Introduction
Laryngeal squamous cell carcinoma (LSCC), a common
type of head and neck squamous cell carcinoma (HNSCC), accounts for
approximately 2.4% of new malignancies worldwide every year
(1,2). LSCC patients often display
considerable variability in survival, despite new advances in
treatment (2).
In the past few years, it has become clear that the
complex interaction between LSCC and immune system members plays an
important role in determining tumor progression (3). Among these members, CD4+
regulatory T cells (Tregs), from either the thymus or the
periphery, play an important role in antitumor immune responses, in
which they have been associated with suppressive activities against
tumor-specific T-cell responses (3,4).
Several studies have shown that Treg prevalence increases in
peripheral blood and many types of human malignancies, such as
ovarian, gastric and esophageal cancer (5–9).
Despite efforts to better understand the role of
Tregs in human malignancies, isolating Treg subsets is difficult
due to the lack of Treg-specific markers. Human Tregs with
suppressive activities were initially described in 1995 based on
elevated CD25 expression (10).
However, subsequent studies showed that these
CD4+CD25+ T cells are mixed populations,
composed of suppressor CD4+CD25high as well
as CD4+CD25low T cells, which are
nonsuppressive, and activated CD4+ T cells (11). Until 2003, Foxp3 was thought to be a
key transcription factor for the development and function of Tregs
(12,13). Although
Foxp3 expression is specific for Tregs, it cannot be
used to isolate living cells to study Treg heterogeneity since
Foxp3 is located intracellularly. Hence, several surface markers,
such as CD127 and CD45RA, have been studied during the functional
evaluation of Tregs (14–16). For example, Liu et al
(14) demonstrated that CD127
expression is inversely correlated with FoxP3 and the suppressive
function of human Tregs. In particular, Miyara et al
(16) recently found that Tregs in
healthy populations and patients with autoimmune disease can be
defined by three functionally distinct subsets on the basis of
CD45RA expression: CD45RA+Foxp3low resting
Tregs, CD45RA−Foxp3high activated Tregs, both
of which are suppressive in vitro, and cytokine-secreting
CD45RA−Foxp3lowCD4+ nonsuppressive
T cells. The frequency and function of these Treg subsets vary in
different diseases (16–18).
When assessing the role of Tregs it is important not
only to examine them directly, but also to investigate their
relationship to other important antitumor members, as Tregs have
been associated with suppressive activities against tumor-specific
T cells. Studies have shown that Th1 cells and CD8+ T
cells play an important role in the control of tumor growth. For
example, CD8+ T cells were shown to mediate antitumor
immunity (19). Th1 cells, a subset
of CD4+ T cells, constitutively express IFN-γ and TNF-α,
and play a role in priming tumor-specific cytotoxic T lymphocytes
(CTLs) through the release of soluble IL-2 in the proximity of CTLs
(20). Moreover, induction of MHC
class I-restricted tumor-specific immunity requires epitope linkage
between Th1 and CTL epitopes, important for CTL induction (21). Recently, we reported that
functionally distinct Treg subsets
(CD45RA+Foxp3low resting Tregs,
CD45RA−Foxp3high activated Tregs, and
cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells) vary
in the peripheral circulation of HNSCC patient subgroups (22); however, the details of distinct Treg
subsets and the correlation between these Treg subsets and
tumor-specific T cells in the peripheral circulation of LSCC have
not been demonstrated.
To investigate the potential implication of
functionally distinct Treg subsets in LSCC immunity, we used the
CD45RA, Foxp3, and CD25 markers to evaluate both the frequency and
various functions of distinct Treg subsets in the peripheral blood
of LSCC patients in relation to CD4+ and CD8+
T cells, tumor stage and nodal status.
Materials and methods
Patients and healthy donors
From March to November 2013, 42 LSCC patients were
enrolled. Patients were diagnosed at the Department of
Otorhinolaryngology, The First Affiliated Hospital of Sun Yat-Sen
University without any previous oncological treatment. Healthy
age-matched donors (20 males and 1 female with a mean age of 43
years) were enrolled as controls. The main clinical and
pathological characteristics of the patients are presented in
Table I. Clinical staging and the
anatomic site of the tumors were assessed according to the 6th
edition of the Union Internationale Contre le Cancer (UICC, 2008)
tumor-node-metastasis classification of malignant tumors.
 | Table IClinicopathological features of the
LSCC patients. |
Table I
Clinicopathological features of the
LSCC patients.
