Introduction
Colorectal cancer (CRC) is a common type of
malignant tumor with a high rate of morbidity and mortality. Early
detection of CRC results in radical resection of the tumor in the
majority of cases. However, radical resection is curative for only
~50% of the patients who will present local recurrence or distant
metastases (1). The survival rate
of patients with metastatic CRC (mCRC) has significantly improved
with applications of molecularly targeted drugs, such as
oxaliplatin (L-OHP), and has led to a substantial improvement in
the overall survival rate. Chemotherapy drugs may exist for
pathological parts of non-specific selection and result in the
decrease of curative effect and side-effects on the body (2,3).
Liposomes, as carriers of chemotherapeutic agents,
can alter the distribution of these agents within the body and
decrease drug toxicity (4,5). Therefore, drug-loaded liposomes offer
a new approach to the treatment of CRC. The polyethylene glycol
(PEG) modification increases the affinity of liposomes for cancer
cells and thus increases the cellular uptake of drugs. Cellular
uptake of PEG-liposomal L-OHP induces bioactive changes in cells,
resulting in apoptosis.
The transcription factor nuclear factor-κB (NF-κB)
is a dimeric complex composed of members of the Rel protein family,
which includes p65 (RelA), p105/p50, p100/p52, RelB, c-Rel and the
viral oncoprotein (v-Rel) (6). In
its classic form, NF-κB is a dimer that consists of the
transcriptionally inactive p50 subunit and the p65/RelA (p65)
subunit. NF-κB is a transcription factor that participates in
proliferation and metastasis (7–9). The
abnormality of NF-κB is closely associated with human malignant
tumor and different molecular modification of tumor cells results
in abnormal activation regulation of NF-κB which loses its
inducibility, thus remaining at an activated state, leading to
abnormal regulation of apoptosis, cell cycle, adherence and
metastatic genes by NF-κB (10,11).
NF-κB participates in the transcription and regulation of multiple
cell factors in the apoptotic pathway (11,12).
Pyrrolidine dithiocarbamate (PDTC), a selective NF-κB inhibitor,
has been used more widely in experimental study as the inhibitor of
NF-κB for the treatment of inflammatory diseases (13). Moreover, it has been reported that
PDTC could induce apoptosis via inhibition of NF-κB activation
(14,15).
In the present study, we investigated the effect of
PEG-liposomal L-OHP on apoptosis, as well as regulation of
apoptotic cell death by NF-κB, and we prepared PEG modified L-OHP
liposomes to treat SW480 cells of human colorectal cancer in an
effort to study the apoptosis.
Materials and methods
Reagents
Human colorectal cancer SW480 cells were provided by
the Life Science Academy of Chongqing Medical University. L-OHP was
purchased from Sigma Co. 1,2-Distearoyl-sn-glycero-
3-phosphoethanolamine-N-[ma-leimide(polyethylene glycol)-2000]
(DSPE-PEG2000) was obtained from Avanti Polar Lipids, Inc.
DIOC18(3),
3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) was obtained from
Vigorous Biotech Co., Ltd. Rabbit polyclonal anti-β-actin, goat
anti-rabbit IgG and peroxidase conjugated secondary antibodies were
obtained from Bioscience Co., USA. Rabbit polyclonal antibodies of
Bcl-2 and Bax were purchased from Santa Cruz Biotechnology, Inc.
Activated-caspase-3 (P17) antibodies were obtained from Bioworld
Technology, Inc. The TUNEL kit was purchased from Promega. NF-κB
inhibitor, ammonium PDTC, was purchased from Beyotime Institute of
Biotechnology.
Cell culture, cell uptake of liposomes
observed by laser focus or scanning electron microscopy (SEM)
Dio-labeled PEG-liposomes and PEG-liposomal L-OHP
were prepared, as previously described (16). Human colorectal cancer SW480 cells
in the logarithmic growth phase were trypsinized and incubated on
glass slides (5×103/slide) in 1 ml of RPMI-1640 medium
containing 10% FBS and pre-incubated for 24 h. After removal of the
culture medium, 1 ml of fresh medium containing the Dio-labeled
PEG-liposomes (2 μmol/ml) was added, followed by incubation at
37°C. At 8 h post-incubation, the cells were washed twice with cold
phosphate-buffered saline (PBS) and fixed with freshly prepared 4%
paraformaldehyde (dissolved in pH 7.4 PBS). The cellular uptake of
Dio-labeled PEG-liposomes was determined by measuring fluorescence
and by SEM.
