Introduction
Nasopharyngeal carcinoma (NPC) is a common tumor in
the head and neck (1,2). NPC is one of the most common
malignancies in Southern China and Southeast Asia with an incidence
rate of 20–30 cases per 100,000 individuals (3,4), and
is believed to be associated with EB virus infection (4,5).
However, its pathogenesis remains unclear. It is known that tumor
development is a multistep process of cumulative genetic
alterations that lead to cell autonomy. During this process,
inflammatory mechanisms are thought to play a critical role
(6,7).
Complement activation is believed to play a critical
role in inflammatory responses in vivo (8). The recent finding that complement may
contribute to tumor growth suggests an insidious relationship
between complement and cancer, particularly in light of evidence
that complement facilitates cellular proliferation and regeneration
(9,10). During carcinogenesis, tumor cells
acquire genetic and epigenetic alterations that dictate their
malignant growth. Because of these alterations, the complement
system can recognize tumor cells, as can be shown by the complement
deposition found in different tumors. In fact, new findings on the
roles of complement in tumor growth have challenged the recognition
that complement always protects against tumors. Some studies have
demonstrated that the generation of complement especial
anaphylatoxin C5a in the tumor microenvironment leads to
significant tumor progression, such as lung cancer (9–11).
Current research has revealed that the signal
transducer and activator of transcription 3 (STAT3) plays a pivotal
role in NPC development. Activation of STAT3 may contribute to both
the development and progression of NPC (12–14).
Thus, targeting aberrant STAT3 signaling may provide an effective
and novel strategy for the treatment of NPC (15). Despite the fact that STAT3
activation is common in NPC, the mechanism of STAT3 activation in
NPC has not been fully elucidated.
It is well known that protein function is often
regulated by post-translational modifications such as acetylation
(16,17), and mounting evidence suggests that
protein acetylation plays important roles in various biological
events including transcriptional regulation, DNA damage repair,
cell proliferation and autophagy (18–22).
Many studies have revealed that signaling molecule STAT3 undergoes
phosphorylation, which is necessary for STAT3 activation in various
diseases (23). However, the STAT3
acetylation and its roles in regulating STAT3 activation in NPC
remain largely unclear.
Lysine (K) acetyltransferase 2B, also known as
P300/CBP-associated factor (PCAF), is a trancriptional
co-activator, which has been shown to interact with Myc, β-catenin,
Homeobox A10 (HOXA10) and histone (24–28).
PCAF has acetyl transferase activity with various transcription
factors and signaling molecules (28–31),
indicating that PCAF may play direct roles in regulating the
activity of transcription factors and signaling molecules. PCAF has
also been reported to be related to cancer (32). However, the roles of PCAF in
mediating STAT3 activation and the proliferation of NPC need to be
explored.
In the present study, we evaluated the implication
of C5a in the proliferation of human NPC cells in vitro.
PCAF-mediated STAT3 acetylation and its role in regulating the
proliferation of NPC cells were subsequently explored. Our results
revealed that C5a stimulated the proliferation of human NPC cells
in vitro. The level of STAT3 acetylation was elevated in
human NPC cells induced by C5a. Moreover, PCAF induction was
required for STAT3 acetylation in human NPC cells by exposure to
C5a. Functionally, PCAF-mediated STAT3 acetylation contributed to
the proliferation of human NPC cells stimulated by C5a. Taken
together, our findings suggest that C5a promotes the proliferation
of NPC cells through PCAF-mediated STAT3 acetylation.
Materials and methods
Reagents
Monoclonal antibodies against human PCAF were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Monoclonal antibodies against human β-actin, STAT3, phospho-STAT3
(Tyr705), phospho-STAT3 (Ser727) and acetylated-lysine were from
Cell Signaling Technology (Danvers, MA, USA). For the western blot
analysis, horseradish peroxidase-conjugated anti-mouse IgG
antibody, 20× LumiGLO reagent and 20× peroxide were purchased from
Cell Signaling Technology. PVDF membranes were from Millipore
(Billerica, MA, USA). TRIzol reagent was purchased from Invitrogen
(Carlsbad, CA, USA). MMLV was provided by Promega (Madison, WI,
USA). A RevertAid™ First Strand cDNA Synthesis kit was from
Fermentas (Pittsburgh, PA, USA). TaqMan® Fast Advanced
Master Mix was from ABI (Foster, CA, USA). The pGCsi.U6.neo.GFP
plasmid was obtained from Shanghai Genkan Biotechnology Co., Ltd.