|
Characteristics | Number |
|---|
| Age (years) |
| Mean (range) | 46 (38–81) |
| Gender |
| Male | 40 |
| Female | 2 |
| Total | 42 |
| Tumor site |
| Glottic
region | 37 |
| Supraglottic
region | 3 |
| Subglottic
region | 2 |
| Tumor stage |
| T1 | 12 |
| T2 | 10 |
| T3 | 14 |
| T4 | 6 |
| Nodal stage |
| N0 | 35 |
| N1 | 5 |
| N2 | 2 |
| N3 | 0 |
| M stage |
| M0 | 42 |
| M1 | 0 |
| Alcohol
history |
| Yes | 31 |
| No | 5 |
| Prior | 3 |
| Unknown | 3 |
| Smoking
history |
| Yes | 36 |
| No | 2 |
| Prior | 3 |
| Unknown | 1 |
Ethics statements
The study protocol (No. 2012-349) was approved by
The Ethics Committee of The First Affiliated Hospital of Sun
Yat-Sen University, and was used for research purposes only.
Patient and healthy donor (HD) informed consent was obtained before
enrollment.
Collection of peripheral blood
Peripheral blood lymphocytes (PBLs) were isolated
from peripheral venous blood as we previously described (22). Isolated cells were immediately
resuspended in 100 μl flow cytometry staining buffer (eBioscience,
San Diego, CA, USA) for surface and intracellular staining.
Antibodies and reagents
Freshly obtained human PBLs were stained with the
following anti-human monoclonal antibodies: anti-CD3-eFluor 605NC
(0.25 μg/100 μl), anti-CD4-FITC (1.0 μg/100 μl), anti-CD8-PE-Cy7
(0.06 μg/100 μl), anti-CD25-APC (0.125 μg/100 μl) and
anti-CD45RA-eFluor 450 (0.5 μg/100 μl) for surface staining; and
anti-Foxp3-PE (0.25 μg/100 μl), antitumor necrosis factor-α
(TNF-α)-Alexa Fluor 700 (0.25 μg/100 μl), anti-interleukin-2
(IL-2)-PE-Cy7 (0.125 μg/100 μl), anti-interferon-γ
(IFN-γ)-APC-eFluor 780 (0.25 μg/100 μl) and anti-interleukin-17
(IL-17)-PerCP-Cy5.5 (0.125 μg/100 μl) for intracellular staining.
Soluble anti-CD3 (OKT3, 0.5 μg/ml) and anti-CD28 (CD28.2, 2 μg/ml)
mAb were used for in vitro activation of T cells. All
antibodies and isotype controls were purchased from eBioscience
(San Diego).
Multicolor flow cytometry
Multicolor flow cytometry was conducted using a
Ten-Color (3 laser: 488 nm blue, 638 nm red and 405 nm violet)
Gallios Flow Cytometer (Beckman Coulter, Hercules, CA, USA)
equipped with Gallios software v1.0. The acquisition and analysis
gates for PBLs (5×104) were determined by characteristic
forward and side-scatter properties of lymphocytes. Furthermore,
analysis gates were restricted to the
CD3+CD4+, CD3+CD8+, and
CD4+CD25−CD45RA− T-cell subsets,
as appropriate. Cells expressing surface and intracellular markers
were acquired and analyzed on a logarithmic scale from FL1 to FL9.
Following doublet discrimination, a CD25 vs. Foxp3 dot plot gated
on CD3+CD4+ T cells was created to determine
Tregs (Fig. 1Aa). In particular,
following doublet discrimination, a CD45RA vs. Foxp3 dot plot gated
on CD3+CD4+ T cells was created to determine
the different levels of Foxp3 expression (Foxp3low and
Foxp3high); CD45RA+ T cells with Foxp3
expression were defined as CD45RA+Foxp3low T
cells (I), CD45RA− T cells exceeding a certain level of
Foxp3 expression on CD45RA+ T cells were defined as
CD45RA−Foxp3high T cells (II),
CD45RA− T cells with the same level of Foxp3 expression
by CD45RA+Foxp3low T cells were defined as
CD45RA−Foxp3low T cells (III) (Fig. 1Ab).
 | Figure 1Functionally distinct subsets of
CD4+ Tregs in 42 LSCC patients and 21 HD. (A)
CD4+ Tregs defined by Foxp3 and CD25 expression (a).
Flow cytometry of each Treg subset (I,
CD45RA+Foxp3low resting Tregs; II,
CD45RA−Foxp3high activated Tregs; III,
cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells)
isolated from HD and LSCC patients. Flow dot plots for one
representative HD and LSCC patient (b). (B) The percentage of Tregs
(a) and each Treg subset (b–d) isolated from HD and LSCC patients
(HD vs. LSCC, mean ± SD, p<0.001). Each dot represents an
individual sample. Red horizontal bars represent mean percentage.
