Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies in China due to the high prevalence of chronic
HBV infection (1,2). According to the data published by the
Chinese Society of Liver Cancer in 2009, HCC in China accounts for
~55 and 45% of the annual new cases of and deaths attributed to
global HCC, respectively (3).
Despite the fact that surgical resection, transplantation, local
ablation (percutaneous ethanol injection, radiofrequency ablation,
percutaneous acetic acid injection and microwave ablation, and
transcatheter arterial chemoembolization) and immunotherapy are
potentially curative modalities (4), many patients with HCC cannot be
treated by these therapies due to the metastasis of cancer cells,
severe adverse effects of antineoplastic drugs, or the expensive
costs. Seeking alternative therapies to improve the curative rate
of HCC has been an urgent task for oncologists.
Traditional Chinese medicine (TCM) herbs are widely
used as complementary and alternative medicines for cancers in
China (5–9). The underlying anticancer mechanisms of
TCM have been extensively investigated for several decades. TCM can
induce cell apoptosis and differentiation, inhibit cell division
and angiogenesis, and promote immune function (reviewed in refs.
10,11). In addition, several randomized
controlled trials have shown that TCM therapy can improve the
quality of life of patients and alleviate chemoradiotherapy-induced
adverse effects (12). TCM is
considered as a valuable addition to standard cancer therapy,
despite the fact that the ingredients of TCM are not completely
understood.
According to the TCM theory, the combined use of
several herbs may have synergic effects by acting on multiple
targets. Typically, a polyherbal formula has a principal component,
and others serve as adjuvant agents to enhance the pharmacological
actions or facilitate the delivery of the principal component. This
principle of formulation has been practiced for more than 5,000
years and is unanimously accepted by TCM physicians. For example,
Realgar-Indigo naturalis formula has been verified to be
effective in treating human acute promyelocytic leukemia (APL). The
formula is composed of Realgar-Indigo naturalis, Salvia
milliorrhizae and Radix psudostellariae. In this
polyherbal formula, Realgar is a principal element and is capable
of clearing heat and removing toxicity to fight APL cells.
Indigo naturalis can cool the blood and detoxify the body.
Salvia milliorrhizae and Radix psudostellariae have
the ability to tonify Qi and invigorate blood circulation. The
latter three herbs enhance the effect of Realgar in clearing heat
and removing toxicity. Indeed, a pharmacological study showed that
the formula has the strongest effect on fighting cancer cells
compared to Realgar alone or in combination with one of the other
three herbs (13).
Jiedu Xiaozheng Yin (JXY) is a polyherbal decoction
to treat HCC and is composed of Hedyotis diffusa Willd
(HDW), Sophora flavescens (SF), Prunella and
Pseudobulbus Cremastrae (PC). In this compound decoction,
HDW is the principal component intended to clear heat and toxins
and resolve hard mass (including cancer). Prunella acts to
clear liver fire. SF and PC can assist HDW to clear heat, detoxify
the body and resolve hard mass. Pharmacological studies have shown
that the four herbs are capable of inducing apoptosis and
inhibiting proliferation and angiogenesis of tumor cells (14–17). A
randomized control trial showed that addition of JXY to standard
treatment of stage III HCC patients can improve the immune function
of patients, decrease recurrence and increase overall survival
(18). Experimental studies have
also demonstrated that JXY can inhibit the angiogenesis of tumors
via downregulation of VEGF-A and VEGFR-2 expression (19) and inhibit tumor cell proliferation
via induction of G0/G1 phase arrest (9). In addition, we also found that JXY can
downregulate the expression of cancer stem cell-related markers
CD133 and c-kit (20). In the
present study, we investigated whether the antitumor effects of JXY
involve the Wnt/β-catenin pathway and the polycomb gene product,
which are important regulators of cancer stem cells (21,22).
Materials and methods
EE-JXY preparation
JXY is composed of HDW (30 g), Prunella (15
g), PC (15 g) and SF (15 g). The ethyl acetate extract from JXY
(EE-JXY) was prepared. Briefly, JXY (7.5 kg) was refluxed with 75%
ethanol for 2 times, 3 h each time. The extract was pooled. The
alcohol was removed under vacuum using a rotary evaporator. The
residue was dissolved with water. The solution was partitioned
sequentially with petroleum ether, chloroform, ethyl acetate and
n-BuOH. The extract was evaporated in vacuum and stored at 4°C
prior to use. EE-JXY was diluted using dimethyl sulfoxide (DMSO)
into 200 mg/ml for the in vitro experiments. For the in
vivo study, EE-JXY was dissolved in normal saline to a final
concentration of 6 mg/ml.
Cell lines and culture
Human hepatocellular carcinoma PLC/PRF/5 and Huh7
cell lines were purchased from the Shanghai Institute of Life
Science, Chinese Academy of Sciences (Shanghai, China), and grown
in high glucose Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal calf serum (both from Gibco, Carlsbad, CA,
USA).
Cellular growth assay
The growth of cells was evaluated using the methyl
thiazolyl tetrazolium (MTT) method. Briefly, adherent PLC/PRF/5 or
Huh7 cells in 96-well plates were treated with EE-JXY with final
concentrations of 0 (control group), 0.0625, 0.125, 0.25 and 0.5
mg/ml for 24, 48 and 72 h, respectively. Then the culture medium
was discarded and MTT (Invitrogen-Life Technologies, Carlsbad, CA,
USA) was added. After incubation for another 4 h, the purple-blue
MTT formazan precipitate was dissolved using DMSO. The OD was
measured at 570 nm. Cell growth was represented by cell viability
as: Cell viability (%) = average ODJXY group/average
ODcontrol group × 100%.
Flat colony formation assay
After cells were exposed to 0.25 mg/ml EE-JXY for 24
h, cells were detached by trypsin, seeded in a 6-well plate at a
final concentration of 3×103 cells/ml and incubated for
10 days. Then colony formation was examined using a crystal violet
cell colony staining kit (Genmed Scientifics Inc., Manassas, VA,
USA) according to the manufacturer’s instructions. The colony
formation ability was evaluated via the OD value at 570 nm.
Mouse xenograft model
Six-week old female BALB/c nu/nu mice were housed at
23±2°C in a humidified pathogen-free facility. Food and water were
provided ad libitum. Huh7 cells at a concentration of
5×106 cells/ml were mixed with Matrigel basement
membrane matrix (1:1; BD Biosciences, Franklin Lakes, NJ, USA) on
ice. The 200-μl cell mixture was injected subcutaneously. When the
tumor volume (TV) reached ~100 mm3, the mice were
randomized into two groups and received 0.13 g/kg/day EE-JXY and
normal saline, respectively by gorge. After 3 weeks, the tumors
were removed for polymerase chain reaction (PCR),
immunofluorescence (IF) and immunohistochemistry (IHC) assays. All
procedures on treating mice were performed according to the Animal
Care Guidelines issued by the Ministry of Science and Technology of
P.R. China, and the Animal Care Committee of Fujian University of
Traditional Chinese Medicine approved our protocol.