(Shanghai, China). The pcDNA3.1 vector was from Invitrogen. The
incision enzymes HindIII and BamHI as well as T4 DNA
ligase were purchased from Takara (Tokyo, Japan). A QIAprep Spin
Miniprep kit was obtained from Qiagen (Hilden, Germany). Human
complement 5a (C5a) was obtained from R&D Systems (Minneapolis,
MN, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Laboratories (Kumamoto, Japan).
Cell culture
The human NPC cell lines, CNE1 and C666-1, and the
control human nasopharyngeal epithelial cell line, NP69, were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). Cell lines was cultured in RPMI-1640 medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum
(FBS; Gibco) and antibiotics (50 U/ml penicillin and 100 μg/ml
streptomycin; Invitrogen, Carlsbad, CA, USA) at 37°C in 5%
CO2.
Generation of the overexpression
plasmids
The pcDNA3.1/PCAF-His plasmids were constructed by
inserting the ORF of human PCAF (NM_003884.4) cDNA into the
mammalian expression plasmids of pcDNA3.1. The PCAF gene containing
a His tag was amplified by polymerase chain reaction (PCR) from
cDNA of normal human nasopharyngeal epithelial cells. The PCR
products and pcDNA3.1 vector were further digested with the two
restriction enzymes, HindIII and BamHI, and then
ligated by using T4 DNA ligase. The recombinant plasmids were
amplified in E.coli strain DH5α and purified with a
QIAprep Spin Miniprep kit. Finally, the constructed plasmids were
sequenced across both junctions to confirm the nucleotide sequence
and the predicted orientation.
Construction of the shRNA expression
plasmids
To silence the PCAF gene in human NPC cells, there
different shRNA sequences against human PCAF mRNA (NM_003884.4)
were designed. The different DNA segments of PCAF shRNA were
constructed into the pGCsi.U6.neo.GFP plasmids, and the most
effective shRNA expression plasmid was chosen for further
functional experiments.
Cellular transfection
NPC cell lines were transfected using Lipofectamine
2000 according to the manufacturer’s instructions. Briefly, 4 μg of
the plasmids was mixed with 250 μl of serum-free medium, and then
10 μl of Lipofectamine 2000 mixed with 250 μl of serum-free medium
was added and incubated for 20 min at room temperature. Finally,
the resultant mixture was added to the cells in each well. The
medium was replaced with serum-containing medium 5 h after
transfection (33,34).
Co-immunoprecipitation experiment
Three hundred micrograms of extract, prepared from
the NPC cell lines (CNE1 and C666-1) and the normal nasopharyngeal
epithelial cell line (NP69) was mixed with 40 μl protein
G-Sepharose beads in co-IP assay buffer, incubated for 2 h and
centrifuged for 2 min. The recovered supernatant was then incubated
with the corresponding antibody (2 μg, preimmune IgG as a control
reaction) at 4°C overnight. Then, 40 μl of protein G-Sepharose
beads was added, and incubation was continued for 2.5 h. Protein
G-precipitated protein complex was recovered by centrifugation and
harvested beads resuspended in 30 μl of 2× SDS-PAGE sample buffer
were boiled for 5 min. The samples were then analyzed by western
blot analysis with specific antibodies. A 40-μg aliquot of
whole-cell extract (WCE) was used as an input control (35).
RNA isolation and real-time quantitative
PCR
The total RNA was isolated from the cells using
TRIzol reagent. An equal amount (4 μg) of total RNA was synthesized
as a first-strand cDNA using the RevertAid™ First Strand cDNA
Synthesis kit. The cDNA was amplified using TaqMan® Fast
Advanced Master Mix to detect the expression of the PCAF gene. The
reaction program consisted of an initial denaturation step at 95°C
for 10 min, denaturation at 95°C for 15 sec and annealing at 60°C
for 60 sec for 40 cycles. The 7500 Real-time PCR system (ABI) was
used for the experiment. Each sample was assayed in triplicate. The
β-actin gene was used as an internal control. The relative level of
gene expression was obtained by calculating the ratio of cycle
numbers of the initial exponential amplification phase as
determined by the sequence detection system for the specific target
gene and β-actin using the following formula:
2−ΔΔCt.