Statistical comparisons were performed using the Kruskal-Wallis
test. (C) CFSE dilution by labeled
CD4+CD25−CD45RA+ responder T cells
assessed after TCR-stimulated co-culture with the indicated Treg
subset. Percentage of suppression is indicated. Flow plots for one
representative LSCC patient (a). The histogram represents the mean
percentages of suppression ± SD (n=6). Statistical comparisons were
performed using the Student’s t-test (b). (D) Production of IL-17,
IL-2, IFN-γ, and TNF-α by each fraction after stimulation with PMA
+ ionomycin. Flow dot plots for one representative LSCC patient
(a). The histogram represents the cytokine expression profiles of
each Treg subset (n=5). Res, responder cells; I,
CD45RA+Foxp3low resting Tregs; II,
CD45RA−Foxp3high activated Tregs; III,
cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells; IV,
CD4+CD25− T cells. Statistical comparisons
were performed using the Student’s t-test (b). |
Surface and intracellular staining
To determine the frequency of CD4+ and
CD8+ T cells, and their naïve phenotypes, mAbs against
surface markers CD3, CD4, CD8 and CD45RA were added to the cell
suspension (1×107 cells/100 μl) and incubated on ice for
30 min in the dark. Appropriate isotype Ab controls were used.
Cells were washed twice and examined by multi-color flow
cytometry.
To determine the frequency of Treg subsets,
Foxp3+CD8+ T cells, and Th1 cells, both cell
surface and intracellular staining was performed. To examine the
secretory function, intracytoplasmic expression of IL-2, IL-17,
TNF-α and IFN-γ
was assessed after stimulation of freshly isolated
PBLs for 5 h with a cocktail of phorbol 12-myristate 13-acetate
(PMA), ionomycin, and Golgi stop (brefeldin A and monensin)
(eBio-science). Briefly, following surface staining, cells were
fixed and permeabilized on ice with fixation/permeabilization
buffer (eBioscience) for 1 h in the dark. Cells were then washed
twice and incubated with intracellular mAbs against Foxp3, IL-2,
IL-17, TNF-α, and IFN-γ for 1 h at room temperature in the dark.
After intracellular staining, cells were washed twice and examined
by multicolor flow cytometry. Appropriate isotype Ab controls were
included for each sample.
In vitro suppression of Treg subsets and
isolation by flow cytometry
Six separate experiments were performed. Stained
cells (mAbs against CD3, CD4, CD25, and CD45RA) at a concentration
of 5×107 cells/100 μl were sorted using a FACS cell
sorter (BD Influx, BD Biosciences). Three Treg subsets were
prepared as live cells as we previously described (22): Foxp3lowCD45RA+
cells, which were CD25++ (I),
Foxp3highCD45RA− cells, which were
CD25+++ (II), and Foxp3lowCD45RA−
cells, which were CD25++ (III) were prepared by sorting
as CD25++CD45RA+,
CD25+++CD45RA−, and
CD25++CD45RA− cells, respectively.
After sorting, 1×104 responder cells
(CD25−CD45RA+CD4+ T cells) were
labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester
(CFSE) (eBioscience) and co-cultured with unlabeled
CD25++CD45RA+,
CD25+++CD45RA−, or
CD25++CD45RA−CD4+ T cells and
assessed for their suppressive activity. Soluble anti-CD28 (2
μg/ml) and plate-bound anti-CD3 (0.5 μg/ml) were used to activate T
cells in 96-well round-bottom plates, and cells were harvested and
analyzed by flow cytometry after 86 h of co-culture. All CFSE data
were analyzed using the ModFit software provided by Verity Software
House (Topsham, USA). The percentages of suppression were
determined based on the proliferation index (PI) of responder cells
alone (100% proliferation, 0% suppression) compared with the PI of
responders co-cultured (1:1 ratio) with Treg subset.
Statistical analysis
Statistical analysis was performed with SPSS
software (SPSS standard version 13.0, IBM, Chicago, IL, USA).
Differences between groups were assessed using the Mann-Whitney U
test, Student’s t-test, or Kruskal-Wallis test. The correlation
between Treg subsets and clinical factors (tumor stage and nodal
status) was determined by one-way ANOVA.
Results
Distinct Treg subsets in LSCC
patients
We first examined the frequency of Tregs. Our
results showed that the Treg percentage was increased in PBLs of 42
LSCC patients compared with 21 HD (8.42±1.30%, median: 8.57% vs.
