Introduction
Chondrosarcomas rank as the third most common type
of bone tumors, after myelomas and osteosarcomas (1). Due to its recalcitrance to
chemotherapy and radiotherapy, chondrosarcoma is primarily treated
with surgery (2). After adequate
resection, the 10-year survival of patients with grade I
chondrosarcoma is excellent, whereas the rate is only 64% for grade
II and 29% for grade III tumors (3). Thus, in recent years, several novel
therapeutic approaches have been evaluated in experimental studies
(3,4).
The apolipoprotein B mRNA-editing enzyme, catalytic
polypeptide-like 3 (APOBEC3) family of proteins is a major
component of the innate immunity system, acting against a variety
of viruses (5,6). The APOBEC family is comprised of a
series of molecules with conserved cytidine deaminase domains
(CDAs), including AID, APOBEC1, APOBEC2, APOBEC3A to H and APOBEC4
(7,8). APOBEC3B expression is relatively high
in many different cancer cell lines, such as breast cancer and in
lymphocytes (9,10). Prostate and renal clear cell
carcinomas showed statistically significant upregulation of
APOBEC3B in the tumors (11). Six
different cancers, breast, uterus, bladder, head and neck, and
lung, show evidence of strong APOBEC3B upregulation in the majority
of tumors (11).
However, the role of APOBEC3B in chondrosarcoma
remains unclear. In the present study, to better understand this
issue, we performed quantitative analysis on the association of
APOBEC3B with the risk of developing chondrosarcoma in a Chinese
Han population.
Materials and methods
Tissue samples
All chondrosarcoma and adjacent non-tumor tissue
samples were obtained from the First Hospital of China Medical
University from June 1993 to June 2013, following the consent of
each patient. The procedure was approved by the China Medical
University Ethics Committee. The study population consisted of 34
men and 18 women and the mean age was 44 years (range, 19–68
years). Twenty-three of the 52 cases were histologic grade I
tumors, 15 were grade II tumors, and the remaining 14 were grade
III.
Cell culture
The human chondrosarcoma cell lines, SW1353 (derived
from a human grade II chondrosarcoma) (12) and OUMS-27 (derived from a human
grade III chondrosarcoma) (13),
and RUNX3-positive SW1353 cells (14) were stored in our laboratory and
maintained in minimum essential medium (MEM) (Life Technologies,
Gaithersburg, MD, USA) supplemented with 10% (v/v) fetal bovine
serum (FBS) and antibiotics (100 U/ml of penicillin and 100 mg/ml
of streptomycin) at 37°C in a 5% (v/v) CO2
incubator.
Plasmid and transfection
SW1353 and OUMS-27 cells were seeded in 10-cm dishes
and grown overnight to 70% confluency, trypsinized and transfected
with the APOBEC3B shRNA plasmid (sc-72515-SH; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) using Lipofectamine™ 2000
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions.
Colony formation assay
Cells were seeded at 200 cells/well in 24-well
tissue culture plates. Plates were incubated for 3 weeks in a
humidified incubator at 37°C. Three weeks after seeding, colonies
were stained with 0.05% crystal violet containing 50% methanol and
counted. The colonies were counted in 4 to 5 random fields for each
of the duplicate samples by using a microscope at ×100
magnification.
Measurement of apoptotic cell death
Cells were harvested 48 h after transfection, and
immunostained with Annexin V-FITC and propidium iodide (PI)
according to the manufacturer’s instructions (Apoptosis Detection
kit; KeyGen, Nanjing, China). Data analysis was performed using
CellQuest software (BD Biosciences, Baltimore, MD, USA).
Transwell migration assay
Cells were plated at 2×105 cells/well in
0.5 ml of serum-free medium in 24-well Matrigel-coated Transwell
units with polycarbonate filters (8-μm pore size; Costar Inc.,
Milpitas, CA, USA). The lower chamber was loaded with 600 μl of MEM
containing 10% FBS. After incubation for 24 h in normal culture
conditions, the top surface of the membrane was gently scrubbed
with a cotton bud and fixed in 4% paraformaldehyde (Sigma-Aldrich,
St. Louis, MO, USA) and stained with crystal violet, and the cells
that had invaded through the membrane filters were counted using a
light microscope. Ten microscopic fields (x400) were randomly
selected to count the cells.