Western blot analysis
The proteins (40 μg) were subjected to 10–15% SDS
polyacrylamide gel electrophoresis and transferred onto PVDF
membranes by the Mini-Protean System (Bio-Rad, Hercules, CA, USA).
The membranes were incubated for 1 h at room temperature (RT) in
blocking buffer (5% skimmed milk in TBS-T) and then incubated with
the appropriate antibodies overnight at 4°C. After washing with
TBST-T, the membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit and anti-mouse for 1 h at 37°C.
The bands were visualized by the ECL detection system with 3–10 min
exposure after washing the membranes. The radiographic band density
was measured using Quantity One® software (Bio-Rad).
Control for protein loading was identified by β-actin as the
internal standard. Each sample was assayed in triplicate.
CCK-8 assay
The NPC cell lines and the normal human
nasopharyngeal epithelial cell line after the different treatments
were incubated with CCK-8 for a final 4 h. The formazan product was
visualized at an absorbance of 450 nm, and the absorbance was
directly proportional to the cell number (35,36).
Each sample was assayed in triplicate.
Statistical analysis
All statistical analyses were carried out using
SPSS® 11.5 software. All data are expressed as mean ±
SD. The statistical significance (defined as P<0.05) of the
groups was evaluated by one-way ANOVA with simultaneous multiple
comparisons between groups by the Bonferroni method.
Results
C5a promotes the proliferation of human
NPC cells
Purified human C5a was added to the human NPC cell
lines (CNE1 and C666-1) to observe its ability to induce the
proliferation of NPC cells. The in vitro studies showed that
C5a enhanced the proliferation of both human NPC cell lines in a
dose-dependant manner, but had no marked effect on the
proliferation of the normal human nasopharyngeal epithelial cell
line (Fig. 1A). In order to confirm
whether this was due to the effect of C5a, anti-C5aR neutralizing
antibodies were added to the media of NPC cells 30 min before
adding C5a, and the proliferation of NCP cells induced by C5a was
subsequently evaluated. The data showed that blockade of the C5a
receptor completely reduced the C5a-triggered NPC cell
proliferation (Fig. 1B–D). These
findings indicate that anaphylatoxin C5a promoted the proliferation
of human NPC cell lines but not that of the normal nasopharyngeal
epithelial cell line.
STAT3 acetylation is enhanced in human
NPC cells induced by C5a
Since C5a was found to stimulate human NPC cell
proliferation (Fig. 1A–C), and
STAT3 activation is believed to be involved in human NPC cell
proliferation (12,13), the effect of C5a stimulation on
STAT3 acetylation was subsequently determined in NPC cells exposed
to C5a. We found that the level of STAT3 acetylation was
significantly enhanced in both human NPC cell lines induced by C5a
in vitro, while the expression of STAT3 was not altered
(Fig. 2). On the other hand, the
results showed that the level of STAT3 phosphorylation was also
increased in the human NPC cells following exposure to C5a
(Fig. 4A).
PCAF expression and its association with
STAT3 are increased in human NPC cells exposed to C5a
The expression of PCAF at both the mRNA and protein
levels was detected in human NPC cells stimulated by C5a, and we
found that C5a markedly enhanced the expression levels of PCAF mRNA
and protein in both human NPC cell lines (Fig. 3A and B). The interaction of PCAF
with STAT3 at the protein level was also found to be enhanced in
both human NPC cell lines in response to C5a stimulation relative
to the normal human nasopharyngeal epithelial cell line (Fig. 3B). These findings indicate that C5a
stimulation induced the expression of PCAF and further enhanced the
interaction of PCAF with STAT3 at the protein level in human NPC
cells, suggesting a potential role of PCAF in acetylating
STAT3.