Reverse transcription-PCR (RT-PCR)
assay
Total RNA from the tumor xenografts was extracted
and subjected to reverse transcription according to the
manufacturer’s recommended protocol (Invitrogen-Life Technologies
and Promega, Madison, WI, USA, respectively). A 20-μl RT-PCR
reaction mixture contained 10 μl of 2X Taq MasterMix (containing
dNTPs, DNA polymerase, buffer, Mg2+), 400 nmol/l of each
primer and 0.4 μg template DNA. Amplification conditions were as
follows: 2 min at 94°C followed by 35 cycles for 30 sec at 94°C, 30
sec at 55°C and 30 sec at 72°C. The primer pairs are shown in
Table I. The amplified products
were size-fractionated on 1.5% agarose gel and detected by a gel
imaging system (Bio-Rad, Hercules, CA, USA). The mRNA levels of
samples were normalized with that of GAPDH as follows: mRNA level =
(gray-scale value)sample/(gray-scale
value)GAPDH.
 | Table IPrimer sequences of cyclin D1, c-myc,
β-catenin, Bmi1, p16INK4A and GAPDH. |
Table I
Primer sequences of cyclin D1, c-myc,
β-catenin, Bmi1, p16INK4A and GAPDH.
| Name | Length (bp) | Forward | Reverse |
|---|
| Cyclin D1 | 496 | 5′-CAT CCC AAT GTT
GTC CG-3′ | 5′-GCA GCC CAA TCA
GGT CA-3′ |
| c-myc | 244 | 5′-AGA GAA GCT GGC
CTC CTC CTA CC-3′ | 5′-CGT CGA GGA GAG
CAG AGA AT-3′ |
| GAPDH | 257 | 5′-AGA AGG CTG GGG
CTC ATT TG-3′ | 5′-AGG GGC CAT CCA
CAG TCT TC-3′ |
| β-catenin | 365 | 5′-GCT GAT TTG ATG
GAG TTG GAC ATG G-3′ | 5′-GCC AAA CGC TGG
ACA TTA GTG G-3′ |
| Bmi1 | 271 | 5′-CCA GGG CTT TTC
AAA AAT GA-3′ | 5′-GCA TCA CAG TCA
TTG CTG CT-3′ |
|
p16INK4A | 162 | 5′-CTT CCT GGA CAC
GCT GGT-3′ | 5′-GCA TGG TTA CTG
CCT CTG GT-3′ |
IF analysis
The cells were seeded in a 96-well plate for 24 h.
After treatment with 0 and 0.25 mg/ml EE-JXY, the cells were fixed
with 4% paraformaldehyde for 10 min and incubated in blocking
buffer containing Triton X-100 for 20 min. The rabbit
anti-β-catenin and Bmi1 antibodies (1:250, ab32572 and ab38295;
Abcam, Cambridge, MA, USA) were added and incubated at 4°C
overnight. After rinsing in PBS 3 times for 10 min, the cells were
incubated in the Alexa Fluor 555-labeled donkey anti-rabbit
secondary antibody (1:1,000, A0453; Beyotime, Shanghai, China) for
2 h in dark. After rinsing another 3 times, 300 ng/ml
4′,6-diamidino-2-phenylindole (DAPI; C1006; Beyotime, Shanghai,
China) was added to the wells. After 5 min, fluorophore was
detected using a high content analysis system (BD Biosciences).
IHC analysis
The tumor xenografts were paraformaldehyde-fixed,
and embedded in paraffin following standard protocols. The
deparaffined tumor sections were subjected to immunostaining for
proliferating cell nuclear antigen (PCNA), c-myc and cyclin D1 with
appropriate antibodies (Maxin, MAB-0145, RMA-0664 and RMA-0541;
Fuzhou, Fujian, China). The average percentage of positive cells
was determined by counting the brown-colored cells under a
microscope (Leica Microsystems, Bensheim, Germany).
Statistical analysis
Data were analyzed and processed using the SPSS 18.0
statistical software, and are presented as the mean ± standard
deviation (SD) of at least three samples. Statistical comparisons
between two groups were performed using the independent samples
t-test, and between multiple groups were analyzed by one-way ANOVA
analysis. A P-value <0.05 was considered significant.
Results
EE-JXY inhibits the proliferation of HCC
cells in vitro
Our previous study demonstrated that EE-JXY inhibits
the proliferation of HepG2 cells (9). To further evaluate the anticancer
potential of EE-JXY for HCC, we investigated the effects of EE-JXY
on the proliferation of Huh7 and PLC/PRF/5 cells by MTT assay.
