Introduction
Chondrosarcoma is the second most common malignant
tumor originating from bone tissue. It appears mostly in
30–60-year-old patients. With complex clinical characteristics,
pathological manifestations and various differentiation degrees,
chondrosarcoma is not sensitive to either radiotherapy or
chemotherapy and surgical resection remains the most common
treatment in the clinic (1).
Hedgehog (HH) pathway is a crucial signaling pathway
in regulating cell growth and differentiation, particularly in bone
and cartilage tissues (2,3). Indian hedgehog (Ihh) ligands first
bind to patched protein 1 (PTCH1) receptor and then release the
repression of Smoothened (Smo) receptor which initiates the
activation of downstream transcription factor GLI family (2,4). This
process leads to downstream gene expression and regulation of
multifarious physiological functions. Our previous studies showed
that an HH/parathyroid hormone-related protein (PTHrP) negative
feedback loop exists to regulate the development of different
levels of cartilage cells. Ihh facilitates PTHrP expression to
promote the growth plate cell proliferation, inhibition of cell
differentiation and maturation. Otherwise, PTHrP reduces Ihh
production conversely (2,3). More specifically, HH pathway controls
transcription factor GLI2 to control secretion of PTHrP protein
which can promote osteolytic destruction, especially in primary or
metastatic bone tumor (5,6). Recently, that abnormal activation of
HH pathway induces carcinogenesis was found in various tumor types,
such as rhabdomyosarcoma, breast cancer, gastrointestinal malignant
tumor and human renal cell carcinomas (7–11).
These studies confirmed that overexpression of GLI transcription
factors caused the tumorigenesis by affecting cell growth and
differentiation progression. Moreover, the malignant properties of
these neoplasms were determined by the differentiation and
maturation degree of tumors (7–11). In
this study, we found that human chondrosarcoma tissue expressed the
seemingly abnormal level of HH signaling-related proteins, such as
Ihh, GLI1, GLI2 and PTHrP. However, to date, there is no evidence
to illustrate how HH signaling pathways affect the growth process
of chondrosarcoma and whether targeting the HH pathway could be an
optional therapy to treat this tumor (2). Based on our previous findings, we
speculated that the HH signaling pathway may be a potential
antitumor target for drug therapy. To test this hypothesis, we
treated human chondrosarcoma SW1353 cells with the HH pathway
inhibitor, HH pathway inhibitor-4 (HPI-4). HPI-4 is a special type
of inhibitor which may affect the downstream of Smo but does not
directly act on transcription factor GLIs (13,14).
Primary cilia, as a crucial part of the HH signaling
pathway, are special external cellular organelles. Since they
contain various receptor proteins, they are always regarded as
important extracellular chemical and physical sensors in regulating
the cell growth and development process. Cilia can influence the
process of differentiation through regulating cell division cycle.
Primary cilia’s abnormal occurrence, morphology and function are
closely related to tumorigenesis (15–19).
We focused on the cilia to study the effects of HPI-4 on
chondrosarcoma cells by investigating the effects on tumor
malignant characteristics, such as proliferation, invasion and
migration ability, so as to determine whether this drug has a
negative regulation on human chondrosarcoma. In addition, the
potential mechanism of HPI-4 through working on primary cilia to
regulate Ihh-PTHrP signaling in vitro was investigated. In
conclusion, these results indicated that HPI-4 blocking HH
signaling may be a feasible chemotherapeutic option for human
chondrosarcoma treatment.
Materials and methods
Cells and reagents
The human chondrosarcoma cell line SW1353 was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s
modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12) with 10%
fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin
(100 U/ml) at 37°C with 5% CO2. HPI-4, also known as
Ciliobrevin A, whose molecular formula is
C17H9Cl2N3O2,
was purchased from Sigma-Aldrich (St. Louis, MO, USA). The study
was approved by the Ethics Committee of Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, Hubei,
China).