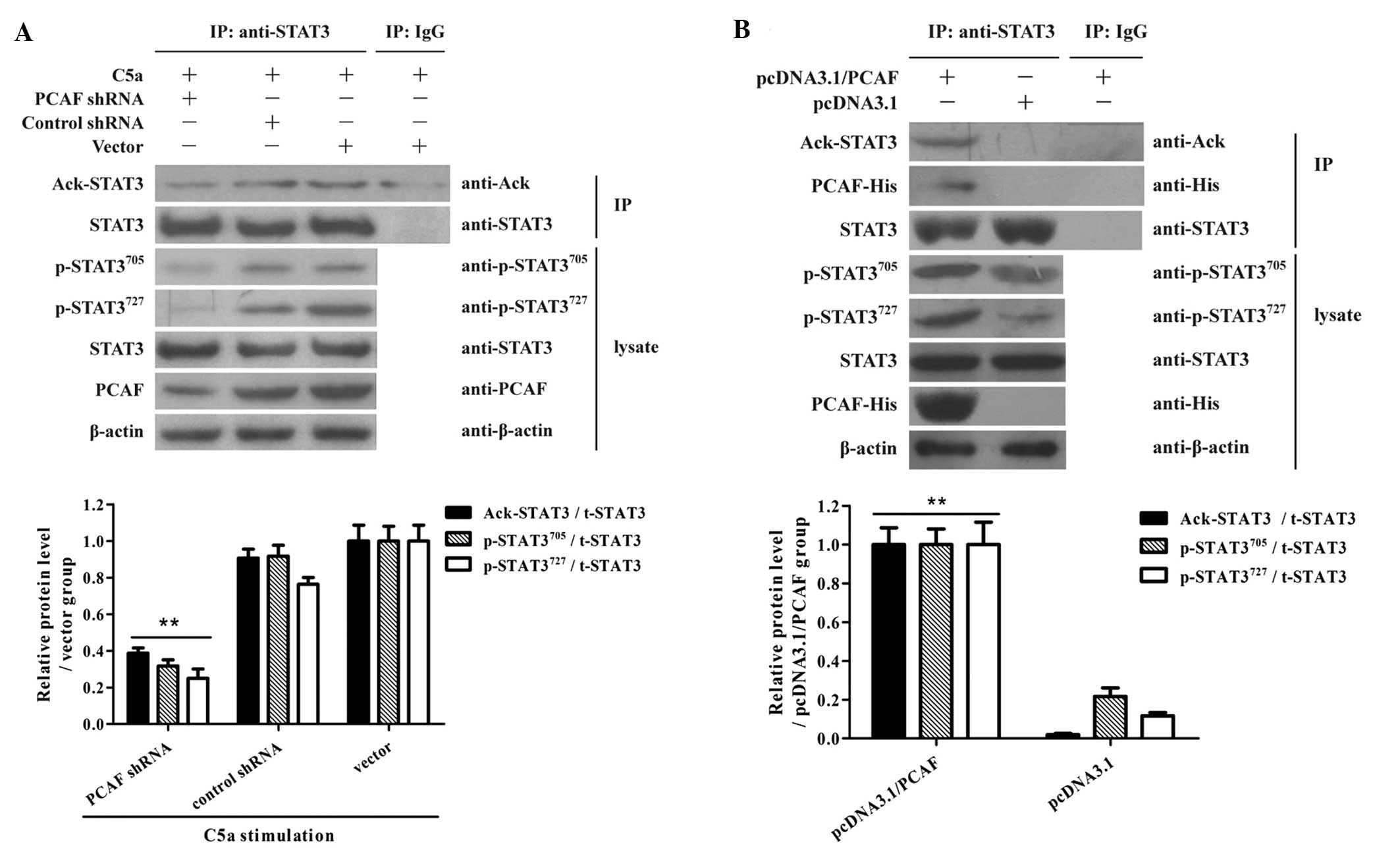
PCAF induction is required for STAT3
acetylation in NPC cells by exposure to C5a
In order to test the requirement of PCAF expression
in STAT3 acetylation and phosphorylation, CNE1 cells were treated
with PCAF shRNA for 48 h followed by C5a stimulation.
Co-immunoprecipitation assay showed that shRNA depletion of PCAF
suppressed STAT3 acetylation and phosphorylation in the NPC cells
following exposure to C5a (Fig.
4A). Furthermore, overexpression of PCAF elevated STAT3
acetylation and phosphorylation in NPC cells (Fig. 4B). Taken together, these data
suggest that acetyltransferase activity of PCAF is required for
Akt1 acetylation and phosphorylation in NPC cells.