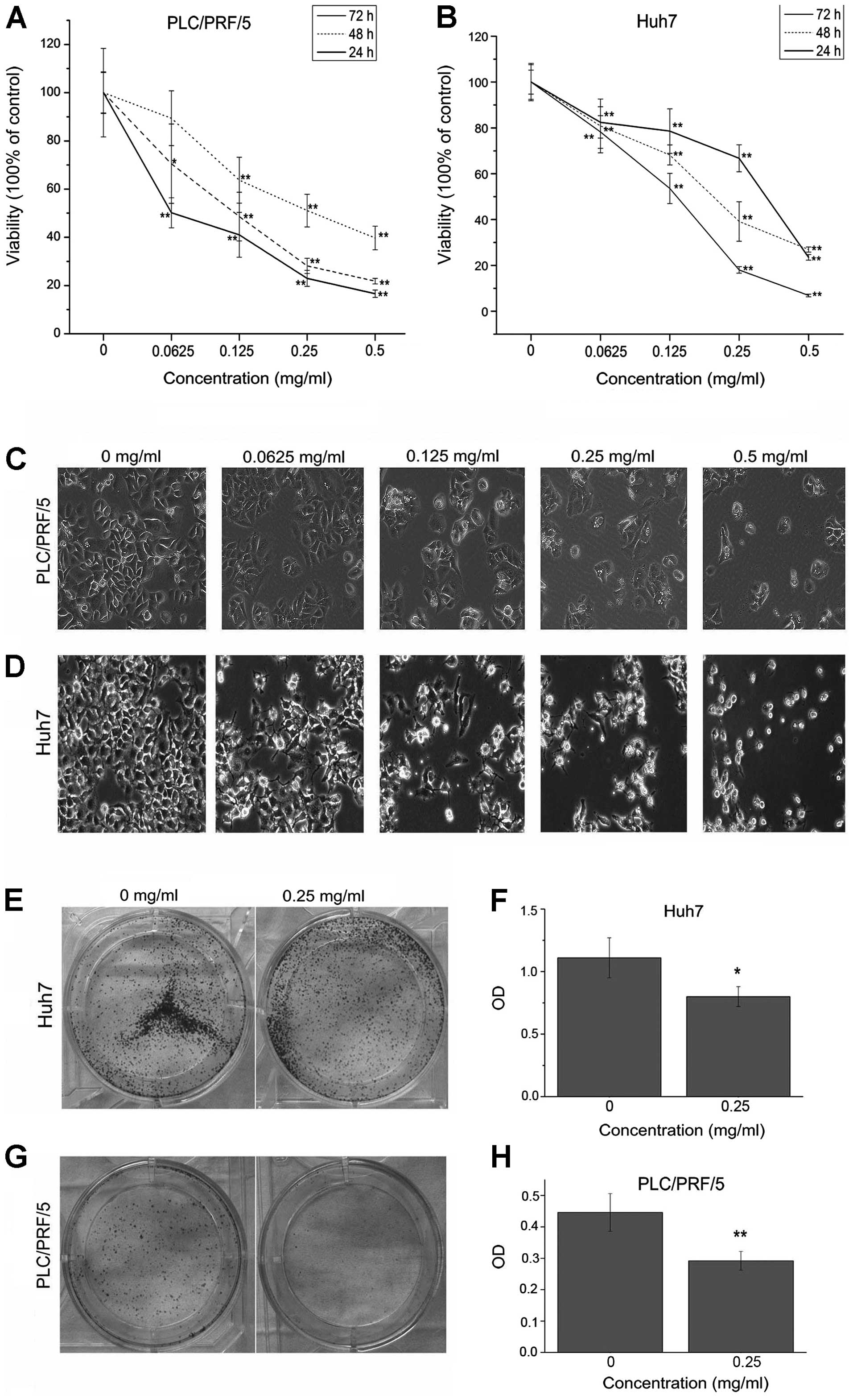
EE-JXY inhibited the proliferation of both HCC cell lines in dose-
and time-dependent manners (Fig. 1A and
B). The half maximal inhibitory concentration (IC50)
was estimated to be 0.29 mg/ml for both Huh7 and PLC/PRF/5 cells at
24 h, which is similar to our previous finding for HepG2 cells
(0.30 mg/ml) (9). Cells exposed to
EE-JXY showed marked morphological changes: originally polygon- or
spindle-shaped cells became round and collapsed, and many vacuoles
appeared in the cytoplasm (Fig. 1C and
D).
Colony formation assay have been used to identity
cancer stem cells in vitro (23). We next evaluated the colony
formation capacity of Huh7 and PLC/PRF/5 cells under the treatment
of EE-JXY. Colony formation assay showed that the numbers of
colonies formed by both EE-JXY-treated Huh7 and EE-JXY-treated
PLC/PRF/5 cells were significantly fewer than the number in the
untreated cells (Fig. 1E–H). Taken
together, these data revealed that a significant growth inhibition
was exerted by EE-JXY on the two HCC cell lines and possibly on the
cancer stem cells.
In the following experiments, Huh7 cells were used
to evaluate the anticancer mechanisms of EE-JXY.
EE-JXY regulates the Wnt/β-catenin
signaling pathway
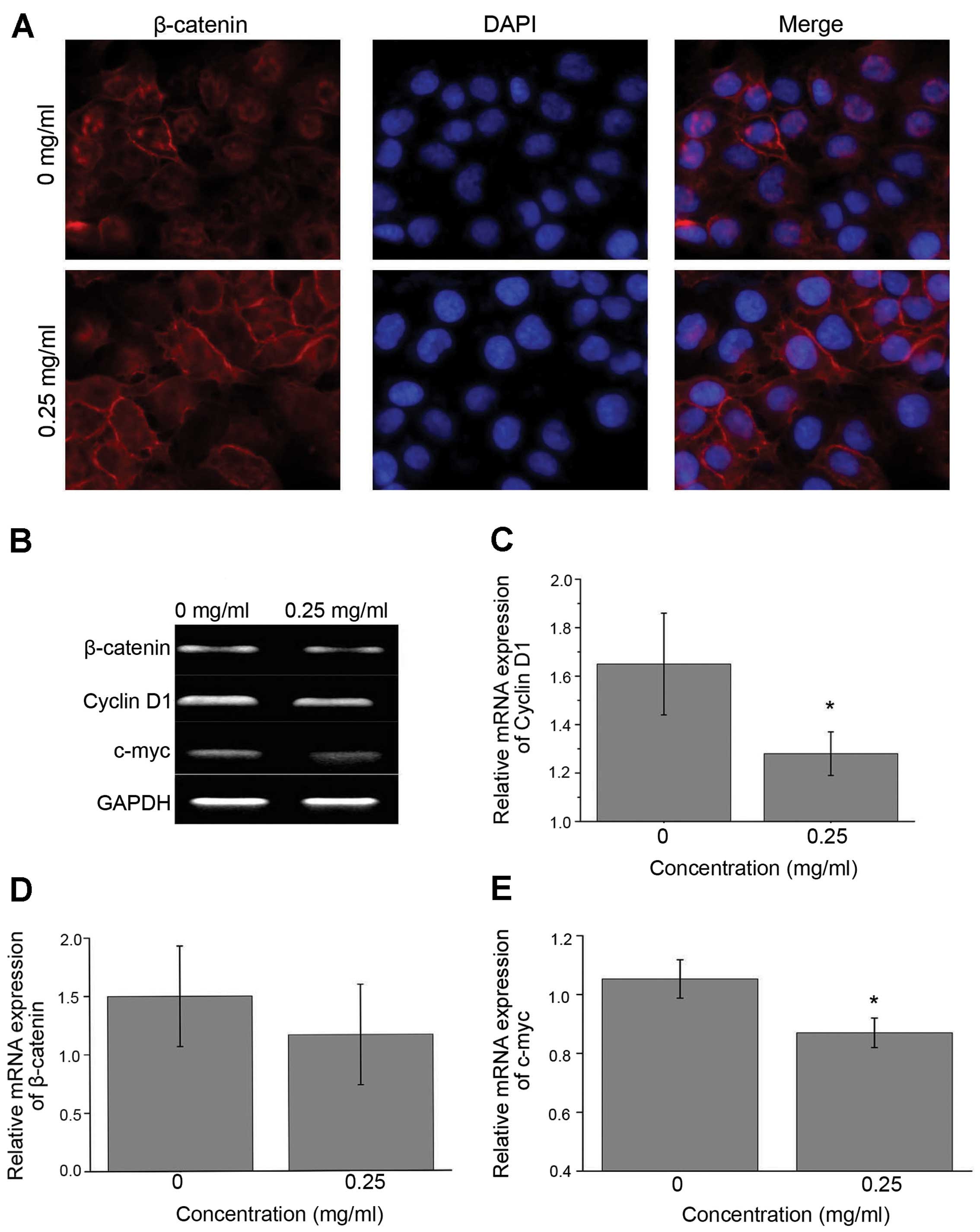
IF analysis showed that β-catenin mostly accumulated
in the cytoplasm or nuclei in the untreated Huh7 cells (Figs. 2A and 3A). After treated with EE-JXY, β-catenin
was mostly observed in the cytomembrane. Obviously, EE-JXY could
facilitate β-catenin translocation to the cytomembrane.
