confidence level >95%
(p<0.05).
Results
Retroviral transfection and multiclonal
selection allows for stable overexpression of the WWOX gene in T98G
glioblastoma cells
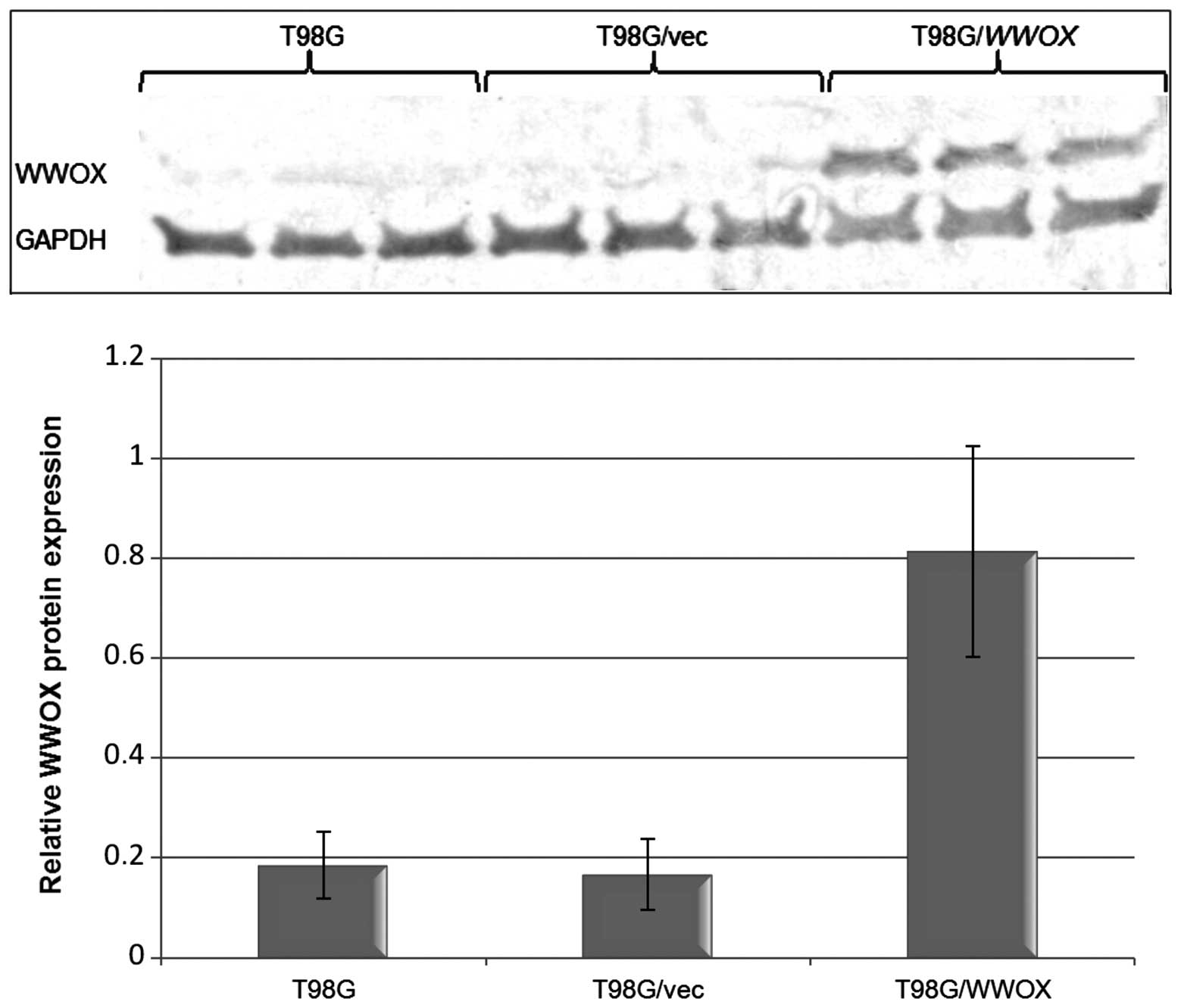
The level of WWOX expression was assessed by
real-time RT-PCR and western blot analysis. The amount of
WWOX mRN A in the T98G/WWOX transfectants was
>29-fold greater than that noted in the T98G/vec control cells.
The higher gene expression resulted in an elevated protein level.
The protein level in the T98G/vec control was comparable to that
found in the untreated T98G cells (Fig.
1).