Cell proliferation-toxicity test
To evaluate the change of cell proliferation
ability, we used the 3-(4,5-dimethylthiazol-2-yl)-
5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS-8)
assay to measure cell proliferation and the toxicity of this drug.
The procedure was as follows: 2,000 cells were plated in 96-well
plates per well. HPI-4 was added to cells at concentrations of 0, 5
and 10 μM in 100 μl DMEM/F12 with 10% FBS and incubated for 0, 1,
3, 6 and 9 days. Then, 10 μl MST-8 (Beyotime, China) was added to
the media in each well and incubated in an environment without
light for 90 min. The absorbance value was measured using an enzyme
microplate reader at 450 nm wavelength. The relative viability of
cells was expressed by OD value.
Tablet colony formation assay
SW1353 cells were harvested by trypsin enzyme
digesting and 200 cells were seeded in a 6-well plate. After cells
attached, HPI-4 was added to cells at different concentrations of
0, 5 and 10 μM in each well. Cells were cultured in DMEM/F12 medium
with 10% FBS and incubated at 37°C with 5% CO2 and fresh
medium was changed every 3 days. Four weeks later, cells were fixed
with 4% paraformaldehyde and stained with 0.2% crystal violet.
Finally, images were captured to record the size and number of
colonies.
Transwell invasion assay
The invasion assay was conducted using Transwell
plates. The filter membrane (pore size, 8 μm) of the Transwell
plates was coated with 20 mg Matrigel (BD Biosciences, USA) at 37°C
for 30 min and the lower chamber was filled with culture medium
DMEM/F12 containing 10% FBS. SW1353 cells were starved with
serum-free DMEM/F12 for 6 h and then 1×105 cells were
transferred onto the upper surface of the chamber. HPI-4 was added
at different concentrations of 0, 5 and 10 μM. Twenty-four hours
later, the culture medium was gently removed and the upper surface
Matrigel was wiped by cotton swab. We used 4% paraformaldehyde to
fix the cells that had invaded through and adhered to the lower
surface for 15 min, then stained with 0.2% crystal violet. Ten
random fields in each plate were selected and counted using an
Olympus microscope.
Wound healing assay
Tumor migratory behavior was imitated with a scratch
assay by measuring migration distance. First, SW1353 cells were
seeded in 6-well plates at a density of 1×105/well. When
they reached nearly confluent monolayer, a single scratch was made
vertically through each well by use of a sterile pipette tip. After
washing with PBS three times, the cells were incubated with 2%
bovine serum DMEM/F12 and cells were stimulated with different
concentrations of HPI-4, and observed under a microscope (10×
objective lens) after 0, 24 and 48 h. Images were recorded at the
same position in order to assess the repair process accurately.
Finally, the percentage of cell migration distance was
calculated.
Immunohistochemical studies
We collected 10 human chondrosarcoma tissues from
surgical resection and these tissues were firstly fixed in 4%
paraformaldehyde. They were then embedded in paraffin and sectioned
for immunohistochemical assays. All experimental processes were
conducted using standard techniques (12). Ihh (1:100; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), GLI1 (1:400; Epitomics Inc.,
Burlingame, CA, USA), GLI2 (1:100; Boster, Wuhan, China) and PTHrP
(1:100; Santa Cruz Biotechnology, Inc.) were used as markers for
activation of HH signaling pathway. Ki67 staining was used to
detect proliferation cells in tissue sections at a dilution of
1:200 (Cell Signaling Technology, USA). All sections were observed
under a microscope at ×200 magnification.
Immunofluorescence assay
SW1353 cells were cultured on the cover glass and
treated with different concentrations of HPI-4. Twenty-four hours
later, they were fixed with 4% paraformaldehyde for 15 min, blocked
with 5% BSA at room temperature for 60 min and then incubated with
acetylated α-tubulin antibody (1:400; Abcam, Cambridge, UK)
overnight at 4°C. Subsequently, the SW1353 cells were incubated
with Cy3-conjugated goat anti-Mouse IgG secondary antibody (Boster)
at room temperature and nuclei were stained with 1 μg/μl DAPI. We
used PBS to wash cells three times during each step during this
process. In the end, images were visualized with fluorescent
microscope at ×200 magnification.