Real-time PCR
Total RNA was isolated using an RNeasy Mini kit
(Biomed, Beijing, China). First-strand cDNA was reverse transcribed
with 1 μg of total RNA, using the Takara reverse transcription kit
and oligo(dT)15 primers (both from Takara, Dalian,
China). The resultant cDNA was then used for quantitative PCR
reactions. The APOBEC3B primers were: 5′-TAGGTGCCACCCCGAT-3′
(sense) and 5′-TTGAGCATAATCTTACTCTTGTAC-3′ (antisense). The
housekeeping gene, GAPDH, was used as the internal control
for normalization of the results. The GAPDH primers were:
5′-AGAAGGCTGGGGCTCATTTG-3′ (sense) and 5′-CGATCCACACGGAGTACTTGC-3′
(antisense). Amplification of APOBEC3B and GADPH was
performed with 1 cycle at 95°C for 10 min, and 40 cycles at 95°C
for 15 sec and 60°C for 60 sec. Calculation of the relative
expression of each transcript was performed using the
2−ΔΔCt method.
Western blot analysis
Equal amounts (30 μg) of cell lysates were separated
by 10% SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes, and incubated with specific
antibodies. The reaction was followed by probing with
peroxidase-coupled secondary antibodies, including anti-rabbit IgG
or anti-mouse IgG antibodies at dilutions ranging from 1:1,000 to
1:2,000 (Amersham Biosciences, Needham, MA, USA). The binding
results were visualized by enhanced chemiluminescence (Amersham
Pharmacia, Piscataway, NJ, USA). The primary antibodies are
summarized in Table I.
 | Table IThe antibodies used in the western
blot analysis. |
Table I
The antibodies used in the western
blot analysis.
| Protein | Manufacturer | Catalog no. | Dilution |
|---|
| AKT | Santa Cruz
Biotechnology | sc-5298 | 1:500 |
| p-AKT | | sc-135650 | 1:500 |
| Caspase 3 | | sc-65495 | 1:200 |
| Caspase 8 | | sc-56070 | |
| Caspase 9 | | sc-8355 | 1:200 |
| β-actin | | sc-103656 | 1:1,000 |
Immunohistochemical staining
Tissues were fixed with 10% buffered formalin,
embedded in paraffin and decalcified in 10% EDTA solution.
Representative blocks were then cut to 4 μm, deparaffinized with
xylene, and rehydrated in a series of ethanol washes (100, 90, 80
and 70%). Sections were then incubated with 3%
H2O2 and 5% serum to block endogenous
peroxidase activity and non-specific binding. For the APOBEC3B
protein, sections were incubated with anti-human APOBEC3B antibody.
The sections were then incubated with the biotinylated secondary
antibodies and visualized by DAB. Counterstaining was carried out
with hematoxylin. The sections were dehydrated in alcohol and
coverslipped. For the negative controls, PBS replaced the primary
antibody.
Statistical analysis
All experiments were performed in triplicate, and
the results are expressed as the means ± standard deviation (SD).
Kaplan-Meier survival plots were generated and comparisons were
carried out with log-rank statistics. A P-value <0.05 was
considered to indicate a statistically significant result. All the
statistical analyses and graphics were performed with GraphPad
Prism version 5.00 for Windows (GraphPad Software, San Diego, CA,
USA).
Results
Assessment of levels of APOBEC3B mRNA and
protein in 52 human chondrosarcoma specimens
Western blotting and immunohistochemical staining
were carried out to investigate the protein levels of APOBEC3B in
the chondrosarcoma specimens of grade I, II and III, respectively.
As shown in Fig. 1A and C, the
level of APOBEC3B protein in the chondrosarcoma tissues was higher
than the level in the normal tissues (P<0.05). To examine the
relationship between the level of APOBEC3B protein and the level of
APOBEC3B transcription, real-time PCR of APOBEC3B
mRNA was carried out in the chondrosarcoma specimens. The results
showed that the level of APOBEC3B mRNA was also higher in
the chondrosarcoma specimens than the level in the normal tissues
and coincident with the level of protein (P<0.05, Fig. 1B). The levels of APOBEC3B
mRNA and protein were higher in the cancer tissues of grade III
than levels in tissues of grade I or II (P<0.05, Fig. 1). Kaplan-Meier analysis showed that
APOBEC3B expression was correlated with the unfavorable prognosis
of patients with grade I, II and III stage chondrosarcoma
(P<0.05, Fig. 1D).
