Introduction
Breast cancer, the leading cause of cancer-related
mortality worldwide in females, has high metastastic and recurrence
rates following curative resection. Metastasis after resection, is
the leading cause of mortality in patients with breast cancer
(1,2). Interleukin-8 (IL-8), a cytokine of the
CXC chemokine family (3), is highly
expressed in many tumor tissues (4)
and promotes tumor progression and cancer metastasis (5–10).
Findings of recent studies have shown that the overexpression of
IL-8 is associated with recurrence and poor prognosis in breast
cancer (11–14). The expression of IL-8 in highly
metastatic breast cancer MDA-MB-231 cells is much higher than that
in the non-metastatic MCF-7 breast cancer cell line (15,16).
Recent studies have demonstrated that, during or after treatment,
several chemotherapeutic drugs resulted in resistance and cancer
metastasis associated with upregulated IL-8 in human breast cancer
(17–20). Thus, suppression of IL-8 may be
beneficial for breast cancer treatment.
Chemotherapeutic agents, such as 5-fluorouracil,
adriamycin, dacarbazine and paclitaxel can induce IL-8 upregulation
(5,21–25).
Coptis chinensis Franch (Huanglian), a medicinal herb,
induces cell growth arrest and apoptosis in human breast cancer
cells (26). Berberine, an active
isoquino-line alkaloid in C. chinensis, shows
anti-metastatic activity in breast cancer cells including
MDA-MB-231 and MCF-7 cells (27).
In the clinic, berberine hydrochloride (BER) with better water
solubility than berberine, is used to treat gastrointestinal
inflammation, bacterial diarrhea or infection as well as some
gastrointestinal cancers (10,28,29).
In our previous studies, BER inhibited the proliferation and IL-8
expression of AGS cells and counteracted enhanced IL-8 induced by
evodiamine in AGS cells (10,30).
However, the effect of berberine and BER on IL-8 expression and the
relationship of IL-8 with migration and cell proliferation in
MDA-MB-231 cells remains to be determined.
Induction of apoptosis of cancer cells, mainly
through the mitochondrial- and death receptor-dependent pathways,
is the principal strategy for chemotherapy. In addition, several
other pathways involved in cell apoptosis are influenced by
chemotherapeutic drugs (31–33).
Berberine has been demonstrated to induce apoptosis of cancer cells
including SW60 and HepG2 cells by interfering with the expression
of molecules in pathways including Bcl-2, Bax and caspase-3
(34–36). However, whether these pathways play
a role in BER-induced apoptosis in breast cancer cells remains to
be clarified.
In the present study, the effects of BER on IL-8
expression, and the migration, invasion and cell proliferation of
MDA-MB-231 cells were investigated. Furthermore, the possible
molecular mechanisms involved in the anti-metastatic and
pro-apoptotic effect of BER were discussed. The results suggested
that BER may be an efficient and safe drug candidate for treating
highly metastatic breast cancer.
Materials and methods
Materials and chemicals
Berberine hydrochloride (BER, purity: 98%, no.
8892010) was purchased from Shanghai Tauto Biotech Co., Ltd.
(Shanghai, China). Dulbecco’s modified Eagle’s medium
(DMEM)/high-glucose medium was obtained from Cellgro (Manassas, VA,
USA). Trypsin (no. 1310929) and FBS (no. 1301838) were provided by
Gibco (Grand Island, NY, USA). Propidium iodine (PI) (no. 118
K3583) and dimethyl sulfoxide (DMSO) (no. RNBC 3642) were supplied
by Sigma Chemical Co. (St. Louis, MO, USA). The primers for qPCR,
TRIzol (no. 66205) and Annexin V-FITC (no. 1081948) were from
Invitrogen (Carlsbad, CA, USA). Cell Counting Kit-8 (no. ET758) was
provided by Dojindo Laboratories (Kumamoto, Japan). SYBR Premix Ex
Taq (no. AK2902) and PrimeScript RT reagent kit (no. AK2001) were
from Takara (Dalian, China). IL-8 ELISA kit (no. E15164-105) was
obtained from eBioscience (San Diego, CA, USA). Human IL-8 (no.
120219) was purchased from PeproTech (Rocky Hill, NJ, USA). The ECL
Prime kit (no. 4618945) was purchased from GE Healthcare
(Buckinghamshire, UK). Anti-GAPDH (no. 8), anti-p38 MAPK (no. 4),
anti-ERK(1/2) (no. 14), anti-JNK (no. 9), anti-p85 PI3K (no. 4),
anti-p65 NF-κB (no. 1), anti-Jak2 (no. 8), anti-p-p38 MAPK (no.
10), anti-p-ERK(1/2) (no. 14), anti-p-JNK (no. 9), anti-p-p85 PI3K
(no. 2), anti-p-p65 NF-κB (no. 6), anti-p-Jak2 (no. 10), anti-p-Akt
(T308, no. 17), and anti-p-Akt (S473, no. 13) antibodies were
supplied by Cell Signaling Technology (Danvers, MA, USA). Anti-Akt
(no. YE121003), and anti-cleaved caspase-3 (no. YJ010604C)
antibodies were supplied by Epitomics (Burlingame, CA, USA). JAK
inhibitor I (Jak inhibitor, no. D3010), LY294002 (PI3K inhibitor),
and SB203580 (p38 MAPK inhibitor, no. C3110) were purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Curcumin (AP-1
inhibitor) was obtained from ICN Biomedicals Inc. (Costa Mesa, CA,
USA). BAY-11-7082 (NF-κB inhibitor, no. 01), SP600125 (JNK
inhibitor, no. 01), PD98059 (ERK1/2 inhibitor, no. 03) were
obtained from Selleck Chemicals (Houston, TX, USA). Anisomycin
(activitors of p38 MAPK and JNK, no. D00140631) was from Calbiochem
(San Diego, CA, USA).
Proliferation assay
MDA-MB-231 cells were cultured in DMEM with 10%
fetal bovine serum (FBS). The cells were seeded in 200 μl of medium
at 1.0×104 cells/ml in 96-well culture plates and grown
overnight. Following treatment with BER for 24 or 48 h,
respectively, the culture medium was collected for ELISA assay of
IL-8. An equal volume of fresh medium was then added back to each
well with an additional 20 μl of CCK-8 solution and incubated at
37°C for another 1 h. Absorbance of the dissolved solutions was
detected at 450 nm by a Thermo Scientific Varioskan Flash
microplate reader (Thermo Fisher Scientific). The cell viability
rate was calculated as: (absorbance of drug-treated
sample/absorbance of control sample) × 100.
