Introduction
Breast cancer is the most common malignancy and the
second most common cause of cancer-related deaths in females in the
Western world, with an estimated 192,370 new cases and 40,170
deaths in the US in 2009 (1).
Although advances in chemotherapy have significantly reduced the
risk of disease recurrence and death, the recurrence of breast
cancer due to chemotherapy failure or acquired resistance remains a
major challenge (2).
Mammalian target of rapamycin (mTOR) is a highly
conserved 289-kDa Ser/Thr kinase found in yeast and all eukaryotes,
consisting of two distinct signaling complexes known as mTORC1 and
mTORC2. It belongs to the phosphoinositide 3-kinase (PI3K) family
of protein kinases and regulates two important downstream
substrates, p70S6 kinase (p70S6K) and eukaryotic initiation factor
4B binding protein 1 (4EBP1) (3).
The mTOR pathway is a major regulator of autophagy activated
downstream of PI3K-Akt, a pathway commonly dysregulated in human
cancer (4) and activated by HER2,
insulin-like growth factor receptor, and estrogen receptor in
breast cancer (5–8), suggesting that it may play an
important role in the development of cancer and many other diseases
(9).
Apoptosis plays an important role in regulating cell
death by controlling cell proliferation through p53 and Bcl-2
proteins. The Bcl-2 family is an important regulator of apoptosis
(10,11) that includes anti-apoptotic and
pro-apoptotic members, such as Bcl-2, Bcl-xL, Mcl-1 and Bax
(12,13). The activation of Bcl-2 can be
regulated by post-translational phosphorylation of Akt, mTOR, and
p70S6K (14,15). Akt regulates cell survival via
various molecular mechanisms that include phosphorylation and the
inactivation of pro-apoptotic proteins, such as Bad, glycogen
synthase kinase-3 (GSK-3), forkhead, and caspase-9 (16,17).
As a downstream effector of PI3K/mTOR, Akt is constitutively
activated in many types of human tumors, including breast cancer.
Moreover, NF-κB and p53 signaling pathways are crucial modulators
of cell survival and apoptosis (18,19),
as well as important regulators of Bcl-2 family genes (20–23).
Autophagy begins with the formation of
double-membrane vesicles, known as autophagosomes, which engulf
cytoplasmic constituents. The autophagosomes then fuse with
lysosomes, allowing the sequestered contents to undergo degradation
and recycling. Monoallelic loss of the essential autophagy gene,
Beclin-1, has been found in 40–75% of human breast, prostate, and
ovarian cancers, suggesting that autophagy may play a role in
preventing these tumors (24). The
production of inositol 1,4,5-triphosphate (PtIns3P) by Beclin-1 is
essential for the recruitment of other autophagy-related gene (Atg)
products critical for autophagosome formation. During the
initiation phase, formation of the Atg5-Atg12 complex promotes the
recruitment and conversion of cytosolic-associated protein light
chain 3 (LC3-I) to LC3-II, the membrane-bound and lipidated form
(25).
This study investigated mTOR pathway activation in
MCF-7 cells treated with PPY by assessing LC3 to monitor autophagy.
We observed that the p53/NF-κB and mTOR pathways were affected by
PPY, which contributes to our understanding of the functional
relationship between the Bcl-2 family and mTOR under apoptotic
conditions in MCF-7 cells.
Materials and methods
Peptide preparation.
The PPY method was performed as previously described
(26). Briefly, the peptide PPY,
found in Porphyra yezoensis, was synthesized by PEPTRON
(Daejeon, Korea). Purification of PPY was performed using a
Shimadzu Prominence HPLC apparatus, controlled using the software
package Class-VP, 6.14 (Kyoto, Japan), on a C18 column (Shiesido
Capcell Pak) in 0.1% triflouroacetic (TFA)/water and a gradient of
10–70% acetonitrile in 0.1% TFA with a flow rate of 1 nm/min and UV
detection at 220 nm.
Cell culture
Human breast cancer MCF-7 cells were obtained from
the Korean Cell Line Bank (Seoul, Korea). Cells were maintained in
RPMI-1640 supplemented with 10% fetal bovine serum, 100 μg/ml
penicillin and 100 ng/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2.