Transcriptome analysis of the WWOX
transfectants
The microarray study was used to assess changes in
the expression level of ~29,000 genes. A total of 2,846 genes
showed a significant increase or decrease in expression depending
on the WWOX level (p<0.05, t-test). For 1,802 of the
genes, the change in expression level was 2-fold or greater. All
the genes identified in the microarray experiment were
ontologically classified using the PANTHER classification system
and grouped according to cellular pathways and biological
processes. The cellular pathways containing 10 or more genes
modulated by WWOX overexpression are presented in Table I. Selected biological processes with
the highest number of WWOX-modulated genes are shown in
Table II.
 | Table IOntological analysis of the genes
modulated by WWOX overexpression: cellular pathways. |
Table I
Ontological analysis of the genes
modulated by WWOX overexpression: cellular pathways.
| Cellular
pathway | No. of genes |
|---|
| Unclassified | 2,041 |
| Inflammation
mediated by chemokine and cytokine signaling pathway | 39 |
| Wnt signaling
pathway | 38 |
| Heterotrimeric
G-protein signaling pathway (Gi α and Gs α mediated) | 32 |
| Integrin signalling
pathway | 28 |
| Interleukin
signaling pathway | 25 |
| Angiogenesis | 22 |
| PDGF signaling
pathway | 21 |
| Huntington
disease | 21 |
| Alzheimer
disease-presenilin pathway | 20 |
| p53 pathway | 20 |
| Parkinson
disease | 20 |
| Heterotrimeric
G-protein signaling pathway (Gq α and Go α mediated) | 20 |
| PI3 kinase
pathway | 19 |
| TGF-β signaling
pathway | 18 |
| EGF receptor
signaling pathway | 17 |
| Endothelin
signaling pathway | 15 |
| Transcription
regulation by bZIP transcription factor | 14 |
| Ionotropic
glutamate receptor pathway | 14 |
| FGF signaling
pathway | 14 |
| Cytoskeletal
regulation by Rho GTPase | 14 |
| Apoptosis signaling
pathway | 13 |
| Alzheimer
disease-amyloid secretase pathway | 13 |
| Oxidative stress
response | 13 |
| Nicotinic
acetylcholine receptor signaling pathway | 13 |
| Metabotropic
glutamate receptor group III pathway | 13 |
| Cadherin signaling
pathway | 13 |
| Angiotensin
II-stimulated signaling through G-proteins and β-arrestin | 12 |
| Ras pathway | 11 |
| p38 MAPK
pathway | 11 |
| T cell
activation | 10 |
| Insulin/IGF
pathway-protein kinase B signaling cascade | 10 |
 | Table IIOntological analysis of the genes
modulated by WWOX overexpression: biological processes. |
Table II
Ontological analysis of the genes
modulated by WWOX overexpression: biological processes.
| Biological
process | No. of genes |
|---|
| Metabolic
process | 1,063 |
| Primary metabolic
process | 1,019 |
| Cellular
process | 777 |
| Unclassified | 629 |
| Cell
communication | 556 |
| Signal
transduction | 524 |
| Nucleobase,
nucleoside, nucleotide and nucleic acid metabolic process | 472 |
| Protein metabolic
process | 415 |
| Transport | 370 |
| Transcription | 295 |
| Transcription from
RNA polymerase II promoter | 293 |
| Developmental
process | 288 |
| Cell surface
receptor linked signal transduction | 256 |
| Immune system
process | 243 |
| Regulation of
transcription from RNA polymerase II promoter | 240 |
| System process | 220 |
| Protein
transport | 192 |
| Intracellular
protein transport | 192 |
| Neurological system
process | 176 |
| Protein
modification | 174 |
| System
development | 174 |
| Cell cycle | 163 |
| Response to
stimulus | 156 |
| Intracellular
signaling cascade | 148 |
| Proteolysis | 136 |
| Cell adhesion | 131 |
| Cell-cell
signaling | 123 |
| Cellular component
organization | 122 |
| Ectoderm
development | 120 |
| Lipid metabolic
process | 118 |
| Vesicle-mediated
transport | 117 |
| G-protein coupled
receptor protein signaling pathway | 114 |
| Nervous system
development | 109 |
| Mesoderm
development | 108 |
| Apoptosis | 101 |
Phenotype analysis of the WWOX
transfectants
To investigate how the changes in the transcriptome
translate into cell phenotype, a number of biological experiments
were performed. Proliferation rate, redox potential, apoptosis,
ability to adhere to extracellular matrix proteins, invasiveness
and 3D culture formation were all evaluated in the transfected
cells.
Proliferation, redox potential and
apoptosis assays
Multiplexing assays for proliferation, redox
potential and apoptosis allowed for elimination of population and
culture differences. An analysis of the proliferation rate showed
that T98G/WWOX cells proliferated 53% more slowly than the
control T98G/vec cells (p<0.01). Simultaneously apoptosis was
increased, although without statistical significance (p>0.05). A
test with alamarBlue to assess mitochondrial metabolism revealed
that cells overexpressing WWOX had a considerably higher
rate of metabolizing the substrate, which may signify an
intensification of overall mitochondrial redox potential.