Enzyme-linked immunosorbent assay
(ELISA)
Cells were seeded in 96-well, 6-well or 35-mm plates
and cultured overnight. Following treatment with BER for 24 or 48
h, the culture medium was collected, centrifuged at 3000 rpm for 5
min and subjected to IL-8 assay using an ELISA kit according to the
manufacturer’s instructions. The absorbance at 450 nm was measured
with a microplate reader, and the concentration of IL-8 in medium
was determined by the standard curve.
Wound-healing assay
MDA-MB-231 cells were seeded in 24-well plates.
After the cells reached 90–95% confluence, a scratch was drawn on
the cell monolayer with a sterile 100 μl pipette tip. The detached
cells were removed by washing with PBS. Treatments of BER (30, 60
and 90 μM) and IL-8 (100 ng/ml) prepared in medium were added onto
the cells and the images were captured immediately under an Olympus
CKX41 microscope (Olympus, Tokyo, Japan) and denoted as time T0.
Following incubation for 24 and 48 h, the cells were photographed
again and denoted as time T24 and T48.
Invasion assay
The invasion ability of breast cancer cells was
evaluated according to the methods described by Kuo et al
(27). Briefly, 200 μl of
MDA-MB-231 cells (1×106 cells in serum-free medium) were
seeded onto the upper part of the 24-well Transwell chambers coated
with Matrigel (BD Biosciences, San Jose, CA, USA). FBS (10%) was
used as the chemoattractant in the bottom chambers. After
incubation with IL-8 (100 ng/ml), BER (90 μM) or their combination
at 37°C for 24 h, the non-invaded cells were removed from the top
of the Transwell membrane with a cotton swab. The invaded cells
were fixed with 4% PFA for 10 min, followed by incubation with 2%
crystal violet staining solution for 15 min, and were observed
under an Olympus CKX41 microscope.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from the MDA-MB-231 cells
using TRIzol reagent according to the manufacturer’s instructions.
Reverse transcription was conducted with a PrimeScript RT reagent
kit. Sense and antisense primers used for qPCR were shown in
Table I. qPCR was performed with
SYBR Premix Ex Taq by using following amplification conditions:
95°C for 30 sec; followed by 40 cycles (95°C for 5 sec; 60°C for 34
sec); and 95°C for 15 sec; 60°C for 1 min; and 95°C for 15 sec. The
relative expression level of individual genes was normalized to
that of GAPDH in the same sample.
 | Table IPrimer sequences used in qPCR. |
Table I
Primer sequences used in qPCR.
| Genes | Forward primer | Reverse primer |
|---|
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| E-cadherin |
CGAGAGCTACACGTTCACGG |
GGGTGTCGAGGGAAAAATAGG |
| Fibronectin |
TGAGCTGCACATGTCTTG |
TCCTACGTGGTATGTCTTCC |
| bFGF |
GGCGTGTACATGTGGTCTCAGA |
TTATGGCTCACTGCAACCTTGA |
| EGF |
GACTTGGGAGCCTGAGCAGAA |
CATGCACAAGTGTGACTGGAGGT |
| MMP-2 |
TGGCAAGTACGGCTTCTGTC |
TTCTTGTCGCGGTCGTAGTC |
| Jak 2 |
TCTGGGGAGTATGTTGCAGAA |
AGACATGGTTGGGTGGATACC |
| Akt1 |
CCTCCACGACATCGCACTG |
TCACAAAGAGCCCTCCATTATCA |
Cell cycle distribution analysis
Cells were seeded in 6-well plates at
6×104 cells/well in 3 ml medium and cultured overnight.
After serum starvation for 24 h, the cells were incubated with BER
(30, 60 and 90 μM), IL-8 (100 ng/ml) or a combination of IL-8 and
BER (90 μM) for 24 h. The cells were harvested by trypsinization,
washed twice with phosphate-buffered saline (PBS), and fixed with
cold 70% ethanol overnight followed by staining with PI solution
containing 50 μg/ml RNase A and 0.1% Triton X-100. The distribution
of the cell cycle was examined using a Millipore Guava flow
cytometer (Millipore, Billerica, MA, USA).
Cell apoptosis detection
Cells were seeded in 6-well plates at
7.5×104 cells/well in 3-ml medium and allowed to adhere
to plates overnight. After serum starvation for 24 h, the cells
were incubated with a range of concentrations of BER (30, 60 and 90
μM) with 10% FBS for another 24 h. In experiments for clarifying
cell signaling pathways involved in BER-induced apoptosis, the
cells were treated with BER at 90 μM for 24 h after pre-incubation
of SB203580 (25 μM), LY294002 (10 μM), SP600125 (20 μM), PD98059
(20 μM), BAY-11-7082 (5 μM), JAK inhibitor I (5 μM), curcumin (8
μM) and anisomycin (10 μg/ml) for 1 h. The cells were subsequently
harvested by careful trypsinization, and washed twice with 1X
Annexin V binding buffer. After resuspension in 1X Annexin V
binding buffer, the cells were stained with Annexin V and PI.
Fluorescence of the cells was examined on a Guava flow
cytometer.
Western blotting
After incubation with BER, the cells were lysed with
lysis buffer and sonicated three times each for 15 sec. The cell
lysate was centrifuged at 14,000 × g for 15 min at 4°C, and the
supernatant was collected. Protein samples were separated by
SDS-PAGE (12 or 15%) and transferred onto PVDF membrane by wet
transfer. PVDF membranes were blocked with 5% non-fat milk solution
and incubated with different primary antibodies overnight at 4°C.
After being washed with 1X TBST, PVDF membranes were incubated with
respective secondary antibodies. The protein bands were visualized
with ECL Prime kit.
Statistical analysis
Each value was presented as means ± SEM. Differences
between two groups were analyzed using Student’s t-test. Pairwise
comparisons among groups were conducted by one-way ANOVA with
Dunnett’s analysis using PrismDemo 4. P<0.05 was considered
statistically significant.