Interestingly, mRNA expression of β-catenin was not affected by
EE-JXY. We then investigated the mRNA expression levels of
β-catenin downstream genes c-myc and cyclin D1. The mRNA levels
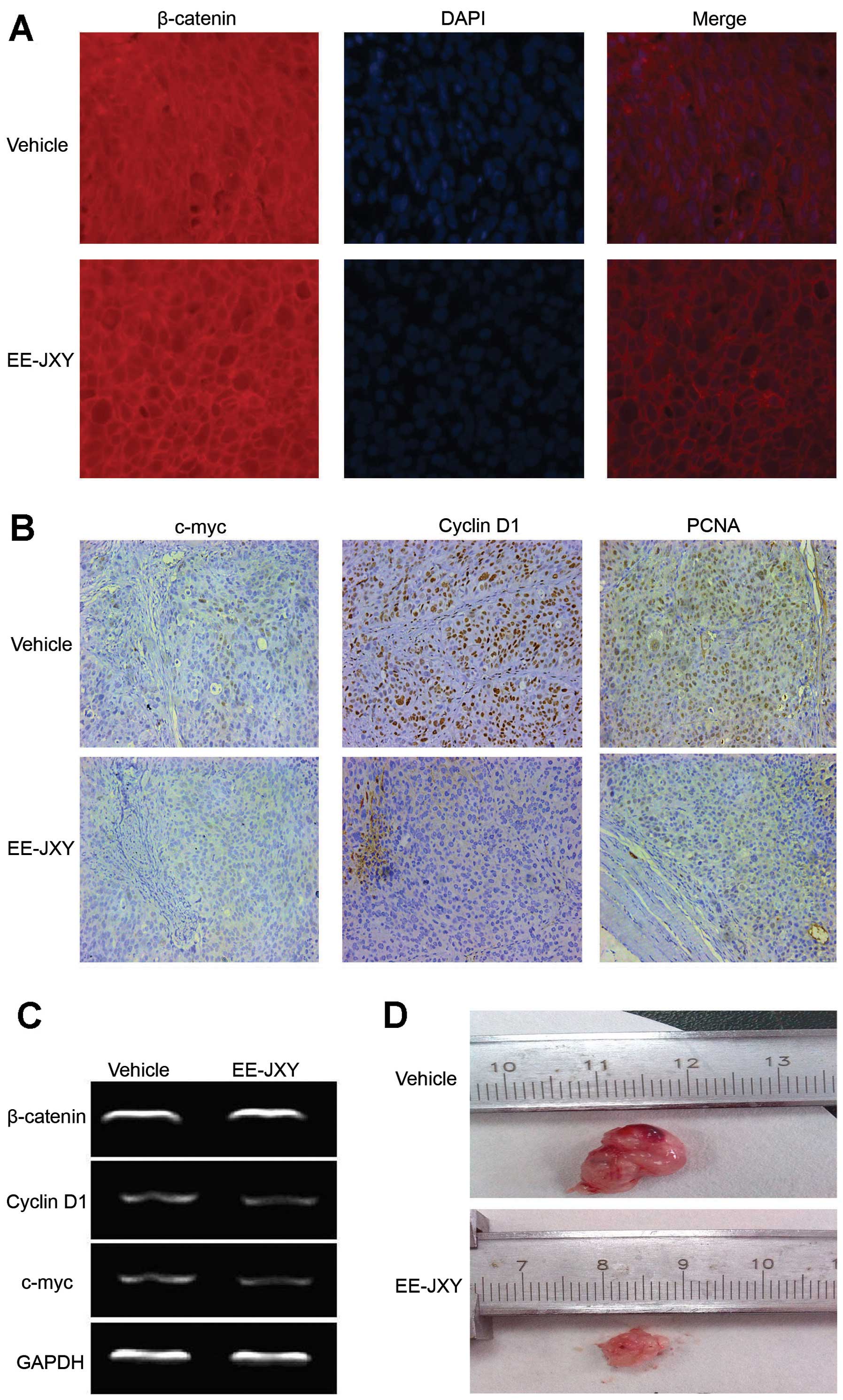
were markedly decreased in the EE-JXY-treated cells (Figs. 2B–E and 3C). IHC staining of tumor tissues from the
tumor-bearing mice also showed that the percentages of
c-myc-positive and cyclin D1-positive cells were decreased in the
EE-JXY-treated mice compared with the control (Fig. 3B).
As PCNA is an indicator of cell proliferation and
regulated by cyclin D1 (24,25),
we evaluated the expression of PCNA in tumor tissues. The findings
demonstrated that PCNA protein expression was inhibited in the
EE-JXY-treated mice compared with the control, which is consistent
with the smaller tumor volume in the EE-JXY-treated mice (Fig. 3B and D).