6.37±1.30%, median: 6.43%, p<0.001) (Fig. 1Ba), consistent with previous
findings (21). The percentage of
the three CD4+ Treg subsets was then evaluated based on
CD45RA and Foxp3 expression. The novelty of this study was that the
percentage of CD45RA−Foxp3high activated
Tregs (2.47±0.75%, median: 2.42% vs. 0.79±0.26%, median: 0.74%,
p<0.001) and of cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells
(5.36±0.93%, median: 5.46% vs. 3.85±0.77%, median: 3.93%,
p<0.001) was increased in the LSCC patient PBLs compared with HD
PBLs. In contrast, the percentage of
CD45RA+Foxp3low resting Tregs (0.59±0.23%,
median: 0.57% vs. 1.73±1.12%, median: 1.31%, p<0.001) was
decreased in the LSCC patient PBLs compared with HD PBLs (Fig. 1Bb–d).
Suppressive and secretory functions of
distinct Treg subsets
The suppressive activity of each Treg subset from
the LSCC patients (n=6) was assessed by their ability to suppress
the proliferation of an autologous T-cell population
(CD25−CD45RA+CD4+). When each Treg
subset isolated from LSCC patients was co-cultured (1:1 ratio) with
autologous CD25−CD45RA+CD4+
responder cells, both activated and resting
Tregs consistently induced a greater percentage of
suppression compared with cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells
(89.12±3.25% vs. 11.29±1.87%, p<0.001; 86.98±5.71% vs.
11.29±1.87%, p<0.001, respectively) (Fig. 1C).
Moreover, the functional cytokine patterns in sorted
Treg subsets from 5 LSCC patients were also studied after ex
vivo stimulation. Those results suggested that
cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells
secreted significantly higher amounts of IL-2, IFN-γ and TNF-α than
did the activated or resting Tregs (p<0.001), whereas IL-17
production remained the same (p>0.05) (Fig. 1D).
Frequency of
Foxp3+CD8+ T cells in LSCC patients
It has been reported that CD8+ T cells
might also express Foxp3, indicating that Foxp3 expression is not
confined to Tregs (23). Thus, we
evaluated Foxp3 expression in CD8+ T cells. The results
revealed that PBL CD8+Foxp3+ T cells in 21
LSCC patients were decreased compared with these cells in 19 HD
(0.23±0.11%, median: 0.20% vs. 0.59±0.53%, median: 0.43%,
p<0.001). Moreover, the percentage of naïve
CD8+Foxp3+CD45RA+ T cells in the
LSCC patients was decreased compared with HD (0.04±0.02%, median:
0.03% vs. 0.37±0.49%, median: 0.21%, p<0.001) (Fig. 2).
CD4+ and CD8+ T
cells in LSCC patients
Cancer patient PBL CD4+ or
CD8+ T cells may decrease because of Treg suppressive
activities (24,25). However, our results showed that
there was no significant difference in the percentage of
CD4+ (36.01±9.75%, median: 34.12% vs. 34.62±9.22%,
median 35.46%, p=0.65) and CD8+ (29.77±8.42%, median:
28.44% vs. 31.98±7.88%, median: 30.48%, p=0.39) T cells (Fig. 3A). Notably, the naïve
CD4+ (23.94±11.92%, median: 23.59% vs. 45.90±10.69%,
median: 50.48%, p<0.001) and naïve CD8+
(51.81±11.29%, median: 51.65% vs. 65.42±13.89%, median: 71.75%,
p=0.002) T cells were significantly lower in the LSCC patients than
in HD (Fig. 3B and C).