Results
BER inhibits proliferation and IL-8
secretion of MDA-MB-231 cells
To examine the efficacy of BER on cell growth of
breast cancer, MDA-MB-231 cells were treated with a range of
concentrations of BER for 24 and 48 h, respectively. As shown in
Fig. 1B, BER dose-dependently
inhibited the proliferation of MDA-MB-231 cells at 24 or 48 h. When
used at concentrations of >90 μM for 24 h, BER suppressed the
growth of cells, and the growth inhibitory rate of was >44.18%.
By contrast, when treated for 48 h, at lower concentrations, such
as 60 μM, BER prevented 38.94% of cells from proliferation.
Accordingly, the IC50 of BER was 78.21 μM for 24-h
treatment, while that for 48 h was 71.87 μM.
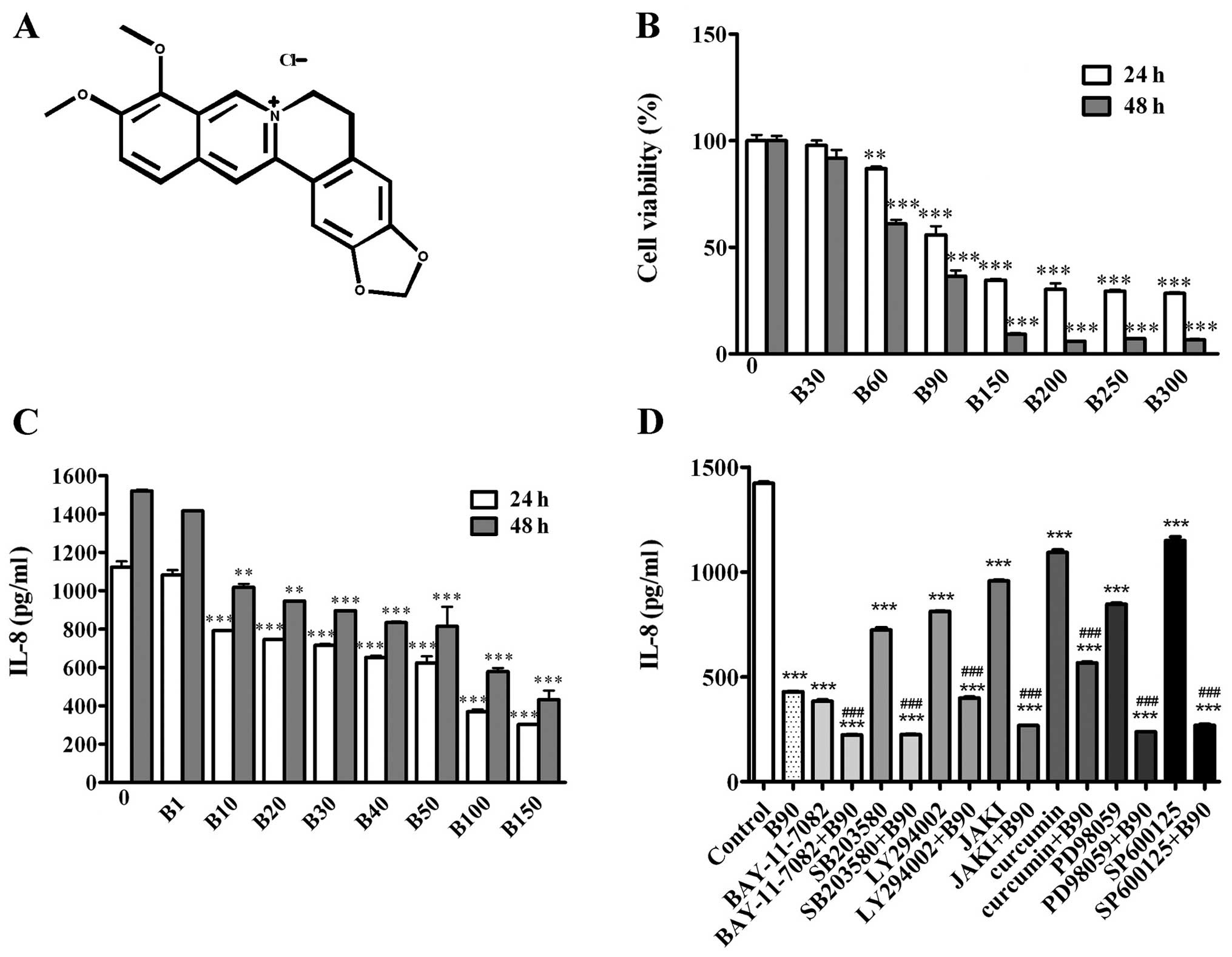 | Figure 1BER inhibits cell proliferation and
IL-8 secretion of MDA-MB-231 cells. (A) Chemical structure of BER.
(B) MDA-MB-231 cells were treated with 0.1% DMSO or BER (30–300 μM)
for 24 and 48 h, then measured by CCK-8 assay to determine the cell
viability. (C) IL-8 secretion was detected by ELISA following BER
treatment (1–150 μM) for 24 and 48 h, respectively. (D) MDA-MB-231
cells were pre-incubated with or without inhibitors of cell
signaling pathways (BAY-11-7082, 5 μM; SB203580, 25 μM; LY294002,
10 μM; JAK I, 5 μM; Curcumin, 8 μM; PD98059, 20 μM; and SP600125,
20 μM) for 1 h, followed by B90 treatment for 24 h. IL-8 level in
culture serum was measured by ELISA. Data are presented as means ±
SEM; **P<0.01, ***P<0.001 vs. control.
###P<0.001 vs. B90. B, berberine hydrochloride. |
Further analysis demonstrated that BER significantly
decreased the IL-8 secretion of MDA-MB-231 cells in a
dose-dependent manner (Fig. 1C). To
determine cellular signaling molecules involved in the modulation
of IL-8 secretion of MDA-MB-231 cells, multiple pathway inhibitors
were used, including LY294002 (10 μM), SB203580 (25 μM), SP600125
(20 μM), PD98059 (20 μM), BAY-11-7082 (5 μM), JAK inhibitor I (5
μM) and curcumin (8 μM). As shown in Fig. 1D, these inhibitors were able to
decrease the IL-8 secretion of MDA-MB-231 cells compared with the
control group. Furthermore, pre-incubation with the above mentioned
inhibitors, with the exception of the AP-1 inhibitor, increased the
inhibitory effect of BER (90 μM) on IL-8 expression in MDA-MB-231
cells.