EE-JXY downregulates Bmi1 and upregulates
p16INK4A expression
As the polycomb group (PcG) transcriptional
repressor Bmi1 is a key regulator in liver cancer cell
proliferation (26–29), we next examined the effect of EE-JXY
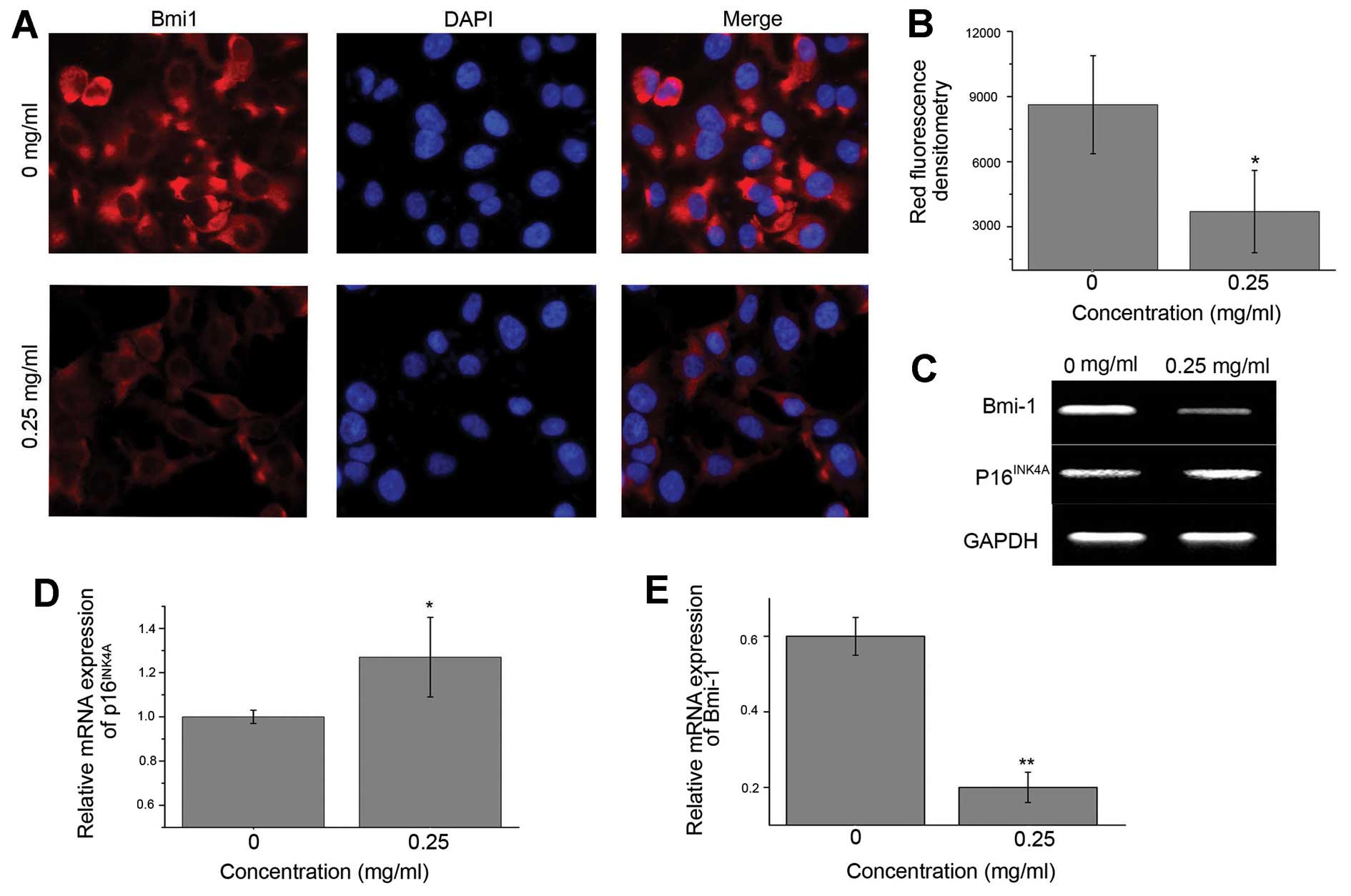
on expression of Bmi1 by IF. Huh7 cells treated with EE-JXY were
observed under a fluorescence microscope (Fig. 4A). Results showed that the
fluorescence intensity of Bmi1 in the EE-JXY-treated cells was
lower than that in the vehicle-treated cells (Fig. 4B), indicating that EE-JXY
downregulated the expression of Bmi1. The result was further
confirmed by RT-PCR (Fig. 4C and
E).
The cyclin-dependent kinase inhibitor 2A (CDKN2A)
gene product, p16INK4A, is a tumor-suppressor protein
that inhibits cyclin dependent kinases 4 and 6. p16INK4A
is regulated by Bmi (30). As
silencing of p16INK4A is a common event in HCC (31), we investigated the expression of
p16INK4A in the EE-JXY-treated cells. As expected,
expression of p16INK4A was upregulated in the
EE-JXY-treated cells (Fig. 4C and
D). Most importantly, similar results were observed in the
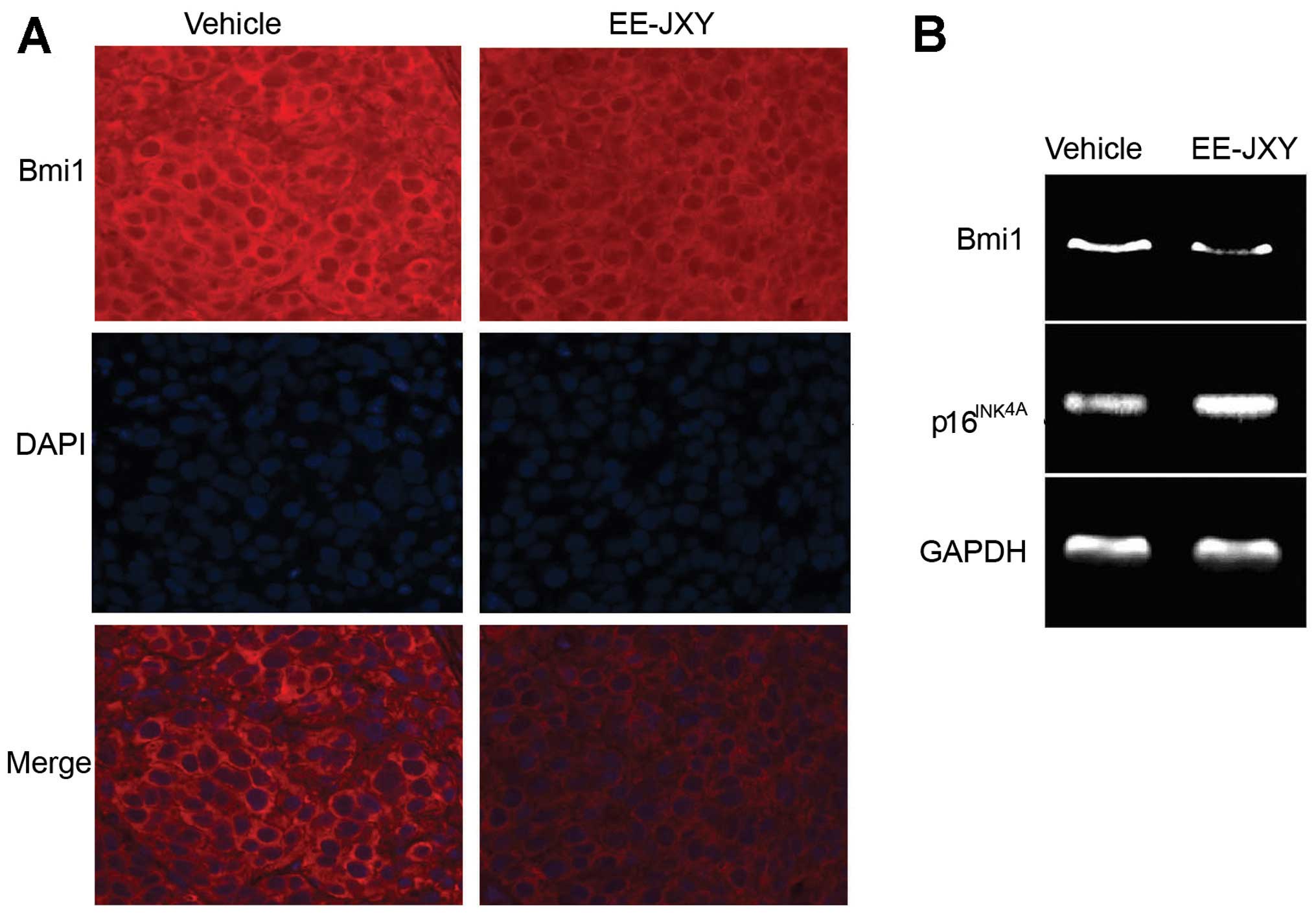
tumor tissues from mice treated with EE-JXY (Fig. 5).