Immunofluorescence and flow cytometry
(FCM) evaluate cellular calcium ion
The cells were placed in wells of a 6-well plate and
glass slides incubated for 24 h, respectively. The cells were
treated with PEG-liposomal L-OHP (containing 28 μg/ml L-OHP),
PEG-liposomal L-OHP + PDTC (10 μmol/l) or PEG-liposomal L-OHP +
PDTC (20 μmol/l) for 12 h, with no treatment as a control. After
treatment, the culture medium was removed and washed by PBS. The
cell glass slides were fixed with freshly prepared 4%
paraformaldehyde, incubated with calcium ion fluorescent probe and
evaluated for laser focus. The cells in 6-well plates were
trypsinized, collected and fixed with 70% ice-cold ethanol
overnight at 4°C. Cells were centrifuged, re-suspended in 400 μl 1X
binding buffer (>1×106/ml) and incubated with calcium
ion fluorescent probe (final concentration 5 nmol/ml) in the dark
for 1 h. The cells were washed with PBS and resuspended in 200 μl,
and analyzed using a BD FACSCalibur (BD Biosciences).
Tagged deoxynucleotide transferase
labeling
SW480 cells (5×103/slide) were incubated
on glass slides for 24 h, treated for 12 h with PEG-liposomal
L-OHP, PEG-liposomal L-OHP + PDTC (10 μmol/l) or PEG-liposomal
L-OHP + PDTC (20 μmol/l) and fixed in freshly prepared 4%
paraformaldehyde (PFA; dissolved in pH 7.4 PBS) for 30 min at room
temperature and finally washed with PBS, as described previously
(17). Cells treated with DNase I,
which fragments the DNA, served as positive controls.
Western blot analysis
The cells were collected and lysed and the protein
concentration of each sample was determined using the Bradford
dye-binding method. Protein samples (50 μg) were electrophoresed on
SDS/12 and 15% PAGE gels and transferred to PVDF membranes
(Millipore). The membranes were incubated overnight at 4°C with
rabbit antibodies to Bcl-2, Bax, activated caspase-3 (1:500) and an
anti-β-actin antibody (1:500), followed by incubation at room
temperature for 1 h with a horseradish peroxidase (HRP)-labeled
goat anti-rabbit secondary antibody added drop-wise concurrently
with the enhanced chemiluminescence illuminating agent (Immobilon ™
Western Chemiluminescent HRP Substrate, kit no. WBKLS0100).
Finally, the membranes were visualized using a Bio-Rad system.
Statistical analyses
All values are expressed as means ± SD using
SPSS17.0 software. Experimental results were analyzed by the
Student’s t-test. P<0.05 was considered as significant for
values obtained for treated compounds compared with controls.
Results
Cellular uptake of liposomes
To study the conjugated effects of the cellular
uptake of liposomes, confocal microscopy and scanning electron
microscopy were adopted, respectively. Confocal microscopy
demonstrated that after incubation in medium containing Dio-labeled
liposomes for 4 h, many of the PEG-liposomes conjugated with cells
and aggregation of liposomes within cells (Fig. 1A–C). Also, scanning electron
microscopy demonstrated that PEG-liposomes adhered to the cell
surface (Fig. 1D).
Change of cellular calcium ion
Apoptosis signal transduction pathway in cells,
calcium ions which activate apoptosis performer caspase-3, and
hydrolysis of various cellular components lead to cell apoptosis.
Cells were treated with PEG-liposomal L-OHP, and the change of
calcium ion was obtained. Pretreatment with the NF-κB inhibitor,
ammonium PDTC, demonstrated increased concentration of cellular
calcium ion. The relative fluorescence intensity was 51.12±2.91,
71.12±2.56 and 82.44±3.27, respectively, control each group,
P<0.05 (Figs. 2 and 3).