PCAF-mediated STAT3 acetylation
contributes to the proliferation of NPC cells stimulated by
C5a
Since PCAF induction was found to be required for
STAT3 acetylation in NPC cells by exposure to C5a (Fig. 4), we further explored the role of
PCAF-mediated STAT3 acetylation in the proliferation of NPC cells
stimulated by C5a. The results showed that suppression of PCAF or
STAT3 could cause significant inhibition with regards to cellular
proliferation in NPC cells induced by C5a (Fig. 5A). Meanwhile, NCP cells
overexpressing PCAF exhibited enhanced cellular proliferation
(Fig. 5B). Taken together, these
data indicate that PCAF-mediated STAT3 acetylation contributes to
the proliferation of NPC cells stimulated by C5a.
Discussion
Nasopharyngeal carcinoma (NPC) is a common tumor in
the head and neck, and is one of the most common malignancies in
Southern China and Southeast Asia (1–4).
However, the molecular pathogenesis of NPC remains largely unclear.
Recently, inflammatory responses have been thought to play a
pivotal role in the process of cancer development (6,7).
Complement activation particularly C5a generation is believed to
play a critical role in regulating inflammatory responses in
vivo (8,37,38),
and contribute to cancer progression (9–11). Our
present studies showed that C5a in vitro enhanced the
proliferation of human NPC cells, but had no significant influence
on the proliferation of normal human nasopharyngeal epithelial
cells. Anti-C5aR neutralizing antibodies were added to the culture
media of NPC cells 30 min before adding C5a, and the proliferation
assay showed that blockade of the C5a receptor completely reduced
the C5a-triggered NPC cell proliferation. These findings indicate
that overproduction of C5a may contribute to the proliferation of
human NPC cells in vivo.
Signal transducer and activator of transcription 3
(STAT3) was found to be activated in the majority of NPC patients
and is clinically correlated with advanced disease (stages III and
IV). Therefore, activation of STAT3 may contribute to both the
development and progression of NPC (14,39,40).
Thus, targeting aberrant STAT3 signaling may provide an effective
and novel strategy for the treatment of NPC (13,15).
Despite the fact that STAT3 activation is common in NPC, the
mechanisms of STAT3 activation in NPC have not been fully
elucidated. Given the importance of STAT3 activation and
C5a-triggered proliferation in NPC pathogenesis (Fig. 1), we examined the effect of C5a
stimulation on STAT3 acetylation in NPC cells. Our data showed that
the level of STAT3 acetylation was significantly elevated in human
NPC cells induced by C5a.
Further experiments were designed to explore the
mechanism of STAT3 acetylation. It is well accepted that PCAF has
acetyl transferase activity and plays direct roles in regulating
the activity of transcription factors and signaling molecules
(24–28). However, the roles of
P300/CBP-associated factor (PCAF) in mediating STAT3 activation as
well as the proliferation of NPC are largely unclear. Our research
revealed that PCAF silencing suppressed STAT3 acetylation and
phosphorylation in NPC cells in response to C5a. In contrast,
overexpression of PCAF elevated STAT3 acetylation and
phosphorylation in NPC cells. Subsequent experiments showed that
suppression of PCAF or STAT3 inhibited the proliferation of NPC
cells induced by C5a, while overexpression of PCAF in NCP cells
enhanced cellular proliferation.
In summary, in the present study, we evaluated the
implication of C5a in the proliferation of human NPC cells in
vitro. C5a-induced PCAF expression and PCAF-mediated STAT3
acetylation as well as their roles in regulating the proliferation
of NPC cells were explored. Collectively, our findings suggest that
C5a promotes the proliferation of NPC cells through PCAF-mediated
STAT3 acetylation. These may provide a potential strategy for
treating human NPC through inhibition of C5a or its receptors.
References
|
1
|
Yan M, Zhang Y, He B, et al: IKKα
restoration via EZH2 suppression induces nasopharyngeal carcinoma
differentiation. Nature Commun. 5:36612014.
|
|
2
|
Du XJ, Tang LL, Mao YP, et al: The
pretreatment albumin to globulin ratio has predictive value for
long-term mortality in nasopharyngeal carcinoma. PloS One.
9:e944732014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wee J, Ha TC, Loong S and Qian CN: High
incidence of nasopharyngeal cancer: similarity for 60% of
mitochondrial DNA signatures between the Bidayuhs of Borneo and the
Bai-yue of Southern China. Chin J Cancer. 31:455–456.