Discussion
In the present study we demonstrated that JXY
suppressed the proliferation of human hepatoma cells (PLC/PRF/5 and
Huh7) at least partially by inhibiting the polycomb gene product
Bmi1 and Wnt/β-catenin signaling. Due to the safety profile of JXY,
JXY is a valuable adjuvant therapy for standard cancer therapy in
patients with HCC.
The main members of the Wnt/β-catenin signalng
pathway include Wnt (an extracellular factor rich in cysteine),
frizzled (a transmembrane protein) and β-catenin (a cytoplasmic
protein) (22,32–35).
In the absence of Wnt, β-catenin normally locates adjacent to cell
membranes. When it forms a β-catenin destruction complex with
adenomatous polyposis coli (APC), axin and glycogen synthase
kinase-3β (GSK-3β), β-catenin is phosphorylated and degraded.
Therefore, β-catenin normally remains at a lower level in cells. In
the presence of the Wnt signal, Wnt associates with frizzled,
unphosphorylated β-catenin translocates from cell membranes to the
cytoplasm and subsequently to the nucleus, where it activates
various downstream events involved in cell apoptosis and
proliferation, such as cyclin D1, c-myc and E-cadherin (34,35).
The Wnt/β-catenin signaling pathway is involved in
the carcinogenesis and metastasis of a number of human cancers,
such as colorectal carcinoma, breast cancer and HCC (22,31–33,36).
Cui et al (37) found
overexpression and abnormal accumulation in the cytoplasm or nuclei
of β-catenin in HCC. Not surprisingly, pharmacological agents
inhibiting the Wnt/β-catenin pathway such as sorafenib can inhibit
the growth of HCC cells (38).
Berberine from Coptis chinensis Franch was also reported to
inhibit β-catenin/Tcf4 reporter activity and exert an
antineoplastic effect (39). In the
present study, cells treated with EE-JXY showed less nuclear
accumulation of β-catenin, which may be responsible for reduced
c-myc and cyclin D1 gene and protein expression in vitro and
in vivo, and subsequent decreased expression of PCNA
(40) and cell proliferation. One
possible ingredient responsible for this effect is matrine in SF.
Matrine was reported to regulate the Wnt/β-catenin signaling
pathway in human acute erythroleukemia cell line TF-1 (41) and hepatic precancerous lesions
(42).
PcG proteins are a group of transcriptional
repressors regulating targeted gene expression through chromatin
modifications. The PcG proteins have two core multiprotein
complexes, polycomb repressive complex (PRC)-1 and -2 (43,44).
Bmi1, one member of PRC-1, has been shown to function as an
oncogene in multiple tumor types (43,45,46).
It regulates cell proliferation, apoptosis and senescence by
repressing downstream p16INK4A and p14ARF,
which are suppressor genes of the INK4A/ARF locus (43,45,46).
Overexpression of Bmil has been reported to be associated with the
progression and aggressive biological behavior of many cancers,
including HCC (47–50). Therefore Bmi1 has been a target for
anticancer drug development (51).
In this study, decreased expression of Bmi1 and increased
expression of p16INK4A were observed in the
EE-JXY-treated cells, supporting our previous finding that EE-JXY
may regulate HCC cell proliferation via the cyclin D-CDK4 pathway
(9).
In addition to inhibiting HCC cell proliferation and
colony formation in vitro, EE-JXY markedly suppressed the
expression of PCNA in vivo, an indicator of cell
proliferation. As our previous study showed that EE-JXY has a
strong antitumor effect in HepG2 tumor-bearing mice (9), we did not evaluate the anticancer
effect of EE-JXY by comparing the tumor volumes of the
EE-JXY-treated mice and the control mice, which requires a higher
number of mice. To spare the animals, only 2 mice were used in each
group in the present study. Notably, with the dose used in our
previous study (9), stronger
inhibition of PCNA expression and a smaller tumor volume were
observed in the EE-JXY-treated mice.
In conclusion, EE-JXY inhibits the proliferation of
PLC/PRF/5 and Huh7 cells at least in part by suppressing the Bmi1
and Wnt/β-catenin signaling pathways. Although the knowledge of the
mechanism of EE-JXY action is still limited, EE-JXY may have
multiple anticancer mechanisms that may deal with multiple targets
in cancer cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81102582 and 81302954),
the Project B of Fujian Province Education Department (no.
JB13101), and the Natural Science Foundation of Fujian Province
(no. 2013J01335).