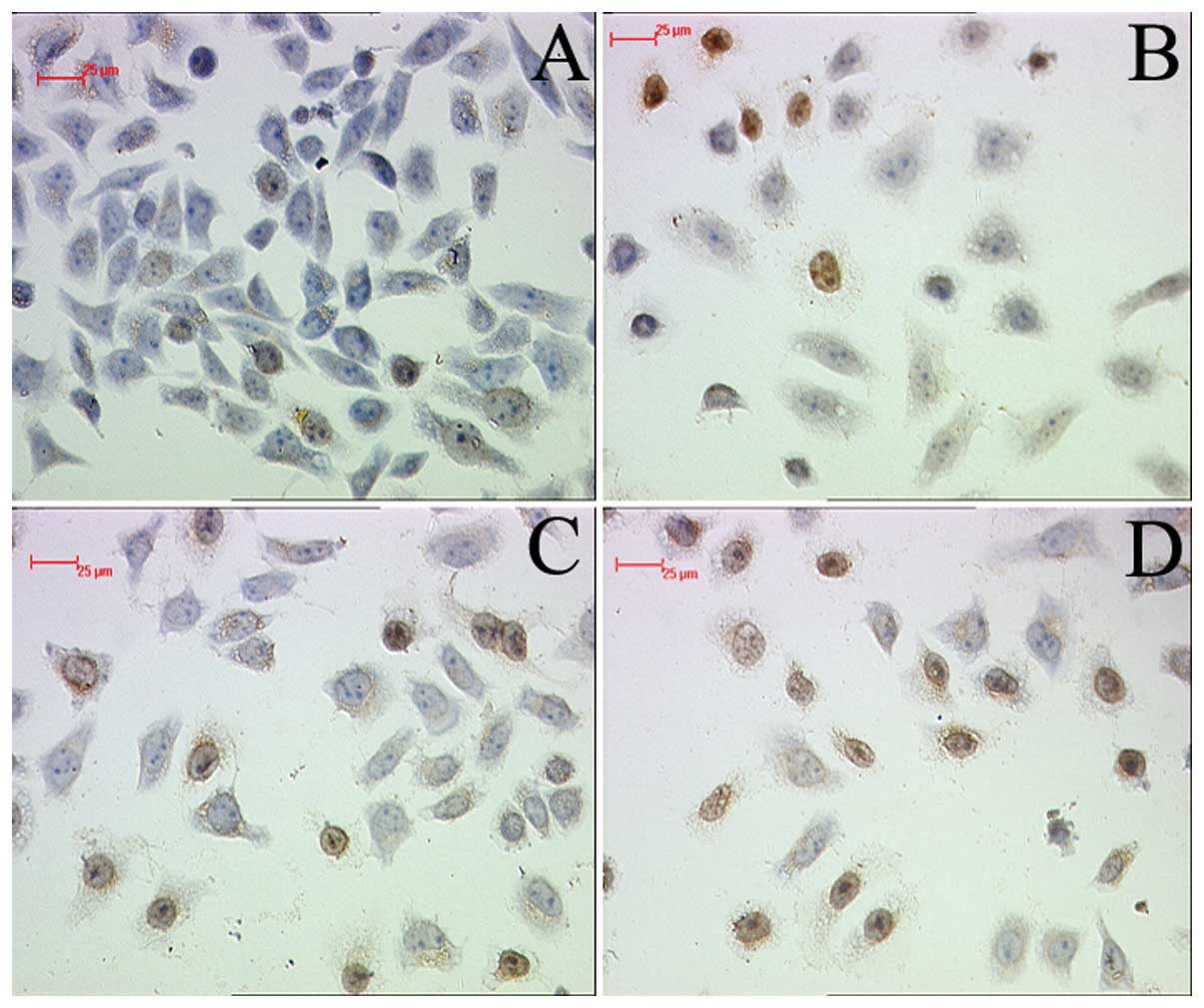
Apoptosis analysis
To establish the cytotoxic effect of PEG-liposomal
L-OHP, apoptosis was assayed by TUNEL. The result demonstrated that
PEG-liposomal L-OHP induced marked apoptotic death of SW480 cells,
compared with control (Fig. 4B).
However, pretreatment with PDTC led to strong detection of
apoptosis in SW480 cells (Fig.
4C–D).
Expression protein of NF-κB (P65)
The phosphorylated NF-κB (p-P65) is able to
translocate to the nucleus, activating genes involved in tumor cell
proliferation and survival. To determine whether apoptosis is
enhanced, after cells were pretreated with NF-κB inhibitor, total
protein was extracted from the SW480 cells. The western blot
results showed that protein of p-P65 was downregulated, compared
with every other group, P<0.05. However, the total protein of
P65 was almost unchanged, compared with every other group,
P>0.05 (Fig. 5).