BER decreases cell invasion and migration
of MDA-MB-231 cells
In order to clarify the relationship between BER and
cell metastasis, invasion and wound-healing assays were carried
out. As shown in Fig. 2A, IL-8
increased the invasion of MDA-MB-231 cells as more cells stained by
crystal violet were found on the bottom chambers of the Transwell
membranes when compared with those in the control groups. BER (90
μM) inhibited the invasion of MDA-MB-231 cells, and could abolish
the increased cell invasion induced by IL-8.
In the wound-healing assays (Fig. 2B), BER (30, 60 and 90 μM) appeared
to dose-dependently prevent the motility of MDA-MB-231 cells after
treatment for 24 and 48 h. However, IL-8 (100 ng/ml) treatment did
not affect cell motility (Fig. 2C)
at 24 or 48 h and showed no interaction with BER treatment.
BER inhibits gene expression of
metastasis-related molecules in MDA-MB-231 cells
To confirm the anti-metastatic effect of BER, the
mRNA expression of MMP-2, EGF, E-cadherin, bFGF and fibronectin was
quantified by qPCR. BER at 90 μM decreased the mRNA expression of
the measured molecules significantly (P<0.05 or P<0.001)
(Fig. 3). By contrast, when used
<90 μM, BER only reduced the mRNA expression of MMP-2, EGF and
fibronectin.
BER induces G2/M phase arrest and
apoptosis in MDA-MB-231 cells
BER induced the G2/M arrest of MDA-MB-231 cells in a
dose-dependent manner (Fig. 4).
IL-8 (100 ng/ml) had no effect on cell cycle distribution. When
combined with IL-8, BER (90 μM) induced G2/M arrest significantly,
although no difference with that induced by BER alone was
observed.
BER appeared to dose-dependently induce the
apoptosis of MDA-MB-231 cells as the ratio of early apoptotic cells
increased with the elevation of BER concentrations (Fig. 5I). By contrast, as shown in Fig. 5II, IL-8 did not markedly affect cell
apoptosis. To our surprise, it also did not influence cell
apoptosis induced by BER (90 μM).
Consistent with the results from flow cytometry, BER
modulated the expression of apoptotic proteins. BER
dose-dependently increased the amount of cleaved caspase-3
(Fig. 5I). By contrast, it also
downregulated the protein expression of Bcl-2 in a dose-dependent
manner but showed no effect on the expression of Bax.
Cellular signaling pathways are involved
in BER-induced apoptosis in MDA-MB-231 cells
To determine the effect of BER on the protein
expression of the cellular signaling molecules, MDA-MB-231 cells
were treated with BER (30, 60 and 90 μM) for 24 h and then
subjected to western blot analysis. Fig. 6A shows that BER dose-dependently
increased the phosphorylation of p38 and SAPK/JNK MAPKs but did not
affect that of ERK. By contrast, BER treatment decreased the
phosphorylation of Jak2, p85 PI3K, Akt and p65 NF-κB in a
dose-dependent manner (Fig. 6B).
Additionally, BER inhibited the mRNA expression of Jak2 and Akt1
(Fig. 6C and D).
To verify the involvement of p38 and JNK MAPKs in
the BER-induced apoptosis, MAPK pathway inhibitors were used
together with BER (90 μM). As shown in Fig. 7A–G, the elevated apoptosis induced
by BER was significantly abrogated by SB203580 and SP600125.
Moreover, the addition of SB203580 and SP600125 reduced the
cellular cleaved caspase-3 compared with that treated with BER
(Fig. 7H). To confirm the effect of
p38 MAPK and JNK on BER-induced apoptosis, anisomycin, the p38 MAPK
and JNK activator, was used. As shown in Fig. 8A, anisomycin (10 μg/ml)
significantly increased the activation of p38 MAPK, which enhanced
the cleavage of caspase-3. When co-treated with anisomycin, BER (90
μM) induced the increased phosphorylation of p38 MAPK compared with
BER treatment alone, leading to an increased production of cleaved
caspase-3. Consistent with the western blot results, anisomycin and
BER induced significant apoptosis compared with the control
(P<0.001) (Fig. 8B–F). When
combined together, anisomycin and BER enhanced apoptosis as
compared to that induced individually.
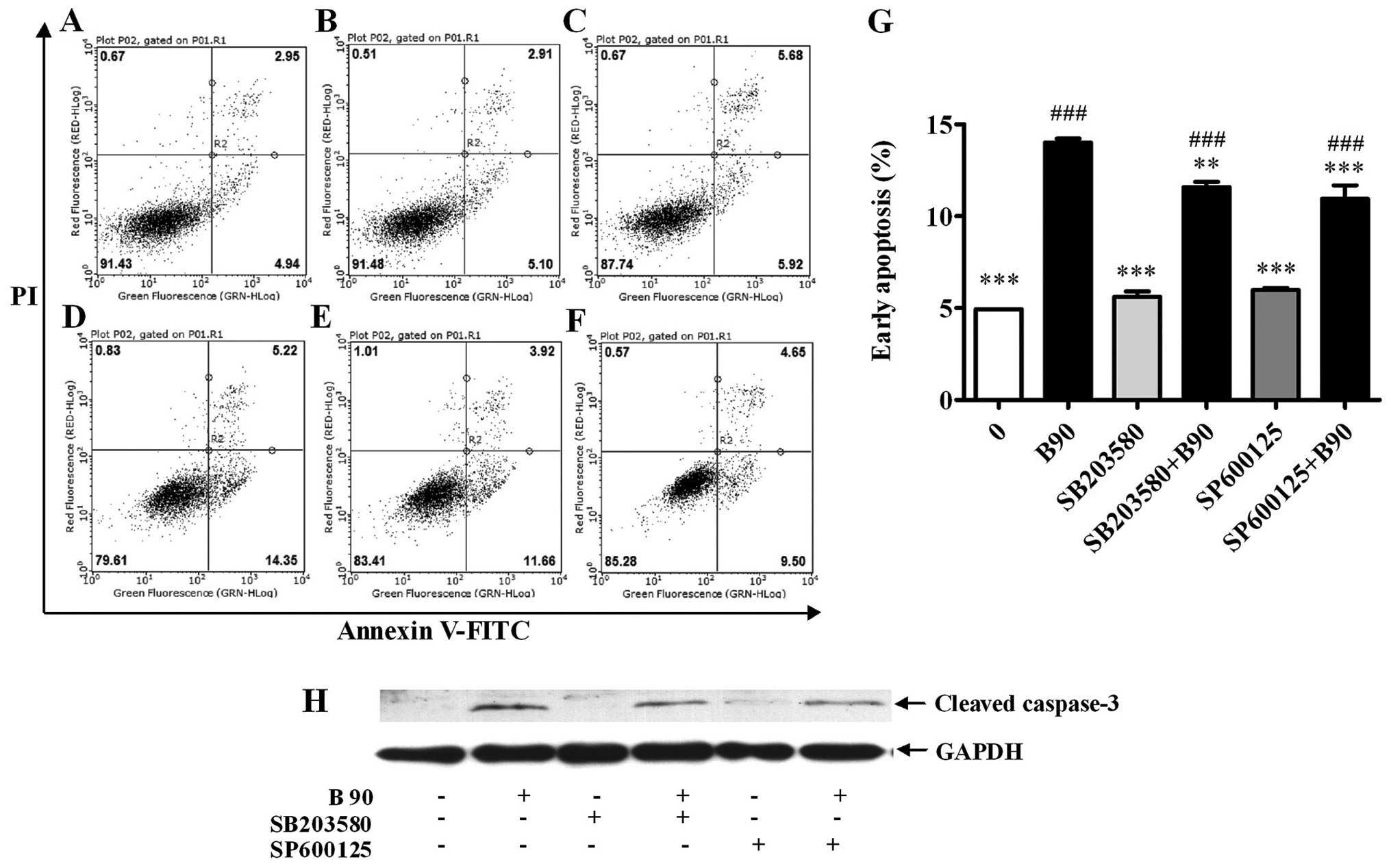 | Figure 7Blockage of p38 MAPK and JNK