Abbreviations:
|
EE-JXY
|
ethyl acetate extract from Jiedu
Xiaozheng Yin
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
HCC
|
hepatocellular carcinoma
|
|
TCM
|
traditional Chinese medicine
|
|
APL
|
acute promyelocytic leukemia
|
|
HDW
|
Hedyotis diffusa Willd
|
|
SF
|
Sophora flavescens
|
|
PC
|
Pseudobulbus cremastrae
|
|
DMSO
|
dimethyl sulfoxide
|
|
IF
|
immunofluorescence
|
|
IHC
|
immunohistochemistry
|
|
PcG
|
polycomb group
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
DAPI
|
4,6-diamidino-2-phenylindole
|
|
PCNA
|
proliferating cell nuclear antigen
|
References
|
1
|
Chen Y, Yu D, Zhang W, Qiu C, Xiang G, Dai
W, Wu S and Wang X: HBV subgenotype C2 infection, A1762T/G1764A
mutations may contribute to hepatocellular carcinoma with cirrhosis
in Southeast China. Iran J Public Health. 41:10–18. 2012.PubMed/NCBI
|
|
2
|
Gao JD, Shao YF, Xu Y, Ming LH, Wu ZY, Liu
GT, Wang XH, Gao WH, Sun YT, Feng XL, Liang LM, Zhang YH and Sun
ZT: Tight association of hepatocellular carcinoma with HBV
infection in North China. Hepatobiliary Pancreat Dis Int. 4:46–49.
2005.PubMed/NCBI
|
|
3
|
Ye SL: Expert consensus on standardization
of the management of primary liver cancer. Zhonghua Gan Zang Bing
Za Zhi. 17:403–410. 2009.(In Chinese).
|
|
4
|
Song MJ and Bae SH: Newer treatments for
advanced hepatocellular carcinoma. Korean J Intern Med. 29:149–155.
2014. View Article : Google Scholar
|
|
5
|
Hu M, Zhao M, An C, Yang M, Li Q, Zhang Y,
Suetsugu A, Tome Y, Yano S, Fu Y, Hoffman RM and Hu K: Real-time
imaging of apoptosis induction of human breast cancer cells by the
traditional Chinese medicinal herb Tubeimu. Anticancer Res.
32:2509–2514. 2012.PubMed/NCBI
|
|
6
|
Tang WM, Chan E, Kwok CY, Lee YK, Wu JH,
Wan CW, Chan RY, Yu PH and Chan SW: A review of the anticancer and
immunomodulatory effects of Lycium barbarum fruit.
Inflammopharmacology. 20:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park BH, Jung KH, Son MK, Seo JH, Lee HS,
Lee JH and Hong SS: Antitumor activity of Pulsatilla koreana
extract in anaplastic thyroid cancer via apoptosis and
anti-angiogenesis. Mol Med Rep. 7:26–30. 2013.
|
|
8
|
Cao Z, Liao L, Chen X, Lan L, Hu H, Liu Z,
Chen L, Huang S and Du J: Enhancement of antitumor activity of
low-dose 5-fluorouracil by combination with Fuzheng-Yiliu granules
in hepatoma 22 tumor-bearing mice. Integr Cancer Ther. 12:174–181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol.
42:202–210. 2013.PubMed/NCBI
|
|
10
|
Wong R, Sagar CM and Sagar SM: Integration
of Chinese medicine into supportive cancer care: A modern role for
an ancient tradition. Cancer Treat Rev. 27:235–246. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo-or radio-therapy for cancer. Biosci Trends. 4:297–307.
2010.PubMed/NCBI
|
|
12
|
Li X, Yang G, Li X, Zhang Y, Yang J, Chang
J, Sun X, Zhou X, Guo Y, Xu Y, Liu J and Bensoussan A: Traditional
Chinese medicine in cancer care: a review of controlled clinical
studies published in Chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, Chen SJ and Chen Z:
Dissection of mechanisms of Chinese medicinal formula
Realgar-Indigo naturalis as an effective treatment for
promyelocytic leukemia. Proc Natl Acad Sci USA. 105:4826–4831.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo YJ, Yang JS, Lu CC, Chiang SY, Lin JG
and Chung JG: Ethanol extract of Hedyotis diffusa willd
upregulates G0/G1 phase arrest and induces apoptosis in human
leukemia cells by modulating caspase cascade signaling and altering
associated genes expression was assayed by cDNA microarray. Environ
Toxicol. Mar 28–2014.(Epub ahead of print). View Article : Google Scholar
|
|
15
|
Chang C, Liu SP, Fang CH, He RS, Wang Z,
Zhu YQ and Jiang SW: Effects of matrine on the proliferation of
HT29 human colon cancer cells and its antitumor mechanism. Oncol
Lett. 6:699–704. 2013.PubMed/NCBI
|
|
16
|
Feng L, Jia X, Zhu M, Chen Y and Shi F:
Chemoprevention by Prunella vulgaris L. extract of non-small
cell lung cancer via promoting apoptosis and regulating the cell
cycle. Asian Pac J Cancer Prev. 11:1355–1358. 2010.
|
|
17
|
Dong H, Guo S, Wang C, Yang J and Xiao P:
Advances in studies on chemical constituents in plants of
Pseudobulbus Cremastrae seu Pleiones and their
pharmacological activities. Zhong Cao Yao. 38:1734–1738. 2007.(In
Chinese).
|
|
18
|
Chen LW, Lin J, Chen W and Zhang W: Effect
of Chinese herbal medicine on patients with primary hepatic
carcinoma in III stage during perioperational period: a report of
42 cases. Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:832–834. 2005.(In
Chinese).
|
|
19
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Jiedu Xiaozheng Yin, a
Chinese herbal formula, inhibits tumor angiogenesis via
down-regulation of VEGF-A and VEGFR-2 expression in vivo and
in vitro. Oncol Rep. 29:1083–1086. 2013.PubMed/NCBI
|
|
20
|
Cao Z, Chen X, Lin Y and Du J: Effect of
Chinese compound prescription on expressions of c-kit and CD133 of
tumor stem cell in mice of hepatocellular carcinoma transplanted
subcutaneously. Fujian J Tradit Chin Med. 20:18–21. 2010.(In
Chinese).
|
|
21
|
Chiba T, Miyagi S, Saraya A, Aoki R, Seki
A, Morita Y, Yonemitsu Y, Yokosuka O, Taniguchi H, Nakauchi H and
Iwama A: The polycomb gene product Bmi1 contributes to the
maintenance of tumor-initiating side population cells in
hepatocellular carcinoma. Cancer Res. 68:7742–7749. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on Cancer Stem Cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar
|
|
24
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D’Amico M, Pestell R and Ben-Ze’ev A: The cyclin D1
gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci
USA. 96:5522–5527. 1999.
|
|
25
|
Pagano M, Theodoras AM, Tam SW and Draetta
GF: Cyclin D1-mediated inhibition of repair and replicative DNA
synthesis in human fibroblasts. Genes Dev. 8:1627–1639. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Yang Z, Song W, Zhou L, Li Q, Tao K,
Zhou J, Wang X, Zheng Z, You N, Dou K and Li H: Overexpression of
Bmi-1 contributes to the invasion and metastasis of hepatocellular
carcinoma by increasing the expression of matrix metalloproteinase
(MMP)-2, MMP-9 and vascular endothelial growth factor via the
PTEN/PI3K/Akt pathway. Int J Oncol. 43:793–802. 2013.PubMed/NCBI
|
|
27
|
Effendi K, Mori T, Komuta M, Masugi Y, Du
W and Sakamoto M: Bmi-1 gene is upregulated in early-stage
hepatocellular carcinoma and correlates with ATP-binding cassette
transporter B1 expression. Cancer Sci. 101:666–672. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Pan K, Zhang HK, Weng DS, Zhou J,
Li JJ, Huang W, Song HF, Chen MS and Xia JC: Increased
polycomb-group oncogene Bmi-1 expression correlates with poor
prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol.