Expression of pro-apoptotic and
anti-apoptotic proteins
Bcl-2, Bax, caspase-3 are associated with apoptosis,
necrosis and autophagy and regulate all major types of cell death.
To establish the cytotoxic effect of PEG-liposomal L-OHP, after
pretreatment with various concentrations of NF-κB inhibitor,
apoptosis was assayed by western blot analysis. The results showed
Bcl-2 was downregulated. However, Bax and caspase-3 were
upregulated, compared with every other group, P<0.05 (Fig. 6).
Discussion
The nature of the active species generated in
vivo, uptake, efflux, intracellular trafficking or insufficient
diffusion in tumor tissues, lead to cytotoxic drugs having no
target selectivity between normal body tissues and pathological
sites (18), resulting in decreased
curative effects and increased toxicity for certain
chemotherapeutic agents.
The ideal therapy is to deliver the drugs directly
to pathological sites. PEG-modified liposomes have been used as a
carrier of anticancer drugs to enhance the affinity of anticancer
drugs to cancer cells and uptake of anticancer drugs by cancer
cells (19–21). Our in vitro study revealed
uptake of PEG-liposomes by cancer cells at 2 h, which increased
with time of exposure and accumulation of a great amount of
liposomes within the cells by fluorescence and SEM. The results are
similar to those reported by Gabizon and Papahadjopoulos, and
Gabizon (22,23). Accordingly, FCM showed that compared
with PEG-liposomal L-OHP, the same dosage of PEG-liposomal L-OHP
had a significantly greater impact on apoptotic cell death, after
pretreatment with PDTC, the plasma Ca2+ level was
consistently higher (Fig. 3).
Moreover, we found that TUNEL experiments showed apoptotic death of
SW480 cells was also consistently increased. PEG-liposomal L-OHP
degraded in cells after pinocytosis; furthermore, the intracellular
drug release enhanced active drug level within cells and slowed
down drug efflux, therefore, liposome entrapment of
chemotherapeutics represents its dual effect on tumor cells
(24–26).
NF-κB, a complex of proteins, is a widespread
transcription factor of eukaryotic cells with extensive actions and
is involved in cellular inflammatory and apoptotic responses
(27). NF-κB plays a key role in
regulating apoptotic cell death. Our study showed that in the
liposomal L-OHP-treated group, the apoptotic cells increased
(Fig. 4B). To further explain the
role of NF-κB in regulating apoptotic cell death, the tumor cells
were administered PDTC pretreatment, which resulted in increased
apoptotic cell death (Fig. 4C and
D). However, the expression protein of NF-κB was markedly
suppressed by PDTC pretreatment, P17, Bax and Bad showed an upward
trend (Figs. 5 and 6). This may be explained by the selective
NF-κB inhibition mechanism of PDTC.
Activated NF-κB is highly expressed in tumor cells
(28,29); the decrease of the activity of NF-κB
inhibits the expression of Bcl-2 and Bax (30,31),
predominantly seen in cytoplasm, and is highly expressed in many
refractory tumors or those with poor prognosis such as gastric
carcinoma and intestinal carcinoma.
Bcl-2 and Bax are members of the Bcl-2 family
(32). They are associated with
apoptosis, necrosis. Bcl-2 is an anti-apoptotic protein and
guardian of the outer membrane. In contrast, Bax is a proapoptotic
protein that induces mitochondrial outer membrane permeabilization,
causing the release of caspase activating proteins and it preserves
its integrity by opposing Bcl-2. The downregulation of Bcl-2 and
upregulation of Bax and induced activation of caspase-3 through
upregulation of intracellular apoptotic pathway (32,33),
results in final apoptotic cell death (34,35).
In the present study, these results indicated that NF-κB plays a
role in mediating regulation L-OHP PEG-liposome-induced aptoptotic
cell death. PDTC was demonstrated to suppress the activation of
NF-κB and to ameliorate the cell death of SW480. Moreover,
apoptosis was correlated with concentration of PDTC. Therefore, the
results obtained in the present study indicate that the inhibition
of NF-κB activation by PDTC in the PEG-liposomes L-OHP is likely to
be associated with the blocking of NF-κB signal pathway, the
downregulation of Bcl-2 expression and upregulation of Bax or
caspase-3 expression.
In conclusion, the experiments presented in this
report indicate that PEG-liposome-targeting approaches represent a
general framework for developing more effective cancer therapies.