Invasion
WWOX-transfected cells were examined to test
whether they exhibit changes in invasiveness. T98G/WWOX
cells demonstrated a slightly greater ability to cross a membrane
coated with basement membrane matrix solution, with 29% more
invasive cells. However, the result was not statistically
significant (p>0.05).
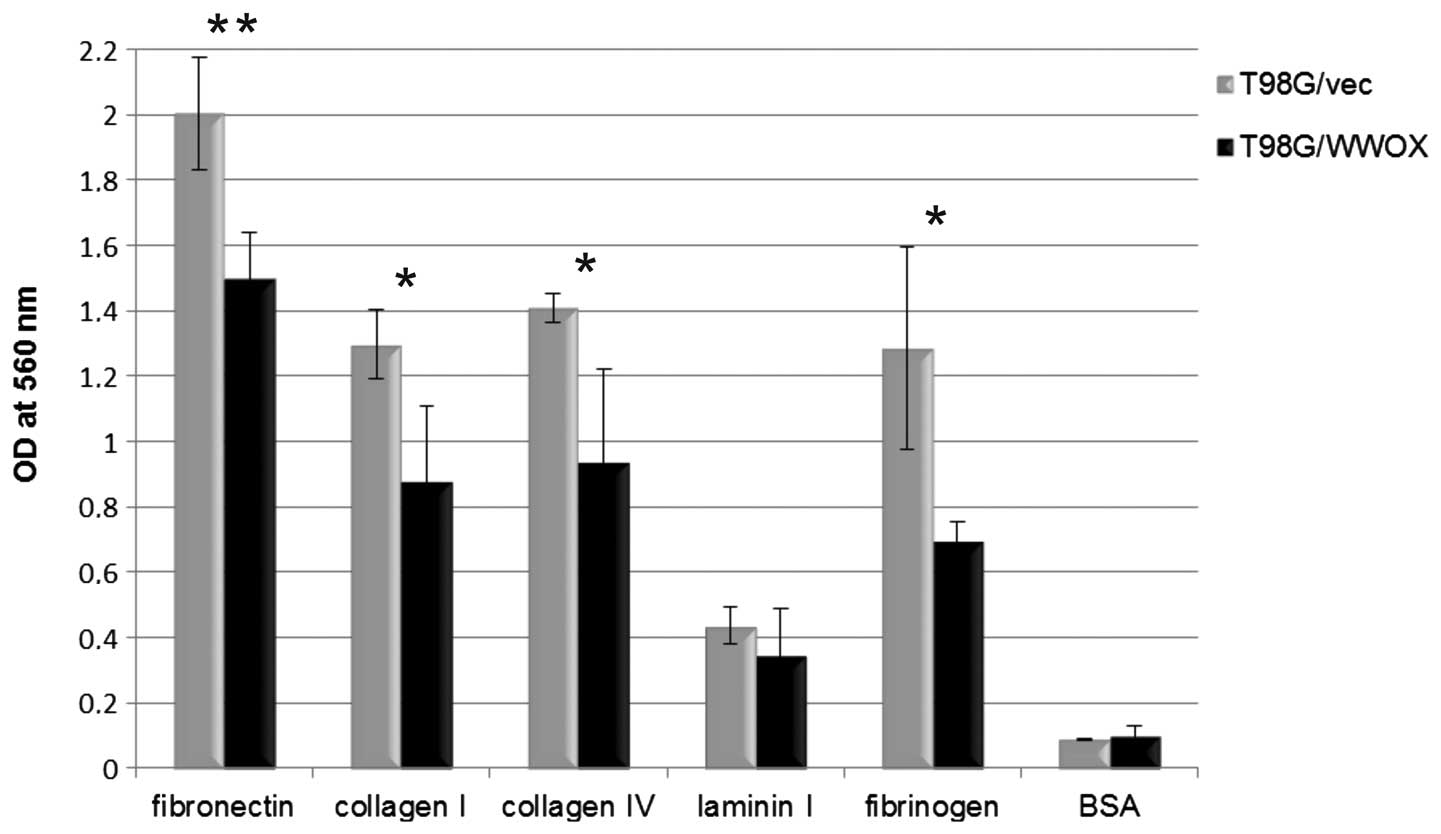
Adhesion
Cells overexpressing WWOX showed a greatly
reduced ability to adhere to extracellular matrix proteins.
Decreased adhesion was noted to all examined proteins: fibronectin
(p<0.01), collagen I (p<0.05), collagen I V (p<0.05),
laminin (p>0.05) and fibrinogen (p<0.05). A graphical
presentation of changes in adhesion between T98G cells
overexpressing WWOX and those with a native WWOX
level is provided in Fig. 2.