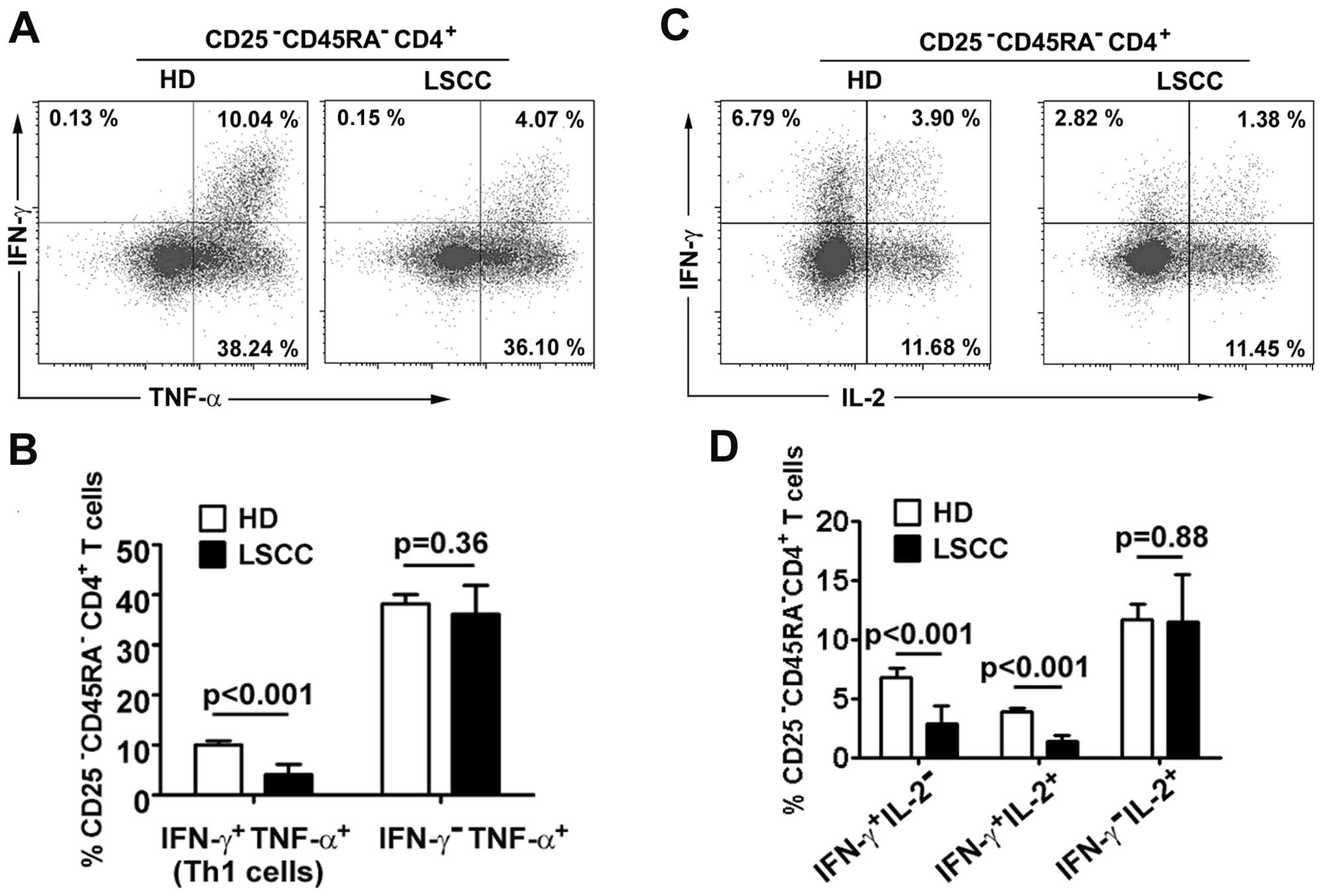
Th1 cells in LSCC patients
Although studies of non-cancerous diseases have
shown that a decrease in Th1 cells can be attributed to Treg
suppressive activities (26,27),
the change in LSCC Th1 cells is unknown. In the present study,
IFN-γ and TNF-α were used to identify Th1 cell levels in a small
cohort of 8 LSCC patients and 5 HD. Our preliminary results showed
that the percentage of Th1 cells in
CD25−CD45RA−CD4+ T cells was
decreased in LSCC patients compared with HD (4.20±2.07% vs.
10.68±0.93%, p<0.001). The percentages of
IFN-γ-TNF-α+ effector
CD25−CD45RA−CD4+ T cells did not
differ between LSCC patients and HD (36.10±5.76% vs. 38.24±1.84%,
p=0.36) (Fig. 4A and B).
To understand which Th1 subsets varied in LSCC
patients, Th1 cells were separated into two subsets
(IFN-γ+IL-2+ and
IFN-γ+IL-2−) using our previously described
method (28). The results showed
that Th1 subsets were decreased in the LSCC patients compared with
HD (IFN-γ+IL-2+ Th1 cells: 1.38±0.53% vs.
3.89±0.28%, p<0.001; IFN-γ+IL-2− Th1
cells: 2.82±1.59% vs. 6.79±0.74%, p<0.001). The percentages of
IFN-γ−IL-2+ effector T cells did not differ
between LSCC patients and HD (11.45±4.01% vs. 11.68±1.28%, p=0.88)
(Fig. 4C and D).
Relationship between circulating Treg
subsets and clinical variables
Glottic squamous cell carcinoma is a common type of
LSCC, and lymph node metastasis is uncommon in patients with
glottic squamous cell carcinoma (especially in patients
with T1 to early T3) due to
the lack of lymphatic drainage in the glottic region. In the
present study, 37 of the 42 LSCC patients had glottic squamous cell
carcinoma; only 7 patients had nodal involvement. Thus, any
conclusions regarding the difference between the two populations
(N0 and N+) must not be overstated since the
number of patients in each category was unbalanced (i.e. 35 vs.
7).