Quantitative real-time PCR (qRT-PCR)
HH signaling pathway-related gene (GLI-1, PTCH1,
Smo, IFT88, PTHrP) expression was measured by RT-PCR. Total RNA was
extracted from cells with TRIzol reagent after 48 h incubation with
HPI-4 in 6-well plates and then 2–5 μg of total RNA was used to
synthesize cDNA with the SuperScript II cDNA synthesis kit
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The total PCR system contained cDNA,
SYBR-Green, no RNA enzyme water and primers; the primer sequences
are listed in Table I.
 | Table IQuantitative real-time PCR primer
sequences. |
Table I
Quantitative real-time PCR primer
sequences.
| Gene | Primer sequences |
|---|
| hPTCH1 | F:
5′-GTGGTGTAGAGGCAGGCAT-3′
R: 5′-GTGCTGGTCTCTGGTTACGA-3′ |
| hGLI1 | F:
5′-TCAAAGTGGGAGGCACAAAC-3′
R: 5′-ATGGGAAGGAGGAGGACTCA-3′ |
| hIFT88 | F:
5′-GTTATGATTGGTGCGTGGAAGT-3′
R: 5′-GGGCTGAGAGATTGGTTGCAG-3′ |
| hPTHrP | F:
5′-AAGGTGGAGACGTACAAAGAC-3′
R: 5′-CAGAGCGAGTTCGCCGTTT-3′ |
| hSmo | F:
5′-GACTTCCTGCGCTGCACTC-3′
R: 5′-AGCCCTCCACGTCCTCGTAC-3′ |
| hβ-actin | F:
5′-CATGTACGTTGCTATCCAGGC-3′
R: 5′-CTCCTTAATGTCACGCACGAT3′ |
Western blot analysis
These procedures were based on previously described
ones (6). Total cell proteins (45
μg/lane) were loaded and separated by sodium dodecyl sulfate (SDS)
polyacrylamide gels, transferred onto PVDF membranes. We used the
following primary antibodies: MMP2 (1:1,000), MMP9 (1:1,000) (both
from Cell Signaling Technology), IFT88 (1:1,000; Abgent, USA),
CyclinD1 (1:100; Boster) and PTHrP (1:100; Santa Cruz
Biotechnology, Inc.); β-actin (1:400; Boster), along with secondary
antibody horseradish peroxidase-labeled goat anti-rabbit and goat
anti-mouse IgG (1:5,000; Boster). We used ECL western blotting
detection kit (Thermo Fisher Scientific, USA) to detect all the
protein bands and were visualized using an enhanced
chemiluminescence system (Bio-Rad, Hercules, CA, USA). All values
were expressed relative to β-actin.
Statistical analysis
Each experiment was performed at least three times
independently. These data are represented as mean ± SD. We used the
Student’s t-test or one-way analysis of variance (ANOVA) to analyze
the differences between means, and P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS 20.0.
Results
Human chondrosarcoma prominently
expresses Ihh-PTHrP signaling-related proteins
Previous studies demonstrated that a variety of
tumors exist with abnormal activation of the HH signaling pathway
(7–11). Using immunohistochemistry assay, we
detected the expression of the Ihh-PTHrP-related proteins in human
chondrosarcoma tissues. These tumor tissues exhibited significantly
high level of staining intensity for Ihh, GLI1, GLI2 and PTHrP
(Fig. 1B–E) compared with the
negative control (Fig. 1A). At the
same time, tumor tissues had a considerable percentage of positive
Ki67 staining in approximately half of total tumor cells
(44.30±4.52%). Obvious expression of Ki67 confirmed that
chondrosarcoma had considerable proliferation ability (Fig. 1F). On the other hand, apparent
expression of Ihh and PTHrP simultaneously indicated that there
appeared no natural negative feedback regulation between Ihh and
PTHrP proteins. These results demonstrated that the distinct
activation of HH signaling pathway may have a positively correlated
relationship with elevated proliferation ability in
chondrosarcoma.