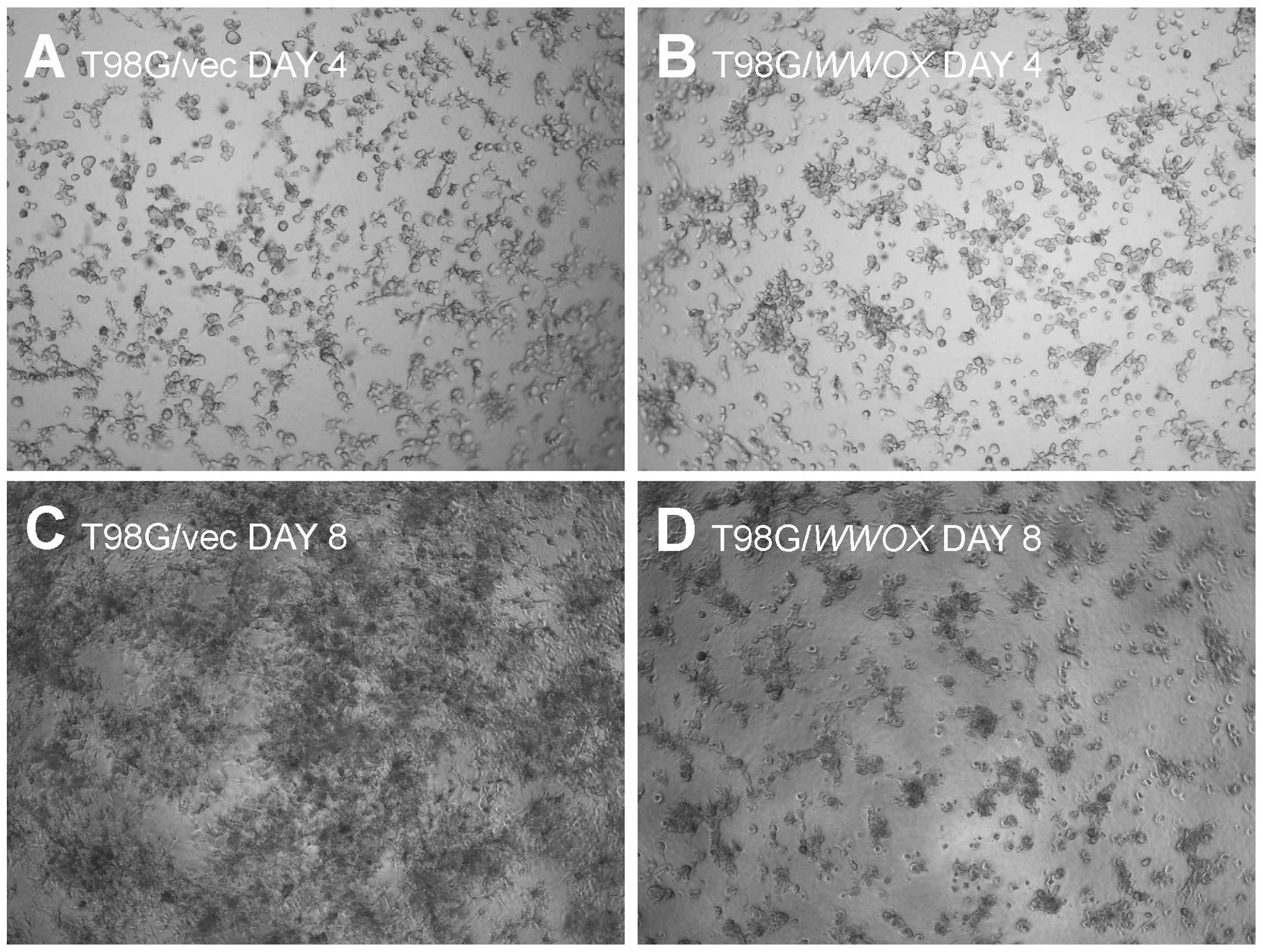
3D culture growth
The T98G/WWOX cells exhibited impaired 3D
culture formation. The cells seeded in a thick Geltrex®
layer after 12 days remained as isolated, single cells that did not
proliferate. In contrast, control T98G/vec cells exhibited
extensive proliferation and network formation (Fig. 3).
Discussion
Our previous report showed that WWOX may be
involved in GBM carcinogenesis and/or tumor progression. The study
describes the association of WWOX expression with the
transcription level of several genes involved in signal
transduction and cell cycle control (10). To specify how WWOX may
influence cancer cell metabolism, we decided to use the T98G
glioblastoma cell line, which has a very low level of expression of
endogenous WWOX to ascertain the effect an increase in
WWOX expression may have on these cells.
WWOX cDNA was introduced into T98G cells by
retroviral transfection. In the stable transfectants, the
expression profile and basic biological processes were examined.