2012.PubMed/NCBI
|
|
4
|
Han BL, Xu XY, Zhang CZ, et al: Systematic
review on Epstein-Barr virus (EBV) DNA in diagnosis of
nasopharyngeal carcinoma in Asian populations. Asian Pac J Cancer
Prev. 13:2577–2581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng X, Ching CB and Chen WN: EBV
up-regulates cytochrome c through VDAC1 regulations and
decreases the release of cytoplasmic Ca2+ in the NPC
cell line. Cell Biol Int. 36:733–738. 2012.PubMed/NCBI
|
|
6
|
Mishra A, Sullivan L and Caligiuri MA:
Molecular pathways: interleukin-15 signaling in health and in
cancer. Clin Cancer Res. 20:2044–2050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh SJ, Kim JM, Kim IK, Ko SH and Kim JS:
Anti-inflammatory mechanism of metformin and its effects in
intestinal inflammation and colitis-associated colon cancer. J
Gastroenterol Hepatol. 29:502–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohmi Y, Ohkawa Y, Tajima O, Sugiura Y and
Furukawa K and Furukawa K: Ganglioside deficiency causes
inflammation and neurodegeneration via the activation of complement
system in the spinal cord. J Neuroinflammation. 11:612014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nitta H, Wada Y, Kawano Y, et al:
Enhancement of human cancer cell motility and invasiveness by
anaphylatoxin C5a via aberrantly expressed C5a receptor (CD88).
Clin Cancer Res. 19:2004–2013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nunez-Cruz S, Gimotty PA, Guerra MW, et
al: Genetic and pharmacologic inhibition of complement impairs
endothelial cell function and ablates ovarian cancer
neovascularization. Neoplasia. 14:994–1004. 2012.PubMed/NCBI
|
|
11
|
Corrales L, Ajona D, Rafail S, et al:
Anaphylatoxin C5a creates a favorable microenvironment for lung
cancer progression. J Immunol. 189:4674–4683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Shi Y, Yuan Q, et al: Epstein-Barr
Virus encoded LMP1 regulates cyclin D1 promoter activity by nuclear
EGFR and STAT3 in CNE1 cells. J Exp Clin Cancer Res. 32:902013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao Q, Zeng Z, Guo X, et al: LPLUNC1
suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation
via inhibiting the Stat3 activation. Oncogene. 33:2098–2109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsang CM, Cheung YC, Lui VW, et al:
Berberine suppresses tumorigenicity and growth of nasopharyngeal
carcinoma cells by inhibiting STAT3 activation induced by tumor
associated fibroblasts. BMC Cancer. 13:6192013. View Article : Google Scholar
|
|
15
|
Pan Y, Zhou F, Zhang R and Claret FX:
Stat3 inhibitor Stattic exhibits potent antitumor activity and
induces chemo- and radio-sensitivity in nasopharyngeal carcinoma.
PloS One. 8:e545652013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beltrao P, Albanese V, Kenner LR, et al:
Systematic functional prioritization of protein posttranslational
modifications. Cell. 150:413–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linares JF, Duran A, Yajima T, Pasparakis
M, Moscat J and Diaz-Meco MT: K63 polyubiquitination and activation
of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol
Cell. 51:283–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pejanovic N, Hochrainer K, Liu T, Aerne
BL, Soares MP and Anrather J: Regulation of nuclear factor κB
(NF-κB) transcriptional activity via p65 acetylation by the
chaperonin containing TCP1 (CCT). PloS One. 7:e420202012.
|
|
19
|
Li T, Diner BA, Chen J and Cristea IM:
Acetylation modulates cellular distribution and DNA sensing ability
of interferon-inducible protein IFI16. Proc Natl Acad Sci USA.
109:10558–10563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie J, Peng M, Guillemette S, et al:
FANCJ/BACH1 acetylation at lysine 1249 regulates the DNA damage
response. PLoS Genet. 8:e10027862012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eckner R: p53-dependent growth arrest and
induction of p21: a critical role for PCAF-mediated histone
acetylation. Cell Cycle. 11:2591–2592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Webster BR, Scott I, Han K, et al:
Restricted mitochondrial protein acetylation initiates
mitochondrial autophagy. J Cell Sci. 126:4843–4849. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mandal T, Bhowmik A, Chatterjee A,
Chatterjee U, Chatterjee S and Ghosh MK: Reduced phosphorylation of
Stat3 at Ser-727 mediated by casein kinase 2 - protein phosphatase
2A enhances Stat3 Tyr-705 induced tumorigenic potential of glioma
cells. Cell Signal. 26:1725–1734. 2014. View Article : Google Scholar
|
|
24
|
Zhao J, Gong AY, Zhou R, Liu J, Eischeid
AN and Chen XM: Downregulation of PCAF by miR-181a/b provides
feedback regulation to TNF-α-induced transcription of
proinflammatory genes in liver epithelial cells. J Immunol.