HPI-4 decreases chondrosarcoma SW1353
cell proliferation ability
As shown in Fig. 2,
in order to examine the effect of HPI-4 treatment on human
chondrosarcoma cell growth, SW1353 cells were stimulated
respectively with 5 and 10 μM HPI-4 and the changes were explored
using MTS-8 assay. With the progress of time, SW1353 cells cultured
with HPI-4 revealed an obvious reduction in proliferation rate
compared with the empty control group (Fig. 2A). In addition, in order to detect
the effect of this drug on a single cell to form a tumor mass, we
conducted a tablet colony formation assay (Fig. 2B). After 4 weeks of incubation, the
empty control group number was 272±19.8 per well compared with 5 μM
(191.00±8.49, P<0.05) and 10 μM (90.50±16.26, P<0.05). The
average size showed the same trends at different doses (Fig. 2B). These results confirmed that
HPI-4 clearly reduced the ability of a single cancer cell to
generate a colony by continuous division, including numbers and
sizes of colonies (Fig. 3C).
Moreover, western blot assay presented a similar conclusion that
inhibiting HH signaling eventually suppressed cell cycle protein
CyclinD1 in HPI-4-treated cells along with the higher concentration
(Fig. 7B). CyclinD1 is considered a
vital symbol of cell cycle progression.
HPI-4 inhibits the invasion and migration
ability of human chondrosarcoma cells
Migration and invasion are important indicators to
evaluate the degree of tumor malignancy, they determine the
severity of the tumor and the prognosis of treatment (7,8). In
order to illustrate the influence of HPI-4 compound on tumor
migration and invasion ability, we performed the Transwell and cell
scratch experiment in vitro. Fig. 3A–D shows that the number of cells
penetrating through the Matrigel matrix decreased significantly as
the drug concentration increased (the white arrows). Matrigel
matrix is regarded as the best component to imitate extracellular
matrix. Scratch experiment was used to simulate the migration of
tumor cells. The results showed that a higher concentration of
HPI-4 observably inhibited tumor cell migration rate as time