decreases cell apoptosis induced by BER in MDA-MB-231 cells. (A–F)
Typical images from flow cytometry with Annexin V/PI double
staining. (G) Blockage of p38 MAPK and JNK decreased cell apoptosis
induced by BER. (H) Western blotting results showed that blockage
of p38 MAPK and JNK decreased cleaved caspase-3 expression induced
by BER. Data are presented as means ± SEM; **P<0.01,
***P<0.001 vs. control; ###P<0.001 vs.
B90. B, berberine hydrochloride (90 μM). A, 0; B, B90; C, SB203580,
25 μM; D, SP600125, 20 μM; E, SB203580+B90; F, SP600125+B90. |
In the present study, the effect of NF-κB, PI3K,
AP-1 and JAK2 on BER-induced apoptosis was clarified. As shown in
Fig. 9, inhibitors of NF-κB and
AP-1 increased the rates of cell apoptosis. Pre-incubation with the
inhibitors of NF-κB, PI3K, AP-1 and JAK2 significantly increased
cell apoptosis induced by BER. Moreover, the protein expression of
cleaved caspase-3 was increased by the pre-incubation of inhibitors
of PI3K, JAK2 and p65 NF-κB, compared with BER used alone.
 | Figure 9Blockage of PI3K, Jak2, NF-κB and
AP-1 elevates cell apoptosis induced by BER in MDA-MB-231 cells.
(A–J) Typical images from flow cytometry with Annexin V/PI double
staining. (K) Blockage of PI3K, Jak2, NF-κB and AP-1 elevated cell
apoptosis induced by BER in MDA-MB-231 cells. (I) Western blotting
results showed that the blockage of PI3K, Jak2 and NF-κB increased
cleaved caspase-3 expression induced by BER. Data are presented as
means ± SEM; ***P<0.001 vs. control;
##P<0.01, ###P<0.001 vs. B90. B90,
berberine hydrochloride (90 μM). A, 0; B, JAK I, 5 μ; C, LY294002,
10 μM; D, BAY-11-7082, 5 μM; E, Curcumin, 8 μM; F, B90; G,
JAKI+B90; H, LY294002+B90; I, BAY-11-7082+B90; J, Curcumin+B90. |
Discussion
The results of the present study have demonstrated
that BER significantly inhibited IL-8 secretion, invasion and
migration of MDA-MB-231 cells and induced cell apoptosis.
Additional experiments revealed that BER suppressed cell
proliferation through G2/M arrest and promoted apoptosis of
MDA-MB-231 cells by modulation of various signaling pathway
molecules such as MAPKs, JAK2, PI3K, Akt and NF-κB.
Chemotherapeutic agents are known to induce IL-8
upregulation in tumor cells, which is closely associated with
chemotherapy resistance and cancer metastasis (3,5–7,10,18,21–25,37–40).
Depletion of IL-8 induces cell cycle arrest and increases the
efficacy of chemotherapeutic agents in breast cancer cells
(41). Therefore, chemotherapeutic
agents with an inhibitory effect on IL-8 production may be more
efficacious in treating breast cancer. MDA-MB-231 is one of the
breast cancer cell lines constitutively expressing a high level of
IL-8 (15,16). Consistent with previous studies
(41), IL-8 enhanced the invasive
ability of MDA-MB-231 cells in our experiments (Fig. 2A). However, IL-8 did not interfere
with cell migration (Fig. 2C) or
cell cycle distribution (Fig. 4).
To the best of our knowledge, IL-8 mainly increases the risk of
breast cancer metastasis through the enhancement of invasive
ability, at least, for MDA-MB-231 cells. In the present study, BER
dose-dependently inhibited the proliferation and IL-8 secretion of
MDA-MB-231 cells (Fig. 1B and C).
Furthermore, BER abrogated the increased invasion induced by IL-8.
Thus, BER counteracted the metastasis of MDA-MB-231 cells at least
partly in an IL-8 dependent manner.
A number of pathways have been found to be actively
involved in the modulation of IL-8 production, including MAPKs,
JAK2, and PI3K/Akt pathways (21,25,42–44).
In agreement with those studies, our results showed that the
activation of ERK1/2, SAPK/JNK, p38 MAPK, JAK2, p85 PI3K, AP-1 and
p65 NF-κB pathways was closely associated with the constitutive
IL-8 secretion in MDA-MB-231 cells (Fig. 1D). Correspondingly, BER was able to
modulate the activation of those molecules. Notably, BER only
deactivated JAK2, p85 PI3K and p65 NF-κB signaling but activated
that of MAPK pathway molecules (Fig.