The clinical impact of circulating Treg subsets on
tumor stage and nodal status was examined. First, the percentage of
Tregs was higher in 20 T3–4 patients (9.07±1.01%) than
in 22 T1–2 patients (7.84±1.28%, p=0.002) and 21 HD
(6.37±1.30%, p<0.001) (Fig.
5Aa). Furthermore, Tregs were increased in 7 N+
patients (9.39±0.98%) when compared with Tregs in 35 N0
patients (8.23±1.28%, p=0.03) and 21 HD (6.37±1.30%, p<0.001)
(Fig. 5Ba).
We also aimed to ascertain whether activated Tregs
correlated with tumor progression. Interestingly, activated Tregs
were elevated in T3–4 patients (2.80±0.70%) relative to
T1–2 patients (2.18±0.67%, p=0.001) and HD (0.79±0.26%,
p<0.001) (Fig. 5Ab). In
addition, activated Tregs were elevated in N+ patients
(3.05±0.88%) relative to N0 patients (2.36±0.67%,
p=0.007) and HD (0.79±0.26%, p<0.001) (Fig. 5Bb). The percentage of resting Tregs
did not differ between patients with T3–4 and
T1–2 (0.58±0.17% vs. 0.61±0.29%, p=0.90) (Fig. 5Ac) or with N+ and
N0 (0.46±0.14% vs. 0.62±0.24%, p=0.58) (Fig. 5Bc). Cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells were
elevated in T3–4 patients compared with T1–2
patients (5.69±0.82% vs. 5.06±0.95%, p=0.02) (Fig. 5Ad), but did not differ between
N+ and N0 patients (5.88±0.52% vs.
5.25±0.97%, p=0.087) (Fig.
5Bd).
Discussion
There is mounting evidence that Tregs are involved
in the control of immune regulation in many types of human
malignancies, with a particular focus on T-cell suppression
(5–9). Tregs have been reported to be negative
prognostic factors for ovarian (5),
hepatocellular (6), gastric and
esophageal cancer (7). However, in
contrast to these observations, Pretscher et al showed that
higher Treg levels did not show any significant influence on the
outcome of oro- and hypopharyngeal carcinoma patients (8). In addition, HNSCC studies indicate
that elevated Treg levels are prognostic factors and predict better
locoregional control and overall survival (9). This apparent contradiction regarding
the role of Tregs in cancer prognosis might be explained by the
functional heterogeneity of Tregs. For example, Zhou et al
showed that CD4+Foxp3− T cells transiently
express lower levels of Foxp3, leading to the generation of T cells
with a pathogenic memory (29).
Allan et al postulated that activated CD4+ T
cells express Foxp3, but lack regulatory activity (30). Hence, identification of distinct
Treg subsets and their functional abilities might be intriguing for
the antitumor immunity field.
Despite a number of studies performed on the role
of Tregs in LSCC (15,23,31,32),
the characteristics of functionally distinct Treg subsets in LSCC
are poorly understood. Recently, one functional study reported by
Drennan et al (15), showed
that suppressive activities of CD127low/− Tregs
(including CD4+CD25interCD127low/−
and CD4+CD25highCD127low/− Tregs)
increased in the peripheral circulation of laryngeal and
oropharyngeal patients. The decrease was associated with advanced
stage and nodal involvement, supporting the need to delineate the
prevalence and function of different Treg subsets in LSCC,
requiring further assessment of immunotherapeutic strategies.
Hence, we sought to analyze the percentage and function of three
distinct Treg subsets in LSCC patients at the time of diagnosis.
Tregs were significantly higher in LSCC patients than in healthy
age-matched donors, in agreement with previous studies (23,31,32).
Nonetheless, a new finding was that the percentage of activated
Tregs with highly suppressive activities increased in LSCC
patients, and that this correlated with tumor stage and nodal
status. Although resting Tregs also showed highly suppressive
activities, the percentage of this Treg subset decreased,
suggesting that resting Tregs may be swiftly converted into
activated Tregs immediately after migrating from the thymus or
having been peripherally generated (16).
Another interesting finding of the present study is
that cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells
increased in parallel with activated Tregs. We found that this Treg
subset secreted elevated levels of effector cytokines, but did not
have suppressive function in vitro. It may be that
cytokine-secreting
CD45RA−Foxp3lowCD4+ T cells are an
heterogeneous Treg subset specific to LSCC. They might also be
non-Tregs that differentiate into effector T cells, as others have
proposed (16). The increased
percentage of this subset might be the result of antigen exposure
in the tumor microenvironment, similar to what is observed for
CD8+ T cells in renal cancer (33). In our opinion, investigators should
carefully identify distinct Treg subsets rather than whole Treg
populations when studying LSCC. Taken together, these data suggest
that activated Tregs might be potential targets in LSCC
immunotherapy. In addition, the Foxp3 expression in CD8+
T cells was evaluated, as this T cell subset might also be
Foxp3+ (23). Our data
showed that the small percentage of
CD8+Foxp3+ T cells in LSCC patients was
decreased compared with HD, and that
CD8+CD45RA+Foxp3+ T cells were
rare in LSCC patients. Nevertheless, the ontogeny and function of
Foxp3 expression in CD8+ T cells are still far from
certain, owing to their scarcity in number and thus the difficulty
in evaluation.