The microarray study for global gene expression allowed the
relevance of WWOX to be investigated, with regard to overall
cellular signaling. The experiment identified 2,846 genes whose
expression levels were significantly altered as a consequence of
WWOX overexpression. The ontological analysis categorized
the differentially expressed genes into 121 signaling pathways. The
most significant were pathways important both for carcinogenesis
and development, such as Wnt, TGFβ, Notch and Hedgehog. The
cellular pathways with the highest number of assigned genes
regulated by WWOX expression level are presented in Table I. The biological experiments on the
WWOX-transfected T98G/WWOX cells revealed a more
intensive energetic mitochondrial metabolism, but a lower rate of
proliferation and higher apoptosis level than the T98G/vec control
cells. Moreover, the T98G/WWOX cells demonstrated a higher
migration potential and reduced attachment to extra-cellular matrix
proteins. Furthermore, cells overexpressing WWOX failed to
grow in an extracellular matrix environment on Geltrex
substrate.
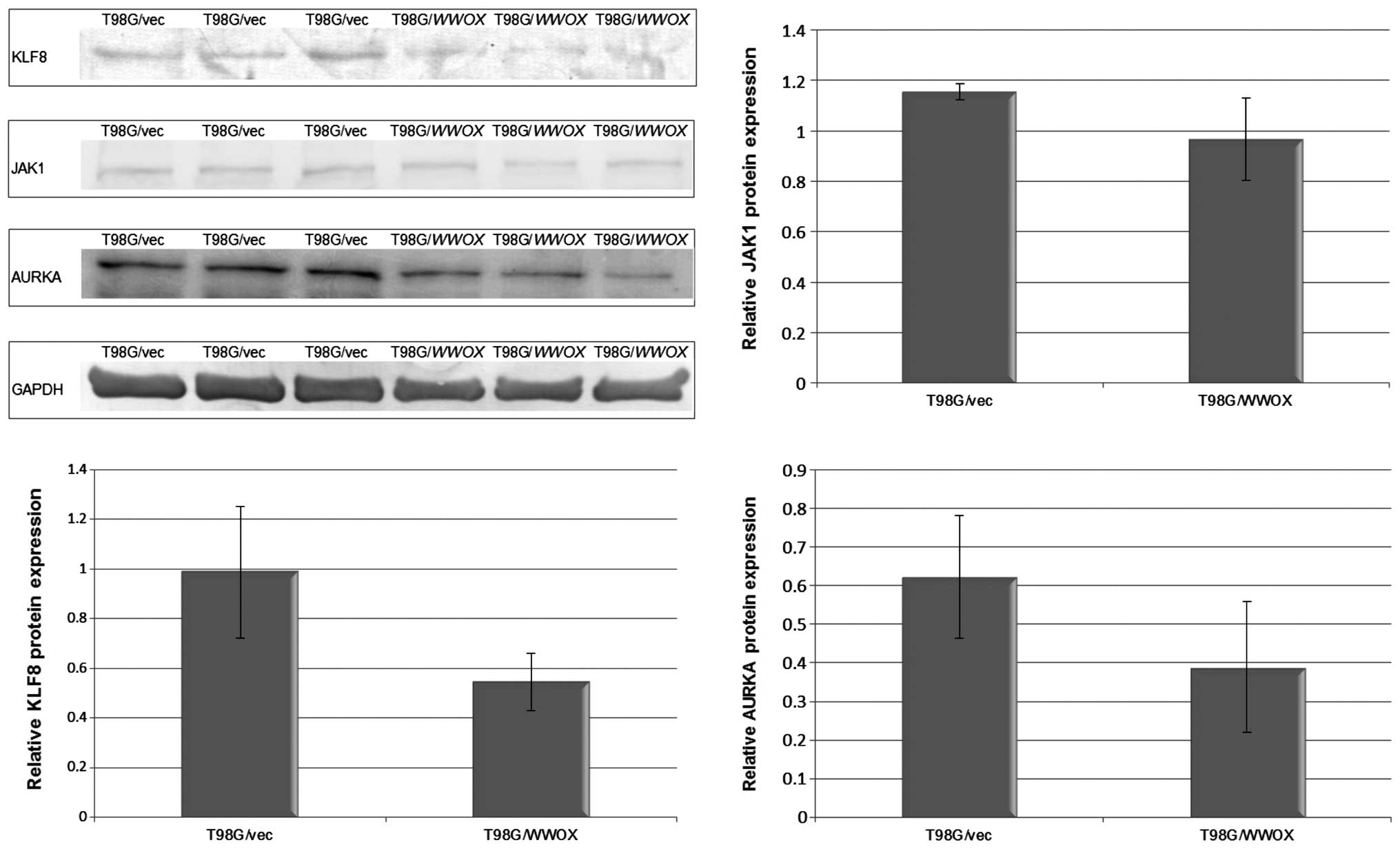
Loss of cell cycle control and intense proliferation
are hallmarks of neoplastic cells. In the proliferation assay with
BrdU, T98G/WWOX cells demonstrated a 53% lower proliferation
rate than the T98G/vec control (p<0.01). An ontological analysis
of the microarray data revealed 163 genes to be involved in cell
cycle regulation, whose expression was modulated by WWOX
overexpression. Among these genes are potent oncogenes linked with
brain cancerogenesis: AURKA, KLF8 and JAK1
(12–16). In our microarray experiment, the
T98G/WWOX cells demonstrated significantly lower expression
of all afore-mentioned genes. Western blot analysis confirmed that
the level of these proteins was considerably lower in the cells
with high WWOX expression (Fig.