Effects of APOBEC3B knockdown on
biological phenotypes of chondrosarcoma cells
SW1353, OUMS-27 and RUNX3-positive SW1353 cells were
transfected with the APOBEC3B shRNA plasmid, and expression of
APOBEC3B was determined by western blotting and immunofluorescence
analysis. As shown in Fig. 2, the
results of the western blot analysis and immunofluorescence
analysis confirmed decreased APOBEC3B protein levels in the three
cell lines after transfection.
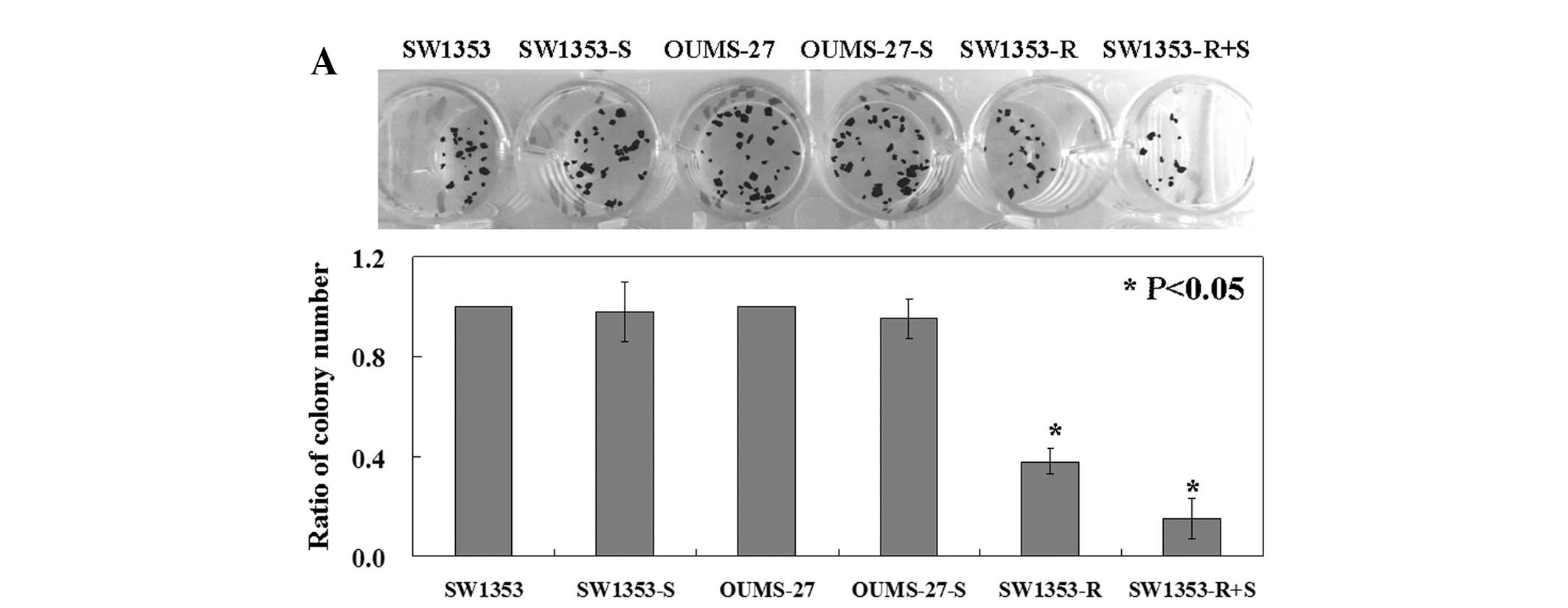
The results from the colony formation assay showed
that the proliferation rates of the SW1353 and OUMS-27 cells after
transfection were slightly lower than the rates in the untreated
cell lines (P<0.05, Fig. 3A).