Liposome entrapment of L-OHP enhanced its anticancer potency. PDTC
can enhance the cell apoptosis induced by PEG-liposomes. Inhibition
of NF-κB activation increases the antitumor effect of the antitumor
agents. The dual treatment regimen is of great significance for the
treatment of colorectal cancer. The success of specific
combinations of targeted therapies may likely depend on the
specificity and efficacy of each individual targeted therapy.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of China (no. 81172295). We thank the Institute
of Life Science of Chongqing Medical University for their technical
support and experimental assistance.
References
|
1
|
Spolverato G, Ejaz A, Azad N and Pawlik
TM: Surgery for colorectal liver metastases: the evolution of
determining prognosis. World J Gastrointest Oncol. 5:207–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alberts SR, Sargent DJ, Nair S, et al:
Effect of oxaliplatin, fluorouracil, and leucovorin with or without
cetuximab on survival among patients with resected stage III colon
cancer: a randomized trial. JAMA. 307:1383–1393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garcia-Foncillas J and Diaz-Rubio E:
Progress in metastatic colorectal cancer: growing role of cetuximab
to optimize clinical outcome. Clin Transl Oncol. 12:533–542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain RL and Shastri JP: Study of ocular
drug delivery system using drug-loaded liposomes. Int J Pharm
Investig. 1:35–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saffari M, Shirazi HF, Oghabian MA, et al:
Preparation and in-vitro evaluation of an antisense-containing
cationic liposome against non-small cell lung cancer: a comparative
preparation study. Iran J Pharm Res. 12:3–10. 2013.PubMed/NCBI
|
|
6
|
Ashour AE, Abd-Allah AR, Korashy HM, et
al: Thymoquinone suppression of the human hepatocellular carcinoma
cell growth involves inhibition of IL-8 expression, elevated levels
of TRAIL receptors, oxidative stress and apoptosis. Mol Cell
Biochem. 389:85–98. 2014. View Article : Google Scholar
|
|
7
|
Nookala AR, Shah A, Noel RJ and Kumar A:
HIV-1 Tat-mediated induction of CCL5 in astrocytes involves NF-κB,
AP-1, C/EBPα and C/EBPγ transcription factors and JAK, PI3K/Akt and
p38 MAPK signaling pathways. PLoS One. 8:e788552013.PubMed/NCBI
|
|
8
|
Zhang F, Lu M, Wang H and Ren T: Aspirin
attenuates angiotensin II-induced inflammation in bone marrow
mesenchymal stem cells via the inhibition of ERK1/2 and NF-κB
activation. Biomed Rep. 1:930–934. 2013.PubMed/NCBI
|
|
9
|
Mezzanotte L, An N, Mol IM, Löwik CW and
Kaijzel EL: A new multicolor bioluminescence imaging platform to
investigate NF-κB activity and apoptosis in human breast cancer
cells. PLoS One. 9:e855502014.PubMed/NCBI
|
|
10
|
Krizanova O, Markova J, Pacak K, et al:
Triptolide induces apoptosis through the SERCA 3 upregulation in
PC12 cells. Gen Physiol Biophys. 33:137–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anttonen M, Pihlajoki M, Andersson N, et
al: FOXL2, GATA4, and SMAD3 co-operatively modulate gene
expression, cell viability and apoptosis in ovarian granulosa cell
tumor cells. PLoS One. 9:e855452014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang Y, Li D, Fu J, et al:
Overexpression of AIOLOS inhibits cell proliferation and suppresses
apoptosis in Nalm-6 cells. Oncol Rep. 31:1183–1190. 2014.PubMed/NCBI
|
|
13
|
Huang Y, Lu Y, Zhang L, et al: Perineural
dexmedetomidine attenuates inflammation in rat sciatic nerve via
the NF-κB pathway. Int J Mol Sci. 15:4049–4059. 2014.PubMed/NCBI
|
|
14
|
Li T, Zhang Q, Zhang J, et al: Fenofibrate
induces apoptosis of triple-negative breast cancer cells via
activation of NF-κB pathway. BMC Cancer. 14:962014.PubMed/NCBI
|
|
15
|
Zhang J, Zhang DL, Jiao XL and Dong Q:
S100A4 regulates migration and invasion in hepatocellular carcinoma
HepG2 cells via NF-κB-dependent MMP-9 signal. Eur Rev Med Pharmacol
Sci. 17:2372–2382. 2013.PubMed/NCBI
|
|
16
|
Yang C, Liu HZ, Fu ZX and Lu WD:
Oxaliplatin long-circulating liposomes improved therapeutic index
of colorectal carcinoma. BMC Biotechnol. 11:212011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang C, Liu HZ and Fu ZX: Effects of
PEG-liposomal oxaliplatin on apoptosis, and expression of Cyclin A
and Cyclin D1 in colorectal cancer cells. Oncol Rep. 28:1006–1012.