6). Therefore, the inhibition of BER on IL-8 production might
occur mainly through JAK2/PI3K/NF-κB pathways.
IL-8 can activate PI3K, protein kinase B (PKB and
Akt), mammalian target of rapamycin (mTOR), ERK1/2, p38 MAPK and
JAK2 pathways to regulate numerous gene and protein expressions
involved in cell proliferation, survival, invasion and migration
(25,45). Activation of PI3K and JAK2 pathways
has been reported to modulate cell invasion and angiogenesis
(25). In experiments of the
present study, we found that BER inactivated the PI3K/Akt, JAK2 and
NF-κB pathways. The results also showed that many
metastasis-related genes, including MMP-2, EGF, E-cadherin, bFGF
and fibronectin, were downregulated by BER, suggesting the
anti-invasive effect of BER was partly, if not all, mediated in an
IL-8 dependent manner.
IL-8 was also found to be independent of cell
migration, cell cycle distribution and apoptosis of MDA-MB-231
cells. By contrast, BER was actively engaged in these processes,
which inhibited cell migration, and induced G2/M arrest and cell
apoptosis in an IL-8 independent manner.
The mitogen-activated protein kinases (MAPK)
pathways, i.e., ERK, SAPK/JNK and p38 MAPK, are actively involved
in drug-induced cell apoptosis of numerous cancer cells (46). Activation of JNK and p38 MAPK
pathways results in enhanced apoptosis induced by berberine in
human hepatoma and colon carcinoma cells (34,35).
When the MAPKs are inhibited, the apoptosis and caspase-3 cleavage
in tumor cells are abrogated (46–48).
Moreover, inhibitors of MAPKs slightly increase cell viability in
MDA-MB-231 cells (49). In the
present study, the phosphorylation of p38 MAPK and SAPK/JNK was
enhanced by BER treatment. In agreement with the previous studies
(31,34), the inhibitors of p38 MAPK and
SAPK/JNK attenuated the apoptosis enhanced by BER treatment in a
caspase-3-dependent manner, while the inhibitors of p38 MAPK and
SAPK/JNK alone showed no obvious effect on cell apoptosis in
MDA-MB-231 cells. Furthermore, anisomycin, an activator of p38 MAPK
and JNK, induced cell apoptosis and significantly elevated cell
apoptosis induced by BER in a caspase-3-dependent manner. Thus, the
activation of p38 MAPK and SAPK/JNK was involved in the cell
apoptosis induced by BER.
In breast cancer cells, activation of the PI3K/Akt
pathway has already been found to prevent cell apoptosis (27,36).
By contrast, inhibition of janus kinase 2 (JAK2) promotes cell
apoptosis (51,52). However, the effect of JAK2 on
apoptosis induced by BER remains to be clarified. In the present
study, BER inhibited the activation of JAK2, p85 PI3K and Akt by
reducing the phosphorylation of Jak2, p85 PI3K and Akt1. Moreover,
the inhibitors of NF-κB, PI3K and JAK2 enhanced the cell apoptosis
induced by BER, suggesting the involvement of these three pathways
in the BER-induced apoptosis of MDA-MB-231 cells. The gene
expression of total Jak2 and Akt1 was also decreased by BER.
Therefore, the enhanced apoptosis induced by BER resulted from
crosstalk among multiple cell signaling pathways including p38
MAPK, JNK, PI3K/Akt/NF-κB and JAK2.
In conclusion, BER inhibited cell metastasis partly
through the downregulation of IL-8 and enhanced cell apoptosis by
activating MAPKs and deactivating the JAK2/PI3K/Akt/NF-κB pathways.
This was different from other chemotherapeutic drugs that induce
apoptosis but simultaneously increase IL-8 expression, therefore,
promote cancer metastasis. Thus, BER showed simultaneous
anti-carcinoma in situ and anti-metastatic effects in
MDA-MB-231 cells, suggesting the effective and safe potential of
BER as a therapeutic candidate to treat highly metastatic breast
cancer.
Acknowledgements
The present study was supported by the Educational
Commission of Shanghai of China (2012JW19); the Key Research
Innovation Project (13ZZ099); the Key Project from the Department
of Education of China (20123107130002); the Shanghai Eastern
Scholar Program (2013–59); and the Excellent Research Team
Cultivation Program of Shanghai University of Traditional Chinese
Medicine.
Abbreviations:
|
BER
|
berberine hydrochloride
|
|
DMSO
|
dimethyl sulfoxide
|
|
PI
|
propidium iodine
|
|
FBS
|
fetal bovine serum
|
|
CCK-8
|
Cell Counting Kit-8
|
|
TBST
|
Tris-HCl-buffered saline with
Tween-20
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
|
PBS
|
phosphate-buffered saline
|
|
RT
|
room temperature
|
|
IL-8
|
interleukin-8
|
|
PCR
|
polymerase chain reaction
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
JAK
|
Janus-activated kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
SAPK/JNK
|
stress-activated protein kinase/c-Jun
N-terminal kinase
|
|
ERK
|
extracellular-regulated protein
kinases
|
|
NF-κB
|
nuclear factor κ-light-chain-enhancer
of activated B cells
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
PI3K
|
phosphatidylinositol 3 kinase
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
EGF
|
epidermal growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
Akt
|
serine/threonine kinase
|
|
AP-1
|
activator protein 1
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 62:10–29. 2012.