The proliferation rate of the RUNX3-positive SW1353 cells was lower
than the rate in the RUNX3-negative cells (P<0.05, Fig. 3A). Notably, the RUNX3-positive
SW1353 cells following APOBEC3B shRNA plasmid transfection had the
lowest proliferative rate among the cell lines (P<0.05, Fig. 3A). The percentage of apoptotic cells
in each group was determined by Annexin V and PI double-staining.
Correspondingly, the RUNX3-positive SW1353 cells after APOBEC3B
shRNA plasmid transfection exhibited increased apoptosis
(6.26±0.42%) compared to the percentage of apoptosis in the SW1353
(0.35±0.09%) and RUNX3-positive SW1353 cells (1.28±0.16%)
(P<0.05, Fig. 3B). Furthermore,
a significantly decreased mobility of SW1353 and OUMS-27 cells
following APOBEC3B shRNA plasmid transfection was noted when
compared to the untreated cells (P<0.05, Fig. 3C). We next assessed whether the
reduced antitumor activities of RUNX3 are correlated with
mutagenesis. The RUNX3-positive SW1353 cells with APOBEC3B shRNA
did not yield any PCR products amplified at lower denaturing
temperatures, suggesting that no editing took place. The
RUNX3-positive SW1353 cells exhibited extensive mutagenesis in the
presence of APOBEC3B (Fig. 3D).
In the western blot analysis, a decreased level of
p-AKT and increased levels of caspase-3, -8 and -9 were detected in
the RUNX3-positive SW1353 cells transfected with APOBEC3B shRNA
when compared with the levels in the untreated cells (Fig. 4), while total levels of AKT showed
no changes (Fig. 4).
Discussion
The APOBEC3 gene family encodes proteins that
play pivotal roles in intracellular defense against viral infection
(8). APOBEC3B is overexpressed in
many types of tumor tissues and lymphoma cells (15,16).
In the present study, we found that APOBEC3B was overexpressed in
chondrosarcoma tissues and cell lines. Furthermore, we also
confirmed that APOBEC3B expression was correlated with an
unfavorable prognosis of the chondrosarcoma patients.
Previous studies found that restoration of RUNX3
induces cell cycle arrest and apoptosis (14,17).
Importantly, in the present study, we found that APOBEC3B knockdown
induced slight apoptosis in the chondrosarcoma cells. However, the
RUNX3-positive SW1353 cells with APOBEC3B knockdown had a higher
apoptotic ratio than the cells without APOBEC3B knockdown. The
APOBEC3 genes have been shown to deaminate 5-methylcytosine and
5-hydroxymethylcytosine, with base excision repair of the resulting
mismatch providing a mechanism for active DNA demethylation
(8). There are numerous reports of
APOBEC3 deaminase editing-independent restriction of HIV, including
APOBEC3-mediated reduction of reverse transcription activity,
strand transfer, or integration (18,19).
To the best of our knowledge, no related studies have shown the
effects of APOBEC3 on mutation of antitumor genes. Mutation
signatures have aided in the identification of environmental
mutagens and carcinogens (20). C→T
transitions in cervix, bladder, lung, head and neck, and breast
cancers have been suggested to be caused by APOBEC3B (21). In the present study, we found that
the reduced antitumor activity of RNUX3 was caused by APOBEC3B.
Furthermore, we found that RUNX3 inhibited p-AKT expression. The
clinical and prognostic significance of AKT and its activated form
(p-AKT) in human cancer have been investigated (11).
In conclusion, this study provides evidence that
APOBEC3B interferes with RUNX3 transcription. This may, at least in
part, contribute to RUNX3-mediated inhibition of chondrosarcoma
cell invasion and proliferation. We are currently investigating
whether other genes in chondrosarcoma are also regulated by
APOBEC3B.
Acknowledgements
We thank Dr Miao Yu for her valuable comments and
excellent technical assistance.