4).
After enhancement of WWOX expression in the
T98G cell line, a tendency for increased apoptosis was observed
(p>0.05). Such an effect of WWOX overexpression was found
to be cosistant across various cell lines (17–24).
While apoptosis seems to proceed through a mitochondrial pathway in
breast, prostate and lung cells, Chiang et al showed that in
U373MG glioblastoma cells, WWOX overexpression triggers a
mitochondrial/caspase-3-independent pathway of apoptosis (25).
WWOX overexpression in T98G cells resulted in
an increased number of invasive cells crossing the basement
membrane. This effect was also observed in breast and colon cancer
cells with ectopic WWOX expression (26,27).
It can be hypothesized that elevated cell motility is related to
the function of WWOX as a regulator of differentiation. The
microarray gene expression analysis revealed a large group of
WWOX-regulated genes whose products are engaged in nervous
system development (108 genes, classification presented in Table II ). Recently, Abdeen et al
reported that WWOX knockdown in MCF-10A normal breast cells
resulted in impaired growth in a 3-D culture Matrigel assay and
mammary ductal formation (28).
This suggests that WWOX expression is required for the
proper development of this gland. Contrary to Abdeen’s results, in
our experiment, the 3-D culture formation by glioblastoma cells was
inhibited by a high level of WWOX.
The increase in mitochondrial redox activity in
T98G/WWOX cells is intriguing. One of the genes whose
expression was elevated in our microarray experiment was
DLD, a gene encoding dihydrolipoamide dehydrogenase,
diaphorase which catalyzes the reduction of resazurin
(alamarBlue®) into resofurin (29). The DLD expression in
T98G/WWOX was >3-fold higher than that in the T98/vec
control cells. This may explain the higher redox potential observed
in the T98G/WWOX cells in the alamarBlue assay. O’Keefe
et al showed in a Drosophila model that WWOX
takes part in aerobic metabolism and the generation of ROS
(30). In their recent study, Dayan
et al claimed that WWOX not only influences
metabolism, but its mRN A level is also related to the metabolic
state of the cell. They reported that switching the metabolism from
glycolysis to oxidative phosphorylation causes a stable increase in
the amount of WWOX mRNA. Consequently, hypoxia, a state
where cells rely on glycolysis, causes its decrease (31). GBM cells are known to switch their
metabolism to glycolysis in response to hypoxic conditions inside
the tumor and the demand for the accelerated production of
substrates needed by the rapidly proliferating cells (32).
The T98G cells overexpressing WWOX exhibited
a significantly lowered ability to adhere to fibronectin, collagen
I, collagen IV and fibrinogen. The decrease in adhesion to laminin,
was also noted, although without statistical significance. A
similar result of WWOX influence on decreased adhesion to
fibronectin was observed by Gourley et al in ovarian cell
lines (33). Adhesion to the
extracellular matrix is fundamental for cancer cell behavior. Cell
adhesion-mediated drug resistance (CAM-DR) is a phenomenon of
apoptosis resistance caused by close interactions between cancer
cells and ECM proteins (34).
Furthermore, one of the main GBM hallmarks is ability for invasion
and infiltration of surrounding tissue; this is the reason for the
almost inevitable relapse of the disease. The invasion of GBM
corresponds with increased adhesion to ECM proteins and its
proteolytic degradation (35). In
the light of the present knowledge in regards to adhesion in the
progression of GBM, the observed reduction in the attachment of
cells to ECM proteins caused by WWOX overexpression can be
interpreted as decreasing cell malignancy.
The decrease in adhesion of the cells to ECM
proteins and their reduced ability to proliferate and form 3D
structures in the extracellular matrix indicate that WWOX
overexpression causes substantial changes in cytoskeleton
organization and the structure of membrane proteins such as
integrins. Indeed, the global gene expression examination showed a
large group of WWOX-regulated genes acting in the integrin
and cadherin signaling pathways (28 and 13 genes, respectively).
Moreover, an ontological classification of the genes affected by
WWOX overexpression with respect to the cellular component
revealed that the largest group of genes was connected with the
cytoskeleton. The organization of the cytoskeleton regulates such
processes as cell adhesion and motility (36,37).