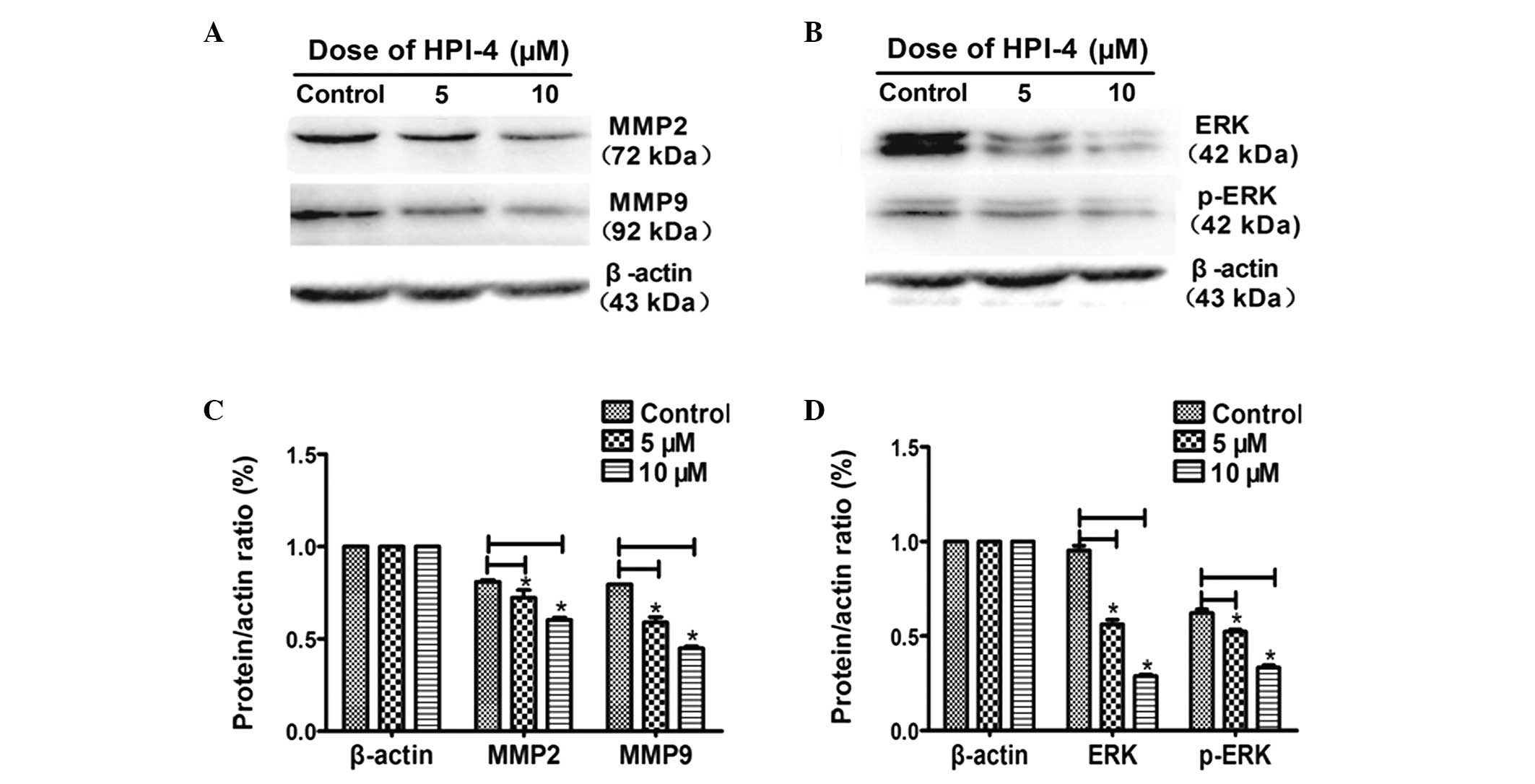
passed. HPI-4 effectively delayed scratch healing time (Fig. 4A and B). At the same time, we
conducted western blot experiment to test the expression of matrix
metalloproteinases (MMPs) which reflects tumor cell decomposition
of the extracellular matrix ability to promote invasion and
migration (22,23). The results demonstrated that after
stimulating for 3 days, the expression level of MMP2, MMP9
decreased in HPI-4-treated cells. Moreover, as MMPs upstream
signaling pathway, the MAPK/ERK pathway was also restrained by
HPI-4, mainly through inhibiting ERK and phosphorylated-ERK (p-ERK)
proteins to downregulate downstream MMPs (Fig. 6A–D).
HPI-4 blocks the HH signaling pathway by
disturbing ciliogenesis
We previously demonstrated that an apparent
activation of HH signaling pathway exists, which may be associated
with tumorigenesis in human chondrosarcoma (Fig. 1). The mechanism of HPI-4 as an
inhibitor of HH signaling pathway is not fully clear, although some
studies have demonstrated that this drug does not act on
conventional Smo receptor (14).
However, primary cilia are an indispensable part of the HH
signaling pathway. This cell organelle can be counted and described
under the field of vision and was regarded as an observed
morphology symbol of cell cycle (15,16).
In order to detect whether HPI-4 realizes its function through
interacting with primary cilia, we used fluorescence microscope to
observe the expression percentage of primary cilia. Normal
chondrosarcoma SW1353 cells present primary cilia 20.55±4.40%,
while adding 5 μM HPI-4 resulted in a decrease to 15.06±5.15%
(P<0.05) and 10 μM to 7.38±1.91% (P<0.001). The results
confirmed that HPI-4 distinctly downregulates ciliogenesis,
particularly at high concentrations (Fig. 5A–D). Moreover, RT-PCR analysis
showed that the expression of cilia microtubule transporters IFT88
significantly decreased (P<0.05) and the HH pathway target genes
PTCH1 and GLI1 decreased distinctly (P<0.05), but only the
higher concentration of HPI-4 suppressed Smo receptor expression.