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R and
Tang XJ: Interleukin-8 associates with adhesion, migration,
invasion and chemosensitivity of human gastric cancer cells. World
J Gastroenterol. 18:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bünger S, Haug U, Kelly FM,
Klempt-Giessing K, Cartwright A, Posorski N, Dibbelt L, Fitzgerald
SP, Bruch HP, Roblick UJ, von Eggeling F, Brenner H and Habermann
JK; BMBF-Consortium ‘Colorectal Cancer Screening Chip’. Toward
standardized high-throughput serum diagnostics: multiplex-protein
array identifies IL-8 and VEGF as serum markers for colon cancer. J
Biomol Screen. 16:1018–1026. 2011.
|
|
5
|
Kitadai Y, Takahashi Y, Haruma K, Naka K,
Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y and Kajiyama G:
Transfection of interleukin-8 increases angiogenesis and
tumorigenesis of human gastric carcinoma cells in nude mice. Br J
Cancer. 81:647–653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuo Y, Ochi N, Sawai H, Yasuda A,
Takahashi H, Funahashi H, Takeyama H, Tong Z and Guha S: CXCL8/IL-8
and CXCL12/SDF-1alpha co-operatively promote invasiveness and
angiogenesis in pancreatic cancer. Int J Cancer. 124:853–861. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ju D, Sun D, Xiu L, Meng X, Zhang C and
Wei P: Interleukin-8 is associated with adhesion, migration and
invasion in human gastric cancer SCG-7901 cells. Med Oncol.
29:91–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohamed MM: Monocytes conditioned media
stimulate fibronectin expression and spreading of inflammatory
breast cancer cells in three-dimensional culture: a mechanism
mediated by IL-8 signaling pathway. Cell Commun Signal. 10:32012.
View Article : Google Scholar
|
|
9
|
Asfaha S, Dubeykovskiy AN, Tomita H, Yang
X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA,
Muthupalani S, Ericksen R, Frucht H, Fox JG and Wang TC: Mice that
express human interleukin-8 have increased mobilization of immature
myeloid cells, which exacerbates inflammation and accelerates colon
carcinogenesis. Gastroenterology. 144:155–166. 2013. View Article : Google Scholar
|
|
10
|
Shi HL, Wu XJ, Liu Y and Xie JQ: Berberine
counteracts enhanced IL-8 expression of AGS cells induced by
evodiamine. Life Sci. 93:830–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milovanovic J, Todorovic-Rakovic N and Abu
Rabi Z: The prognostic role of interleukin-8 (IL-8) and matrix
metalloproteinases -2 and -9 in lymph node-negative untreated
breast cancer patients. J BUON. 18:866–873. 2013.PubMed/NCBI
|
|
12
|
Hartman ZC, Poage GM, den Hollander P,
Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck
SG, Mills GB and Brown PH: Growth of triple-negative breast cancer
cells relies upon coordinate autocrine expression of the
proinflammatory cytokines IL-6 and IL-8. Cancer Res. 73:3470–3480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Todorović-Raković N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013.PubMed/NCBI
|
|
15
|
Freund A, Chauveau C, Brouillet JP, Lucas
A, Lacroix M, Licznar A, Vignon F and Lazennec G: IL-8 expression
and its possible relationship with estrogen-receptor-negative
status of breast cancer cells. Oncogene. 22:256–265. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freund A, Jolivel V, Durand S, Kersual N,
Chalbos D, Chavey C, Vignon F and Lazennec G: Mechanisms underlying
differential expression of interleukin-8 in breast cancer cells.
Oncogene. 23:6105–6114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T,
Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL,
Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB,
Johnson MD, Lippman M, Ethier S, Gazdar A and Gray JW: A collection
of breast cancer cell lines for the study of functionally distinct
cancer subtypes. Cancer Cell. 10:515–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Britschgi A, Andraos R, Brinkhaus H,
Klebba I, Romanet V, Mϋller U, Murakami M, Radimerski T and
Bentires-Alj M: JAK2/STAT5 inhibition circumvents resistance to
PI3K/Mtor blockade: a rationale for cotargeting these pathways in
metastatic breast cancer. Cancer Cell. 22:796–811. 2012. View Article : Google Scholar
|
|
19
|
Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q,
Zhu J, Chen JJ, Huang RC, Chen ZS and Huang RP: Enhanced
chemosensitization in multidrug-resistant human breast cancer cells
by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat.
135:737–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Juvekar A and Wulf GM: Closing escape
routes: inhibition of IL-8 signaling enhances the anti-tumor
efficacy of PI3K inhibitors. Breast Cancer Res. 15:3082013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collins TS, Lee LF and Ting JP: Paclitaxel
up-regulates interleukin-8 synthesis in human lung carcinoma
through an NF-kappaB-and AP-1-dependent mechanism. Cancer Immunol
Immunother. 49:78–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Larco JE, Wuertz BR, Manivel JC and
Furcht LT: Progression and enhancement of metastatic potential
after exposure of tumor cells to chemotherapeutic agents. Cancer
Res. 61:2857–2861. 2001.PubMed/NCBI
|
|
23
|
Lev DC, Onn A, Melinkova VO, Miller C,
Stone V, Ruiz M, McGary EC, Ananthaswamy HN, Price JE and Bar-Eli
M: Exposure of melanoma cells to dacarbazine results in enhanced
tumor growth and metastasis in vivo. J Clin Oncol. 22:2092–2100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kishida O, Miyazaki Y, Murayama Y, Ogasa
M, Miyazaki T, Yamamoto T, Watabe K, Tsutsui S, Kiyahara T,
Shimomura I and Shinomura Y: Gefitinib (Iressa, ZD1839) inhibits
SN38-triggered EGF signals and IL-8 production in gastric cancer
cells. Cancer Chemother Pharmacol. 55:584–594. 2005. View Article : Google Scholar
|
|
25
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang JX, Liu J, Wang J, He C and Li FP:
The extract of huanglian, a medicinal herb, induces cell growth
arrest and apoptosis by upregulation of interferon-beta and
TNF-alpha in human breast cancer cells. Carcinogenesis.
26:1934–1939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuo HP, Chuang TC, Tsai SC, Tseng HH, Hsu
SC, Chen YC, Kuo CL, Kuo YH, Liu JY and Kao MC: Berberine, an
isoquino-line alkaloid, inhibits the metastatic potential of breast
cancer cells via Akt pathway modulation. J Agric Food Chem.
60:9649–9658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan W, Li Y, Chen M and Wang Y: Berberine
hydrochloride: anticancer activity and nanoparticulate delivery
system. Int J Nanomed. 6:1773–1777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu HM, Peng QX, Liu TS, Yang DJ and Chen
XZ: Effect of retro-Zuojin pill on apoptosis and Bcl-2, Bax gene
expression in SGC-7901 cells. Chin J Exp Trad Med Form (Chin).
14:28–31. 2008.
|
|
30
|
Shi HL, Xie JQ and Wu DZ: Effect of
berberine on cell proliferation and IL-8 expression in AGS cells.