Western blot analysis
Proteins (50 μg/ml) were separated by 7.5–15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a polyvinylidene fluoride (PVDF) membrane
(Millipore, Billerica, MA, USA). The membrane was blocked with 1%
bovine serum albumin (BSA) in TBS-T (10 mM Tris-HCl, 150 mM NaCl,
pH 7.5, 0.1% Tween-20) and then incubated overnight with the
indicated primary antibodies (diluted 1:1,000) in TBS-T containing
1% BSA with gentle shaking at 4°C. The secondary antibody was a
peroxidase-conjugated goat anti-mouse or rabbit antibody (diluted
1:10,000). Signals were detected using an enhanced
chemiluminescence (ECL) western blotting kit (Amersham, Piscataway,
NJ, USA).
siRNA transfection in vitro
The control and mTOR siRNA sequences were designed
by cosmo GENETECH (Seoul, Korea). mTOR was targeted using the
following siRNAs: sense, 5′-UGAACCCUGCCUUUGUCAUGC-3′ and antisense,
5′-GCAUGACAAAGGCAGGGUUCA-3′. Briefly, MCF-7 cells were transfected
with the control, non-targeting or mTOR-targeted siRNAs using
Lipofectamine (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The cells were cultured in the
presence of the transfection mixture for 72 h, and on the following
day, the transfection mixture was replaced with fresh RPMI medium.
After transfection, complete medium was added to a final volume of
1 ml, yielding a 50 nM final concentration of siRNA in each well.
After a 24-h incubation at 37°C and 5% CO2, the
transfected cells were refreshed with 1 ml complete media and
returned to the incubator.
Results
Expression of the mTOR pathway in MCF-7
cells
PI3K/Akt signaling is crucial in a variety of
divergent physiological processes, including transcription,
differentiation, apoptosis, and metabolism (27). mTOR is a downstream kinase in the
PI3K/Akt pathway whose activation is correlated with an increase in
PI3K/Akt-dependent Ser2448 phosphorylation (28) and regulates cell growth by
integrating nutrient- and growth factor-derived signals (29,30).
Therefore, we examined activation of the mTOR pathway in MCF-7
cells (Fig. 1). There was a
dose-dependent decrease in mTOR and p70S6K in the MCF-7 cells
treated with PPY, which also decreased the level of
phosphoinositide-dependent kinase 1 (PDK1). We previously
demonstrated in MCF-7 cells that PPY increased the level of
phosphatase and tensin homolog (PTEN) in a dose-dependent manner,
which was accompanied by decreased ribosomal protein S6 (RPS6).
These results demonstrate that PPY inhibits MCF-7 cell growth.
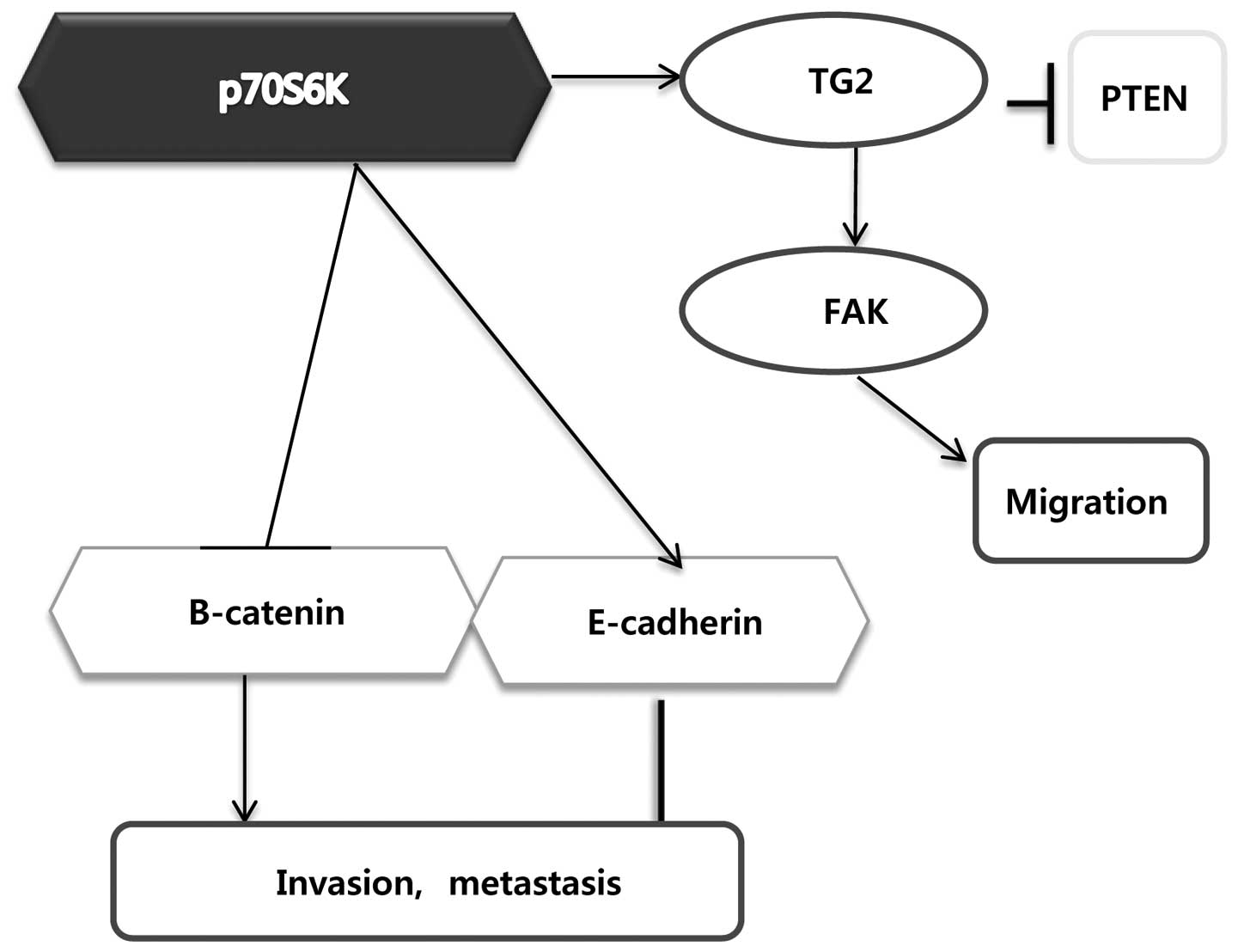
p70S6K plays an important role in
metastasis
p70S6K has been associated with poor prognosis and
metastasis in breast cancer, but the underlying mechanisms are not
well understood. To determine the downstream targets and mechanisms
that may play a role in metastasis, western blot analysis was used
to detect proteins that may be critical in cell attachment,
motility, invasion and metastasis (31). In a dose-dependent manner, PPY
decreased activation of p70S6K in MCF-7 cells and downregulated