Thus, we speculated that HPI-4 may inhibit the action of the HH
pathway by disrupting ciliogenesis rather than by suppressing Smo
(Fig. 7E and F).
HPI-4 inhibits the expression of PTHrP
regulated by reducing GLI2
HH and PTHrP play an important role in regulating
the growth process of bone and cartilage. In normal cartilage
tissue, an Ihh/PTHrP negative feedback loop exists. HH signaling
pathways activate downstream GLI transcription factors to regulate
the generation of PTHrP (2–6). We previously showed that GLI2 and
PTHrP have a certain collaborative expression in the human
chondrosarcoma tissues with apparent activation of the HH signaling
pathway (Fig. 1E). As shown in
Fig. 7, after stimulating
chondrosarcoma cells with different concentrations of HPI-4,
ciliogenesis-related microtubules transporter IFT88 was inhibited,
which led to primary cilia dependency. HH pathway transduction was
blocked and downregulated transcription factor protein GLI2. Thus,
chained along with suppression of PTHrP protein and mRNA expression
simultaneously (Fig. 7A–D).
Discussion
As a type of malignant tumor secreting
cartilage-like matrix, human chondrosarcoma has different general
properties from other bone tumors. Depending on the tumor
differentiation degree, the malignant severity is also different.
Chondrosarcoma always occurs with a proclivity for long and flat
bones, the main parts of weight bearing structures. This tumor can
invade surrounding tissues and the consequences caused by
mechanical structure damage cannot be ignored. Due to secreting
cartilage-like matrix and lacking vascular distribution,
chondrosarcoma is not sensitive to radiotherapy or chemotherapy and
has high risks of recurrence and metastasis after surgical
resection (1). Therefore,
controlling the invasion and metastasis is key in guiding the
treatment and improving prognosis of chondrosarcoma.
The HH signaling pathway is an important pathway in
the progression of bone and cartilage development. Previous studies
have confirmed the existence of HH/PTHrP negative feedback loop in
normal growth plates to regulate cell growth and differentiation
(1–3), but it is absent in cartilage neoplasia
(2,12). Furthermore, abnormal activation of
HH signaling was found in different sources of malignant tumors,
such as breast cancer, human renal cell carcinomas and
gastrointestinal malignant tumor (7–11).
Nevertheless, the specific pathogenesis for the abnormal activation
of HH pathway that induces oncogenesis remains unclear. In the
present study, we detected that apparent expression of protein Ihh,
GLI1, GLI2 and PTHrP existed in human chondrosarcoma and this level
of HH signaling activation was accompanied by strong proliferation
ability. By using HH signaling pathway inhibitor HPI-4 to stimulate
chondrosarcoma cell line SW1353 in vitro, we illustrated
that suppression of HH signaling could decrease tumor cell
proliferation activation as time passed. Furthermore, MAPK/ERK
signaling mediated tumor invasion-related proteins matrix
metalloproteinases MMP2 and MMP9 (22,23)
expression declined visibly by use of HH signaling inhibitor HPI-4.
This phenomenon indicates that there may exist a downstream
crosstalk between HH signaling and MAPK/ERK signaling pathways. HH
signaling inhibitor HPI-4 could have an obvious inhibitory effect
on tumor invasion ability by regulating the expression of MMPs
eventually. Therefore, we concluded that HH signaling pathway
activation is closely linked with tumor cell proliferation activity
and enhancing the decomposition of the extracellular matrix,
thereby affecting tumor invasion and metastasis.