Additionally, besides the cellular functions
discussed above, cell cycle control, metabolism, invasiveness,
apoptosis and adhesion, several other notable aspects of cell
behavior which could be affected by WWOX emerged from the
microarray study. In the group of genes whose expression was
altered by WWOX overexpression were genes involved in
angiogenesis, development and intracellular transport. The precise
mechanism and the nature of WWOX acting on the numerous
cellular functions indicated here is yet to be determined.
In conclusion, the observed reduction in ECM
adhesion, lowering of the proliferation rate and the increase in
apoptosis can be regarded as a decrease in cell malignancy due to
WWOX overexpression. The most striking evidence of the
WWOX tumor suppressor effect is the inhibition of the growth
of glioblastoma cells in an extracellular matrix environment. The
higher migration rates may be linked with the presumed role played
by WWOX in differentiation and the regulation of
development.
The obtained results indicate that WWOX may
be a key regulator of the main cellular processes: cell cycle,
apoptosis, metabolism, cytoskeleton structure and differentiation.
The increase in WWOX expression influences the phenotype of
T98G cells, reducing their malignancy.
Present knowledge of WWOX function suggests
that, contrary to initial assumptions, it does not act as a
classical tumor suppressor. It appears that the role of WWOX
is not limited to that of cell cycle control or genome integrity
protection, but its influence on cell function is more global. It
is probable that it is one of the pivotal regulators of genes
involved in cell differentiation, responsible for maintaining
tissue structure.
Acknowledgements
The present study was funded by the Medical
University of Lodz, grants 503/0-078-02/503-01 and
502-03/0-078-02/502-04-020.
References
|
1
|
Chen J and Xu T: Recent therapeutic
advances and insights of recurrent glioblastoma multiforme. Front
Biosci (Landmark Ed). 18:676–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lwin Z, MacFadden D, Al-Zahrani A, et al:
Glioblastoma management in the temozolomide era: have we improved
outcome? J Neurooncol. 115:303–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanu OO, Hughes B, Di C, et al:
Glioblastoma multiforme oncogenomics and signaling pathways. Clin
Med Oncol. 3:39–52. 2009.PubMed/NCBI
|
|
4
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3–24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
5
|
Aqeilan RI, Trapasso F, Hussain S, et al:
Targeted deletion of Wwox reveals a tumor suppressor
function. Proc Natl Acad Sci USA. 104:3949–3954. 2007.PubMed/NCBI
|
|
6
|
Aqeilan RI, Palamarchuk A, Weigel RJ,
Herrero JJ, Pekarsky Y and Croce CM: Physical and functional
interactions between the Wwox tumor suppressor protein and the
AP-2gamma transcription factor. Cancer Res. 64:8256–8261. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aqeilan RI, Pekarsky Y, Herrero JJ, et al:
Functional association between Wwox tumor suppressor protein and
p73, a p53 homolog. Proc Natl Acad Sci USA. 101:4401–4406. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aqeilan RI, Donati V, Palamarchuk A, et
al: WW domain-containing proteins, WWOX and YAP, compete for
interaction with ErbB-4 and modulate its transcriptional function.
Cancer Res. 65:6764–6772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matteucci E, Bendinelli P and Desiderio
MA: Nuclear localization of active HGF receptor Met in aggressive
MDA-MB231 breast carcinoma cells. Carcinogenesis. 30:937–945. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kosla K, Pluciennik E, Kurzyk A, et al:
Molecular analysis of WWOX expression correlation with
proliferation and apoptosis in glioblastoma multiforme. J
Neurooncol. 101:207–213. 2011.
|
|
11
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borges KS, Castro-Gamero AM, Moreno DA, et
al: Inhibition of Aurora kinases enhances chemosensitivity to
temozolomide and causes radiosensitization in glioblastoma cells. J
Cancer Res Clin Oncol. 138:405–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lehman NL, O’Donnell JP, Whiteley LJ, et
al: Aurora A is differentially expressed in gliomas, is associated
with patient survival in glioblastoma and is a potential
chemotherapeutic target in gliomas. Cell Cycle. 11:489–502. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samaras V, Stamatelli A, Samaras E, et al:
Comparative immunohistochemical analysis of aurora-A and aurora-B
expression in human glioblastomas. Associations with proliferative
activity and clinicopathological features. Pathol Res Pract.
205:765–773. 2009. View Article : Google Scholar
|
|
15
|
Schnell O, Romagna A, Jaehnert I, et al:
Kruppel-like factor 8 (KLF8) is expressed in gliomas of different
WHO grades and is essential for tumor cell proliferation. PLoS One.