The complex functions of human Tregs and their
interaction with other antitumor immune system members make the
study of antitumor immunity challenging (3,4).
Previous HNSCC studies have shown that a decreased frequency of
peripheral CD4+ or CD8+ T cells may be
attributed to Treg suppressive activities (24,25).
However, one striking finding of our study is that the percentages
of naïve CD4+ and naïve CD8+ T cells were
decreased in the LSCC patients compared with these percentages in
HD, whereas the percentages of CD4+ and CD8+
T cells were not. We hypothesized that Tregs may suppress
proliferation of naïve CD4+CD45RA+ and naïve
CD8+CD45RA+ T cells, but not all
CD4+ and CD8+ T cells. The functional study
of Treg subsets partially supported our hypothesis that among
Tregs, both activated and resting Tregs had highly suppressive
activities on autologous naïve CD4+CD45RA+ T
cells.
It is now generally agreed that antitumor cellular
immune responses are induced and maintained by Th1 cells (20,21).
Although Th1 cells can be suppressed by Tregs in other diseases,
such as autoimmune disease and allergies (17,26,27),
the interplay between Tregs and Th1 cells is poorly understood in
human malignancies. Our preliminary results, perhaps most
excitingly, showed that the percentage of Th1 cells (including
IFN-γ+IL-2+ and
IFN-γ+IL-2− Th1 subsets) was decreased in
LSCC patients compared with HD, which suggests that Th1 cells may
be suppressed by activated Tregs. Although we did not test this
hypothesis, the present data support this speculation and suggest a
complex interaction between Th1 cells and activated Tregs in LSCC.
Further studies that focus on the interaction between Tregs and Th1
subsets may shed more light on the immunosuppressive activities of
Tregs with respect to antitumor immunity.
In conclusion, this study provides evidence to
support the notion that activated Tregs suppress
CD4+CD25− T-cell proliferation, and that
functionally activated Tregs are correlated with disease
progression in LSCC patients. An increase in activated Tregs might
reduce T-cell-mediated antitumor immunity, as represented by the
decrease in CD4+ T-cell subpopulations in LSCC patients.
Thus, the present findings provide important information relevant
to the future design of immunotherapeutic strategies for LSCC.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (grant no. 81271055/H1301).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alhamarneh O, Amarnath SM, Stafford ND and
Greenman J: Regulatory T cells: what role do they play in antitumor
immunity in patients with head and neck cancer? Head Neck.
30:251–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curiel TJ, Coukos G, Zou L, et al:
Specific recruitment of regulatory T cells in ovarian carcinoma
fosters immune privilege and predicts reduced survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi N, Hiraoka N, Yamagami W, et al:
FOXP3+ regulatory T cells affect the development and
progression of hepatocarcinogenesis. Clin Cancer Res. 13:902–911.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kono K, Kawaida H, Takahashi A, et al:
CD4(+) CD25high regulatory T cells increase with tumor
stage in patients with gastric and esophageal cancers. Cancer
Immunol Immunother. 55:1064–1071. 2006. View Article : Google Scholar
|
|
8
|
Pretscher D, Distel LV, Grabenbauer GG,
Wittlinger M, Buettner M and Niedobitek G: Distribution of immune
cells in head and neck cancer: CD8+ T-cells and
CD20+ B-cells in metastatic lymph nodes are associated
with favourable outcome in patients with oro- and hypopharyngeal
carcinoma. BMC Cancer. 9:2922009. View Article : Google Scholar
|
|
9
|
Badoual C, Hans S, Rodriguez J, et al:
Prognostic value of tumor-infiltrating CD4+ T-cell
subpopulations in head and neck cancers. Clin Cancer Res.