In the HH pathway inhibitor family, HPI-4 has a
different interaction mechanism from other HH signaling pathway
inhibitors. For example, the classical inhibitor cyclopamine is
able to block Smo receptor and GANT51/GANT61 directly inhibits the
expression of GLI family to interrupt HH pathway (13,14),
while HPI-4 may affect ciliogenesis to block this signaling
pathway. Primary cilia are believed to regulate cell cycle
progression and are considered a sign of ending cell division
(15–18). In normal cells, primary cilia only
present on non-proliferating cells and play an important role in
initiating cilia-dependent HH pathway (4,15–19).
Cilia related IFT88 transfer in primary cilia and reflect the
activity of material transportation in cilia (16). Theoretically, cilia microtubules
disassembling during cell division progression and proliferation
cells should lack primary cilia. However, after stimulating with
HPI-4, the number of extracellular cilia decreased and intra-cilia
transport protein IFT88 expressed in significant decline on gene
and protein level accompanied by multiplication ability
suppression. This illustrated that the drug might disrupt the
normal function of cilia and inhibit cell proliferation
simultaneously, accompanied by restraining the expression of HH
downstream target genes GLI1 and PTCH1. Hence, we can infer that
HPI-4 can downregulate HH activation by disrupting ciliogenesis and
affecting their function. Primary cilia may be a crucial cell
organelle involved in tumor initiation and progression.
Regarded as a vital factor in the regulation of
osteogenesis and osteolysis, PTHrP is closely related to HH
signaling pathway (5,6,20,21).
Previous studies revealed that PTHrP expression could be increased
by TGF-β stimulation, which could upregulate HH downstream
transcription molecule GLI2 expression. GLI2 could be activated and
transferred into nucleus to launch a variety of cytokines
transcription, especially PTHrP (6). By inhibiting the activity of the HH
signaling, we found its downstream GLI2 expression decreased and
endogenous PTHrP was restrained. As a result, we infer that the HH
pathway inhibitor could affect to block osteolysis caused by PTHrP
overexpression in tumor.
In summary, this study revealed that abnormal HH
signaling pathway existed in human chondrosarcoma tissues. HPI-4, a
new special antitumor compound, can perturb tumor cell ciliogenesis
to block HH signaling pathway, consequently suppressing
chondrosarcoma cell proliferation, invasion and migration
abilities. These conclusions indicate that HPI-4 may be an
effective therapy option to treat chondrosarcoma with abnormal
activation of the HH signaling pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant no. 81202121) and the Hubei
Provincial Health Department of Young Scientists Fund (Grant no.
QJX2012-05).
Abbreviations:
|
HPI-4
|
Hedgehog pathway inhibitor-4
|
|
Ihh
|
Indian Hedgehog
|
|
PTHrP
|
parathyroid hormone-related
protein
|
|
PTH
|
parathyroid hormone
|
|
IFT88
|
intraflagellar transport protein
88
|
|
PTCH1
|
patched protein 1
|
|
Smo
|
Smoothened
|
References
|
1
|
Björnsson J, McLeod RA, Unni KK, et al:
Primary chondrosarcoma of long bones and limb girdles. Cancer.
83:2105–2119. 1998.PubMed/NCBI
|
|
2
|
Tiet TD, Hopyan S, Nadesan P, et al:
Constitutive hedgehog signaling in chondrosarcoma up-regulates
tumor cell proliferation. Am J Pathol. 168:321–330. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu K, Guo F, Zhang S, et al: Blocking Ihh
signaling pathway inhibits the proliferation and promotes the
apoptosis of PSCs. J Huazhong Univ Sci Technolog Med Sci. 29:39–44.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hassounah NB, Bunch TA and McDermott KM:
Molecular pathways: the role of primary cilia in cancer progression
and therapeutics with a focus on Hedgehog signaling. Clin Cancer
Res. 18:2429–2435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson RW, Merkel AR, Danilin S, et al:
6-Thioguanine inhibition of parathyroid hormone-related protein
expression is mediated by GLI2. Anticancer Res. 31:2705–2712.