134:535–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steele JC, Torr EE, Noakes KL, Kalk E,
Moss PA, Reynolds GM, Hubscher SG, van Lohuizen M, Adams DH and
Young LS: The polycomb group proteins, BMI-1 and EZH2, are
tumour-associated antigens. Br J Cancer. 95:1202–1211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacobs JJ, Kieboom K, Marino S, DePinho RA
and van Lohuizen M: The oncogene and Polycomb-group gene bmi-1
regulates cell proliferation and senescence through the ink4a
locus. Nature. 397:164–168. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hui AM, Sakamoto M, Kanai Y, Ino Y, Gotoh
M, Yokota J and Hirohashi S: Inactivation of p16INK4 in
hepatocellular carcinoma. Hepatology. 24:575–579. 1996. View Article : Google Scholar
|
|
32
|
Micsenyi A, Tan X, Sneddon T, Luo JH,
Michalopoulos GK and Monga SP: β-catenin is temporally regulated
during normal liver development. Gastroenterology. 126:1134–1146.
2004.
|
|
33
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: a
review. Gynecol Oncol. 131:772–779. 2013.
|
|
34
|
Rakheja D, Cunningham JC, Mitui M, Patel
AS, Tomlinson GE and Weinberg AG: A subset of cranial fasciitis is
associated with dysregulation of the Wnt/beta-catenin pathway. Mod
Pathol. 21:1330–1336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikeda S, Kishida S, Yamamoto H, Murai H,
Koyama S and Kikuchi A: Axin, a negative regulator of the Wnt
signaling pathway, forms a complex with GSK-3beta and beta-catenin
and promotes GSK-3beta-dependent phosphorylation of beta-catenin.
EMBO J. 17:1371–1384. 1998. View Article : Google Scholar
|
|
36
|
Pennisi E: How a growth control path takes
a wrong turn to cancer. Science. 281:1438–1441. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cui J, Zhou X, Liu Y, Tang Z and Romeih M:
Wnt signaling in hepatocellular carcinoma: analysis of mutation and
expression of beta-catenin, T-cell factor-4 and glycogen synthase
kinase 3-beta genes. J Gastroenterol Hepatol. 18:280–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei Y, Shen N, Wang Z, Yang G, Yi B, Yang
N, Qiu Y and Lu J: Sorafenib sensitizes hepatocellular carcinoma
cell to cisplatin via suppression of Wnt/β-catenin signaling. Mol
Cell Biochem. 381:139–144. 2013.PubMed/NCBI
|
|
39
|
He B, Kang Q, Yang J, Shang J, He T and
Zhou Q: Correlation between antitumor activity of berberine and the
inhibition of Wnt/beta-catenin signal pathway. Zhongguo Yaolixue
Tongbao. 21:1108–1111. 2005.(In Chinese).
|
|
40
|
Kumar DU and Devaraj H: Expression of Wnt
3a, β-catenin, cyclin D1 and PCNA in mouse dentate gyrus
subgranular zone (SGZ): a possible role of Wnt pathway in SGZ
neural stem cell proliferation. Folia Biol (Praha). 58:115–120.
2012.
|
|
41
|
Zhou CJ, Shi LJ, Fu LH, Wang L, Yu YC, Hu
CL and Chen L: Effect of matrine on expressions of SALL4 gene and
downstream target genes of Wnt/β-catenin signaling pathway in human
acute erythroleukemia cell line TF-1. Chin J Biol. 26:94–98.
2013.
|
|
42
|
Gao JT: Modulating action of matrine on
rat hepatic precancerous lesion and Wnt signaling transduction
pathway. http://d.g.wanfangdata.com.cn/Thesis_Y1354799.aspx.
Shijiazhuang: Heibei Medical University; 2008, (In Chinese).
|
|
43
|
Sparmann A and van Lohuizen M: Polycomb
silencers control cell fate, development and cancer. Nat Rev
Cancer. 6:846–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valk-Lingbeek ME, Bruggeman SW and van
Lohuizen M: Stem cells and cancer; the polycomb connection. Cell.
118:409–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park IK, Morrison SJ and Clarke MF: Bmi1,
stem cells, and senescence regulation. J Clin Invest. 113:175–179.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan L, Xu C, Wang C, Tao J, Ho C, Jiang L,
Gui B, Huang S, Evert M, Calvisi DF and Chen X: Bmi1 is required
for hepatic progenitor cell expansion and liver tumor development.
PLoS One. 7:e464722012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sawa M, Yamamoto K, Yokozawa T, Kiyoi H,
Hishida A, Kajiguchi T, Seto M, Kohno A, Kitamura K, Itoh Y, Asou
N, Hamajima N, Emi N and Naoe T: BMI1 is highly expressed in
M0-subtype acute myeloid leukemia. Int J Hematol. 82:42–47. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Silva J, García V, García JM, Peña C,
Domínguez G, Díaz R, Lorenzo Y, Hurtado A, Sánchez A and Bonilla F:
Circulating Bmi-1 mRNA as a possible prognostic factor for advanced
breast cancer patients. Breast Cancer Res. 9:R552007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu JH, Song LB, Zhang X, Guo BH, Feng Y,
Li XX, Liao WT, Zeng MS and Huang KH: Bmi1 expression predicts
prognosis for patients with gastric carcinoma. J Surg Oncol.
97:267–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sasaki M, Ikeda H, Itatsu K, Yamaguchi J,
Sawada S, Minato H, Ohta T and Nakanuma Y: The overexpression of
polycomb group proteins Bmi1 and EZH2 is associated with the
progression and aggressive biological behavior of hepatocellular
carcinoma. Lab Invest. 88:873–882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao L, Bombard J, Cintron K, Sheedy J,
Weetall ML and Davis TW: BMI1 as a novel target for drug discovery
in cancer. J Cell Biochem. 112:2729–2741. 2011. View Article : Google Scholar : PubMed/NCBI
|