2012.PubMed/NCBI
|
|
18
|
Olszewski U and Hamilton G: A better
platinum-based anticancer drug yet to come? Anticancer Agents Med
Chem. 10:293–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Preiss MR and Bothun GD:
Stimuli-responsive liposome-nanoparticle assemblies. Expert Opin
Drug Deliv. 8:1025–1040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suntres ZE: Liposomal antioxidants for
protection against oxidant-induced damage. J Toxicol.
2011:1524742011.PubMed/NCBI
|
|
21
|
Pagano RE and Weinstein JN: Interactions
of liposomes with mammalian cells. Annu Rev Biophys Bioeng.
7:435–468. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gabizon A and Papahadjopoulos D: Liposome
formulations with prolonged circulation time in blood and enhanced
uptake by tumors. Proc Natl Acad Sci USA. 85:6949–6953. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gabizon AA: Stealth liposomes and tumor
targeting: one step further in the quest for the magic bullet. Clin
Cancer Res. 7:223–225. 2001.PubMed/NCBI
|
|
24
|
Noble GT, Stefanick JF, Ashley JD, et al:
Ligand-targeted liposome design: challenges and fundamental
considerations. Trends Biotechnol. 32:32–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hood RR, Shao C, Omiatek DM, et al:
Microfluidic synthesis of PEG- and folate-conjugated liposomes for
one-step formation of targeted stealth nanocarriers. Pharm Res.
30:1597–1607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abu Lila AS, Doi Y, Nakamura K, et al:
Sequential administration with oxaliplatin-containing PEG-coated
cationic liposomes promotes a significant delivery of subsequent
dose into murine solid tumor. J Control Release. 142:167–173.
2010.
|
|
27
|
Vaiopoulos AG, Athanasoula KCh and
Papavassiliou AG: NF-κB in colorectal cancer. J Mol Med.
91:1029–1037. 2013.
|
|
28
|
Li J, Li J, Yue Y, et al: Genistein
suppresses tumor necrosis factor α-induced inflammation via
modulating reactive oxygen species/Akt/nuclear factor κB and
adenosine monophosphate-activated protein kinase signal pathways in
human synoviocyte MH7A cells. Drug Des Devel Ther. 8:315–323.
2014.
|
|
29
|
Zha Y, Gan P, Yao Q, Ran FM and Tan J:
Downregulation of Rap1 promotes 5-fluorouracil-induced apoptosis in
hepatocellular carcinoma cell line HepG2. Oncol Rep. 31:1691–1698.
2014.PubMed/NCBI
|
|
30
|
Sethi G, Ahn KS, Sung B and Aggarwal BB:
Pinitol targets nuclear factor-kappaB activation pathway leading to
inhibition of gene products associated with proliferation,
apoptosis, invasion, and angiogenesis. Mol Cancer Ther.
7:1604–1614. 2008. View Article : Google Scholar
|
|
31
|
Aggarwal BB: Nuclear factor-kappaB: the
enemy within. Cancer Cell. 6:203–208. 2004.PubMed/NCBI
|
|
32
|
Fan XX, Li N, Wu JL, et al: Celastrol
induces apoptosis in gefitinib-resistant non-small cell lung cancer
cells via caspases-dependent pathways and Hsp90 client protein
degradation. Molecules. 19:3508–3522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park MY, Jeong YJ, Kang GC, et al: Nitric
oxide-induced apoptosis of human dental pulp cells is mediated by
the mitochondria- dependent pathway. Korean J Physiol Pharmacol.
18:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim MH, Kim SH and Yang WM: Beneficial
effects of Astragaloside IV for hair loss via inhibition of Fas/Fas
L-mediated apoptotic signaling. PLoS One. 9:e929842014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis- related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013.PubMed/NCBI
|