7:e304292012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu Y, Zhong Y, Fu J, et al: Activation of
JAK/STAT signal pathway predicts poor prognosis of patients with
gliomas. Med Oncol. 28:15–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aderca I, Moser CD, Veerasamy M, et al:
The JNK inhibitor SP600129 enhances apoptosis of HCC cells induced
by the tumor suppressor WWOX. J Hepatol. 49:373–383. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fabbri M, Iliopoulos D, Trapasso F, et al:
WWOX gene restoration prevents lung cancer growth in vitro
and in vivo. Proc Natl Acad Sci USA. 102:15611–15616. 2005.
View Article : Google Scholar
|
|
19
|
Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X and
Sun ZQ: WWOX induces apoptosis and inhibits proliferation of
human hepatoma cell line SMMC-7721. World J Gastroenterol.
18:3020–3026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iliopoulos D, Fabbri M, Druck T, Qin HR,
Han SY and Huebner K: Inhibition of breast cancer cell growth in
vitro and in vivo: effect of restoration of Wwox expression. Clin
Cancer Res. 13:268–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuroki T, Yendamuri S, Trapasso F, et al:
The tumor suppressor gene WWOX at FRA16D is involved
in pancreatic carcinogenesis. Clin Cancer Res. 10:2459–2465.
2004.
|
|
22
|
Qin HR, Iliopoulos D, Semba S, et al: A
role for the WWOX gene in prostate cancer. Cancer Res.
66:6477–6481. 2006.PubMed/NCBI
|
|
23
|
Xiong Z, Hu S and Wang Z: Cloning of WWOX
gene and its growth-inhibiting effects on ovarian cancer cells. J
Huazhong Univ Sci Technolog Med Sci. 30:365–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang P, Jia R, Ying L, et al:
WWOX-mediated apoptosis in A549 cells mainly involves the
mitochondrial pathway. Mol Med Rep. 6:121–124. 2012.
|
|
25
|
Chiang MF, Yeh ST, Liao HF, Chang NS and
Chen YJ: Overexpression of WW domain-containing oxidoreductase WOX1
preferentially induces apoptosis in human glioblastoma cells
harboring mutant p53. Biomed Pharmacother. 66:433–438. 2012.
View Article : Google Scholar
|
|
26
|
Lewandowska U, Zelazowski M, Seta K,
Byczewska M, Pluciennik E and Bednarek AK: WWOX, the tumour
suppressor gene affected in multiple cancers. J Physiol Pharmacol.
60:47–56. 2009.PubMed/NCBI
|
|
27
|
Nowakowska M, Pospiech K, Lewandowska U,
Piastowska-Ciesielska AW and Bednarek AK: Diverse effect of WWOX
overexpression in HT29 and SW480 colon cancer cell lines. Tumour
Biol. View Article : Google Scholar : 2014.PubMed/NCBI
|
|
28
|
Abdeen SK, Salah Z, Khawaled S and Aqeilan
RI: Characterization of WWOX inactivation in murine mammary gland
development. J Cell Physiol. 228:1391–1396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O’Brien J, Wilson I, Orton T and Pognan F:
Investigation of the Alamar Blue (resazurin) fluorescent dye for
the assessment of mammalian cell cytotoxicity. Eur J Biochem.
267:5421–5426. 2000.PubMed/NCBI
|
|
30
|
O’Keefe LV, Colella A, Dayan S, et al:
Drosophila orthologue of WWOX, the chromosomal fragile site
FRA16D tumour suppressor gene, functions in aerobic metabolism and
regulates reactive oxygen species. Hum Mol Genet. 20:497–509.
2011.
|
|
31
|
Dayan S, O’Keefe LV, Choo A and Richards
RI: Common chromosomal fragile site FRA16D tumor suppressor WWOX
gene expression and metabolic reprograming in cells. Genes
Chromosomes Cancer. 52:823–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wolf A, Agnihotri S and Guha A: Targeting
metabolic remodeling in glioblastoma multiforme. Oncotarget.
1:552–562. 2010.PubMed/NCBI
|
|
33
|
Gourley C, Paige AJ, Taylor KJ, et al:
WWOX gene expression abolishes ovarian cancer tumorigenicity in
vivo and decreases attachment to fibronectin via integrin alpha3.
Cancer Res. 69:4835–4842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morin PJ: Drug resistance and the
microenvironment: nature and nurture. Drug Resist Updat. 6:169–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giese A, Laube B, Zapf S, Mangold U and
Westphal M: Glioma cell adhesion and migration on human brain
sections. Anticancer Res. 18:2435–2447. 1998.PubMed/NCBI
|
|
36
|
Brieher WM and Yap AS: Cadherin junctions
and their cytoskeleton(s). Curr Opin Cell Biol. 25:39–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vitriol EA and Zheng JQ: Growth cone
travel in space and time: the cellular ensemble of cytoskeleton,
adhesion, and membrane. Neuron. 73:1068–1081. 2012. View Article : Google Scholar : PubMed/NCBI
|