188:1266–1274. 2012.PubMed/NCBI
|
|
25
|
Love IM, Sekaric P, Shi D, Grossman SR and
Androphy EJ: The histone acetyltransferase PCAF regulates p21
transcription through stress-induced acetylation of histone H3.
Cell Cycle. 11:2458–2466. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge X, Jin Q, Zhang F, Yan T and Zhai Q:
PCAF acetylates {beta}-catenin and improves its stability. Mol Biol
Cell. 20:419–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patel JH, Du Y, Ard PG, et al: The c-MYC
oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and
TIP60. Mol Cell Biol. 24:10826–10834. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu LH, Sun LH, Hu YL, et al: PCAF impairs
endometrial receptivity and embryo implantation by down-regulating
β3-integrin expression via HOXA10 acetylation. J Clin Endocrinol
Metab. 98:4417–4428. 2013.PubMed/NCBI
|
|
29
|
Wang LX, Wang J, Qu TT, Zhang Y and Shen
YF: Reversible acetylation of Lin28 mediated by PCAF and SIRT1.
Biochim Biophys Acta. 1843:1188–1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schlottmann S, Erkizan HV,
Barber-Rotenberg JS, et al: Acetylation increases EWS-FLI1 DNA
binding and transcriptional activity. Front Oncol. 2:1072012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang CY, Yang SF, Wang Z, et al: PCAF
acetylates Runx2 and promotes osteoblast differentiation. J Bone
Miner Metab. 31:381–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rajendran R, Garva R, Ashour H, et al:
Acetylation mediated by the p300/CBP-associated factor determines
cellular energy metabolic pathways in cancer. Int J Oncol.
42:1961–1972. 2013.PubMed/NCBI
|
|
33
|
Zhou HB, Yin YF, Hu Y, et al: Suppression
of vascular endothelial growth factor via siRNA interference
modulates the biological behavior of human nasopharyngeal carcinoma
cells. Jpn J Radiol. 29:615–622. 2011. View Article : Google Scholar
|
|
34
|
Kedjarune-Leggat U, Supaprutsakul C and
Chotigeat W: Ultrasound treatment increases transfection efficiency
of low molecular weight chitosan in fibroblasts but Not in KB
cells. PloS One. 9:e920762014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiu W, Zhang Y, Liu X, et al: Sublytic
C5b-9 complexes induce proliferative changes of glomerular
mesangial cells in rat Thy-1 nephritis through TRAF6-mediated
PI3K-dependent Akt1 activation. J Pathol. 226:619–632. 2012.
View Article : Google Scholar
|
|
36
|
Liu Y, Li Z, Wu L, et al: MiRNA-125a-5p: a
regulator and predictor of gefitinib’s effect on nasopharyngeal
carcinoma. Cancer Cell Int. 14:242014.PubMed/NCBI
|
|
37
|
Di Paolo NC, Baldwin LK, Irons EE,
Papayannopoulou T, Tomlinson S and Shayakhmetov DM: IL-1α and
complement cooperate in triggering local neutrophilic inflammation
in response to adenovirus and eliminating virus-containing cells.
PLoS Pathog. 10:e10040352014.
|
|
38
|
Hoth JJ, Wells JD, Jones SE, Yoza BK and
McCall CE: Complement mediates a primed inflammatory response after
traumatic lung injury. J Trauma Acute Care Surg. 76:601–609. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsiao JR, Jin YT, Tsai ST, Shiau AL, Wu CL
and Su WC: Constitutive activation of STAT3 and STAT5 is present in
the majority of nasopharyngeal carcinoma and correlates with better
prognosis. Br J Cancer. 89:344–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma N, Kawanishi M, Hiraku Y, et al:
Reactive nitrogen species-dependent DNA damage in EBV-associated
nasopharyngeal carcinoma: the relation to STAT3 activation and EGFR
expression. Int J Cancer. 122:2517–2525. 2008. View Article : Google Scholar : PubMed/NCBI
|