Pharmacol Clin Chin Mater Med (Chin). 28:45–48. 2012.
|
|
31
|
Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang
HI, Wang CJ, Yang SF, Liou YS and Kuo WH: Berberine induces
apoptosis in SW620 human colonic carcinoma cells through generation
of reactive oxygen species and activation of JNK/p38 MAPK and Fas
L. Arch Toxicol. 81:719–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hur JM, Hyun MS, Lim SY, Lee WY and Kim D:
The combination of berberine and irradiation enhances anti-cancer
effects via activation of p38 MAPK pathway and ROS generation in
human hepatoma cells. J Cell Biochem. 107:955–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo HP, Chuang TC, Yeh MH, Hsu SC, Way TD,
Chen PY, Wang SS, Chang YH, Kao MC and Liu JY: Growth suppression
of HER2-overexpressing breast cancer cells by berberine via
modulation of the HER2/PI3K/Akt signaling pathway. J Agric Food
Chem. 59:8216–8224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ho YT, Lu CC, Yang JS, Chiang JH, Li TC,
Ip SW, Hsia TC, Liao CL, Lin JG, Wood WG and Chung JG: Berberine
induced apoptosis via promoting the expression of caspase-8, -9 and
-3, apoptosis-inducing factor and endonuclease G in SCC-4 human
tongue squamous carcinoma cancer cells. Anticancer Res.
29:4063–4070. 2009.PubMed/NCBI
|
|
35
|
Eom KS, Kim HJ, So HS, Park R and Kim TY:
Berberine-induced apoptosis in human glioblastoma T98G cells is
mediated by endoplasmic reticulum stress accompanying reactive
oxygen species and mitochondrial dysfunction. Biol Pharm Bull.
33:1644–1649. 2010. View Article : Google Scholar
|
|
36
|
Xu LN, Lu BN, Hu MM, Xu YW, Han X, Qi Y
and Peng JY: Mechanisms involved in the cytotoxic effects of
berberine on human colon cancer HCT-8 cells. Biocell. 36:113–120.
2012.PubMed/NCBI
|
|
37
|
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason
GA, Christensen JG and Kerbel RS: Accelerated metastasis after
short-term treatment with a potent inhibitor of tumor angiogenesis.
Cancer Cell. 15:232–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paez-Ribes M, Allen E, Hudock J, Takeda T,
Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D and Casanovas O:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Britschgi A, Radimerski T and Bentires-Alj
M: Targeting PI3K, HER2 and the IL-8/JAK2 axis in metastatic breast
cancer: which combination makes the whole greater than the sum of
its parts? Drug Resist Updat. 16:68–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lai Y, Shen Y, Liu XH, Zhang Y, Zeng Y and
Liu YF: Interleukin-8 induces the endothelial cell migration
through the activation of phosphoinositide 3-kinase-Rac1/RhoA
pathway. Int J Biol Sci. 7:782–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shao N, Chen LH, Ye RY, Lin Y and Wang SM:
The depletion of interleukin-8 causes cell cycle arrest and
increases the efficacy of docetaxel in breast cancer cells. Biochem
Biophys Res Commun. 431:535–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chelouche-Lev D, Miller CP, Tellez C, Ruiz
M, Bar-Eli M and Price JE: Different signalling pathways regulate
VEGF and IL-8 expression in breast cancer: implications for
therapy. Eur J Cancer. 40:2509–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bezzerri V, Borgatti M, Finotti A,
Tamanini A, Gambari R and Cabrini G: Mapping the transcriptional
machinery of the IL-8 gene in human bronchial epithelial cells. J
Immunol. 187:6069–6081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Y, Wang W, Wang L, Wang X and Xia J:
Regulatory mechanisms of interleukin-8 production induced by tumour
necrosis factor-α in human hepatocellular carcinoma cells. J Cell
Mol Med. 16:496–506. 2012.
|
|
45
|
Burger M, Hartmann T, Burger JA and
Schraufstatter IU: KSHV-GPCR and CXCR2 transforming capacity and
angiogenic responses are mediated through a JAK2-3-dependent
pathway. Oncogene. 24:2067–2075. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Uehara N, Kanematsu S, Miki H, Yoshizawa K
and Tsubura A: Requirement of p38 MAPK for a cell-death pathway
triggered by vorinostat in MDA-MB-231 human breast cancer cells.
Cancer Lett. 315:112–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS,
Jung JS and Kim JM: Role of ERK activation in cisplatin-induced
apoptosis in OK renal epithelial cells. J Appl Toxicol. 25:374–382.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jo SK, Cho WY, Sung SA, Kim HK and Won NH:
MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by
decreasing inflammation and apoptosis. Kidney Int. 67:458–466.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jayasooriya RGPT, Moon DO, Park SR, Choi
YH, Asami Y, Kim MO, Jang JH, Kim BY, Ahn JS and Kim GY: Combined
treatment with verrucarin A and tumor necrosis factor-α sensitizes
apoptosis by overexpression of nuclear factor-kappaB-mediated Fas.
Envir Toxicol Pharmacol. 36:303–310. 2013.
|
|
50
|
Parada E, Egea J, Romero A, del Barrio L,
García AG and López MG: Poststress treatment with PNU282987 can
rescue SH-SY5Y cells undergoing apoptosis via α7 nicotinic
receptors linked to a Jak2/Akt/HO-1 signaling pathway. Free Radic
Biol Med. 49:1815–1821. 2010.PubMed/NCBI
|
|
51
|
Chen Q, Lu Z, Jin Y, Wu Y and Pan J:
Triptolide inhibits Jak2 transcription and induces apoptosis in
human myeloproliferative disorder cells bearing Jak2V617F through
caspase-3-mediated cleavage of Mcl-1. Cancer Lett. 291:246–255.
2010. View Article : Google Scholar
|
|
52
|
Will B, Siddiqi T, Jorda MA, Shimamura T,
Luptakova K, Staber PB, Costa DB, Steidl U, Tenen DG and Kobayashi
S: Apoptosis induced by JAK2 inhibition is mediated by Bim and
enhanced by the BH3 mimetic ABT-737 in JAK2 mutant human erythroid
cells. Blood. 115:2901–2909. 2010. View Article : Google Scholar : PubMed/NCBI
|