References
|
1
|
Terek RM, Schwartz GK, Devaney K, et al:
Chemotherapy and P-glycoprotein expression in chondrosarcoma. J
Orthop Res. 16:585–590. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hiraoka K, Zenmyo M, Komiya S, et al:
Relationship of p21(waf1/cip1) and differentiation in
chondrosarcoma cells. Virchows Arch. 440:285–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schrage YM, Briaire-de Bruijn IH, de
Miranda NF, et al: Kinome profiling of chondrosarcoma reveals
SRC-pathway activity and dasatinib as option for treatment. Cancer
Res. 69:6216–6222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeLaney TF, Liebsch NJ, Pedlow FX, et al:
Phase II study of high-dose photon/proton radiotherapy in the
management of spine sarcomas. Int J Radiat Oncol Biol Phys.
74:732–739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goila-Gaur R and Strebel K: HIV-1 Vif,
APOBEC, and intrinsic immunity. Retrovirology. 5:512008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heidmann T, Heidmann O and Nicolas JF: An
indicator gene to demonstrate intracellular transposition of
defective retroviruses. Proc Natl Acad Sci USA. 85:2219–2223. 1988.
View Article : Google Scholar
|
|
7
|
Macduff DA and Harris RS: Directed DNA
deamination by AID/APOBEC3 in immunity. Curr Biol. 16:R186–R189.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conticello SG: The AID/APOBEC family of
nucleic acid mutators. Genome Biol. 9:2292008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burns MB, Lackey L, Carpenter MA, et al:
APOBEC3B is an enzymatic source of mutation in breast cancer.
Nature. 494:366–370. 2013.PubMed/NCBI
|
|
10
|
Refsland EW, Stenglein MD, Shindo K, Albin
JS, Brown WL and Harris RS: Quantitative profiling of the full
APOBEC3 mRNA repertoire in lymphocytes and tissues:
implications for HIV-1 restriction. Nucleic Acids Res.
38:4274–4284. 2010.PubMed/NCBI
|
|
11
|
Burns MB, Temiz NA and Harris RS: Evidence
for APOBEC3B mutagenesis in multiple human cancers. Nat Genet.
45:977–983. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim DW, Kim KO, Shin MJ, et al:
siRNA-based targeting of antiapoptotic genes can reverse
chemoresistance in P-glycoprotein expressing chondrosarcoma cells.
Mol Cancer. 8:282009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishida K, Furumatsu T, Takada I, et al:
Inhibition of human chondrosarcoma cell growth via apoptosis by
peroxisome proliferator-activated receptor-γ. Br J Cancer.
86:1303–1309. 2002.PubMed/NCBI
|
|
14
|
Jin Z, Han YX and Han XR: Loss of RUNX3
expression may contribute to poor prognosis in patients with
chondrosarcoma. J Mol Histol. 44:645–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu R, Zhang X, Zhang W, Fang Y, Zheng S
and Yu XF: Association of human APOBEC3 cytidine deaminases with
the generation of hepatitis virus B x antigen mutants and
hepatocellular carcinoma. Hepatology. 46:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiu YL and Greene WC: The APOBEC3
cytidine deaminases: an innate defensive network opposing exogenous
retroviruses and endogenous retroelements. Annu Rev Immunol.
26:317–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han YX and Liang DY: The role of the tumor
suppressor RUNX3 in giant cell tumor of the bone. Int J Oncol.
40:673–678. 2011.PubMed/NCBI
|
|
18
|
Holmes RK, Koning FA, Bishop KN and Malim
MH: APOBEC3F can inhibit the accumulation of HIV-1 reverse
transcription products in the absence of hypermutation. Comparisons
with APOBEC3G. J Biol Chem. 282:2587–2595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwatani Y, Chan DS, Wang F, et al:
Deaminase-independent inhibition of HIV-1 reverse transcription by
APOBEC3G. Nucleic Acids Res. 35:7096–7108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mbisa JL, Barr R, Thomas JA, et al: Human
immunodeficiency virus type 1 cDNAs produced in the presence of
APOBEC3G exhibit defects in plus-strand DNA transfer and
integration. J Virol. 81:7099–7110. 2007. View Article : Google Scholar
|
|
21
|
Lawrence MS, Stojanov P, Polak P, et al:
Mutational heterogeneity in cancer and the search for new
cancer-associated genes. Nature. 499:214–218. 2013. View Article : Google Scholar : PubMed/NCBI
|