2011.PubMed/NCBI
|
|
6
|
Johnson RW, Nguyen MP, Padalecki SS, et
al: TGF-β promotion of Gli2-induced expression of parathyroid
hormone-related protein, an important osteolytic factor in bone
metastasis, is independent of canonical Hedgehog signaling. Cancer
Res. 71:822–831. 2011.
|
|
7
|
Oue T, Uehara S, Yamanaka H, et al:
Hedgehog signal inhibitors suppress the invasion of human
rhabdomyosarcoma cells. Pediatr Surg Int. 29:1153–1158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Che J, Zhang FZ, Zhao CQ, et al:
Cyclopamine is a novel Hedgehog signaling inhibitor with
significant anti-proliferative, anti-invasive and anti-estrogenic
potency in human breast cancer cells. Oncol Lett. 5:1417–1421.
2013.PubMed/NCBI
|
|
9
|
Zhuang Z, Wang K, Cheng X, et al: LKB1
inhibits breast cancer partially through repressing the Hedgehog
signaling pathway. PLoS One. 8:e674312013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basten SG, Willekers S, Vermaat JS, et al:
Reduced cilia frequencies in human renal cell carcinomas versus
neighboring parenchymal tissue. Cilia. 2:22013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan R, Peng X, Yuan X, et al: Suppression
of growth and migration by blocking the Hedgehog signaling pathway
in gastric cancer cells. Cell Oncol (Dordr). 36:421–435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho L, Ali SA, Al-Jazrawe M, et al: Primary
cilia attenuate hedgehog signalling in neoplastic chondrocytes.
Oncogene. 32:5388–5396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lauth M, Bergström A, Shimokawa T, et al:
Inhibition of GLI-mediated transcription and tumor cell growth by
small-molecule antagonists. Proc Natl Acad Sci USA. 104:8455–8460.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu VM, Chen SC, Arkin MR, et al: Small
molecule inhibitors of Smoothened ciliary localization and
ciliogenesis. Proc Natl Acad Sci USA. 109:13644–13649. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goto H, Inoko A and Inagaki M: Cell cycle
progression by the repression of primary cilia formation in
proliferating cells. Cell Mol Life Sci. 70:3893–3905. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Irigoín F and Badano JL: Keeping the
balance between proliferation and differentiation: the primary
cilium. Curr Genomics. 12:285–297. 2011.PubMed/NCBI
|
|
17
|
Proulx-Bonneau S and Annabi B: The primary
cilium as a biomarker in the hypoxic adaptation of bone
marrow-derived mesenchymal stromal cells: a role for the secreted
frizzled-related proteins. Biomark Insights. 6:107–118.
2011.PubMed/NCBI
|
|
18
|
McGlashan SR, Haycraft CJ, Jensen CG, et
al: Articular cartilage and growth plate defects are associated
with chondrocyte cytoskeletal abnormalities in Tg737orpk
mice lacking the primary cilia protein polaris. Matrix Biol.
26:234–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rich DR and Clark AL: Chondrocyte primary
cilia shorten in response to osmotic challenge and are sites for
endocytosis. Osteoarthritis Cartilage. 20:923–930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das S, Tucker JA, Khullar S, et al:
Hedgehog signaling in tumor cells facilitates osteoblast-enhanced
osteolytic metastases. PLoS One. 7:e343742012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mak IW, Turcotte RE and Ghert M:
Parathyroid hormone-related protein (PTHrP) modulates adhesion,
migration and invasion in bone tumor cells. Bone. 55:198–207. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartsch JE, Staren ED and Appert HE:
Matrix metalloproteinase expression in breast cancer. J Surg Res.
110:383–392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung TW, Lee YC and Kim CH: Hepatitis B
viral HBx induces matrix metalloproteinase-9 gene expression
through activation of ERK and PI-3K/AKT pathways: involvement of
invasive potential. FASEB J. 18:1123–1125. 2004.PubMed/NCBI
|