12:465–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunological self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.PubMed/NCBI
|
|
11
|
Baecher-Allan C, Brown JA, Freeman GJ and
Hafler DA: CD4+CD25(high) regulatory cells in human
peripheral blood. J Immunol. 167:1245–1253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of CD4(+)CD25(+)
regulatory T cells. Nat Immunol. 4:330–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu WH, Putnam AL, Xu-Yu Z, et al: CD127
expression inversely correlates with FoxP3 and suppressive function
of human CD4(+) T reg cells. J Exp Med. 203:1701–1711. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Drennan S, Stafford ND, Greenman J and
Green VL: Increased frequency and suppressive activity of
CD127(low/−) regulatory T cells in the peripheral circulation of
patients with head and neck squamous cell carcinoma are associated
with advanced stage and nodal involvement. Immunology. 140:335–343.
2013.PubMed/NCBI
|
|
16
|
Miyara M, Yoshioka Y, Kitoh A, et al:
Functional delineation and differentiation dynamics of human
CD4+ T cells expressing the Foxp3 transcription factor.
Immunity. 30:899–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kordasti S, Marsh J, Al-Khan S, et al:
Functional characterization of CD4+ T cells in aplastic
anemia. Blood. 119:2033–2043. 2012. View Article : Google Scholar
|
|
18
|
Buckner JH: Mechanisms of impaired
regulation by CD4(+) CD25(+)FOXP3(+) regulatory T cells in human
autoimmune diseases. Nat Rev Immunol. 10:849–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benchetrit F, Gazagne A, Adotevi O, et al:
Cytotoxic T lymphocytes: role in immunosurveillance and in
immunotherapy. Bull Cancer. 90:677–685. 2003.(In French).
PubMed/NCBI
|
|
20
|
Bennett SR, Carbone FR, Karamalis F,
Miller JF and Heath WR: Induction of a CD8+ cytotoxic T
lymphocyte response by cross-priming requires cognate
CD4+ T cell help. J Exp Med. 186:65–70. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cassel D and Forman J: Linked recognition
of helper and cytotoxic antigenic determinants for the generation
of cytotoxic T lymphocytes. Ann NY Acad Sci. 532:51–60. 1988.
View Article : Google Scholar
|
|
22
|
Sun W, Li WJ, Wu CY, Zhong H and Wen WP:
CD45RA−Foxp3high but not
CD45RA+Foxp3low suppressive T regulatory
cells increased in the peripheral circulation of patients with head
and neck squamous cell carcinoma and correlated with tumor
progression. J Exp Clin Cancer Res. 33:352014. View Article : Google Scholar
|
|
23
|
Strauss L, Bergmann C, Gooding W, et al:
The frequency and suppressor function of
CD4+CD25highFoxp3+ T cells in the
circulation of patients with squamous cell carcinoma of the head
and neck. Clin Cancer Res. 13:6301–6311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lau KM, Cheng SH, Lo KW, et al: Increase
in circulating Foxp3+CD4+CD25(high)
regulatory T cells in nasopharyngeal carcinoma patients. Br J
Cancer. 96:617–622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chikamatsu K, Sakakura K, Whiteside TL and
Furuya N: Relationships between regulatory T cells and
CD8+ effector populations in patients with squamous cell
carcinoma of the head and neck. Head Neck. 29:120–127. 2007.
View Article : Google Scholar
|
|
26
|
Shevach EM: Mechanisms of
Foxp3+ T regulatory cell-mediated suppression. Immunity.
30:636–645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmidt A, Oberle N and Krammer PH:
Molecular mechanisms of Treg-mediated T cell suppression. Front
Immunol. 3:512012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Foulds KE, Rotte MJ, Paley MA, et al:
IFN-gamma mediates the death of Th1 cells in a paracrine manner. J
Immunol. 180:842–849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou X, Bailey-Bucktrout SL, Jeker LT, et
al: Instability of the transcription factor Foxp3 leads to the
generation of pathogenic memory T cells in vivo. Nat Immunol.
10:1000–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Allan SE, Crome SQ, Crellin NK, et al:
Activation-induced FOXP3 in human T effector cells does not
suppress proliferation or cytokine production. Int Immunol.
19:345–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Erfani N, Khademi B, Haghshenas MR,
Mojtahedi Z, Khademi B and Ghaderi A: Intracellular CTLA4 and
regulatory T cells in patients with laryngeal squamous cell
carcinoma. Immunol Invest. 42:81–90. 2013. View Article : Google Scholar
|
|
32
|
Schaefer C, Kim GG, Albers A, Hoermann K,
Myers EN and Whiteside TL: Characteristics of
CD4+CD25+ regulatory T cells in the
peripheral circulation of patients with head and neck cancer. Br J
Cancer. 92:913–920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Attig S, Hennenlotter J, Pawelec G, et al:
Simultaneous infiltration of polyfunctional effector and suppressor
T cells into renal cell carcinomas. Cancer Res. 69:8412–8419. 2009.
View Article : Google Scholar : PubMed/NCBI
|