transglutaminase 2 (TG2), β-catenin, and focal adhesion kinase
phosphorylation (p-FAK) (Fig. 2).
TG2 is a multifunctional enzyme known for its calcium-dependent
post-translational covalent cross-linking of proteins (32,33),
and TG2 expression on the cell membrane, as a result of its
association with specific integrins, has been reported to promote
cell survival signaling (34).
These results demonstrated that p70S6K was involved in the
metastasis of MCF-7 cells. In addition, p70S6K plays an important
role in metastasis by regulating key proteins such as cyclin D1,
PDCD4 and FAK, whereas E-cadherin, β-catenin and TG2 are essential
for cell attachment, survival, and invasion, as well as metastasis
in breast cancer (Fig. 3).
Activation of NF-κB and Bcl-2 family
members
As shown in Fig. 4,
the activities of NF-κB and Bcl-2 were decreased, while those of
p53, Bad, and Bax were increased by PPY. This indicated that PPY
controls apoptosis regulator gene expression by downregulation of
p53 and upregulation of NF-κB to stimulate PPY-induced apoptosis in
MCF-7 cells. Importantly, this study also showed that the p53/NF-κB
and PI3K/Akt/mTOR pathways were affected by PPY, clarifying the
functional relationship among NF-κB, Bcl-2 family genes and mTOR
following PPY treatment. This demonstrated that PPY might modulate
anticancer and Akt/mTOR signaling. NF-κB, which plays a pivotal
role in cell survival, regulates a vast number of genes related to
apoptosis, such as Bcl-2, Bax and Fas (35). Bcl-2 family members such as Bax and
Bad promote apoptosis, whereas other members such as Bcl-2 and
Bcl-xL exert anti-apoptotic effects (36).
The role of autophagy
Autophagy is important in a variety of other
cellular processes, including the recycling of aged or damaged
organelles, remodeling of cellular structures during development,
cell death, and protection against bacterial infection (37). As shown in Fig. 5, we detected increased expression of
autophagy-associated proteins such as LC3, Beclin-1, Atg5 and Atg7.
When MCF-7 cells were treated with 500 ng/ml PPY for 24 h, an
increase in LC3 protein expression was observed compared with the
untreated cells. As a specific marker for autophagy, LC3 has been
widely used to monitor autophagy. Lipidation of
microtubule-associated protein LC3-1, an autophagy marker, coats
autophagosomes during autophagy and is converted to LC3-II
resulting in delayed electrophoretic mobility (38). Beclin-1 is an essential autophagic
gene that contributes to initial vesicle nucleation and formation
of the autophagosome, whereas Atg5 participates in autophagic
vesicle elongation and completion (39). Fig.
6 summarizes what we know about the anatomy of autophagy and
the role of Atg and other proteins involved in the formation and
maturation of autophagosomes (40).
These results support the idea that PPY induces autophagy, inhibits
tumor growth and induces apoptosis in MCF-7 cells. In addition, we
demonstrated that PPY-induced autophagy occurred via the Akt/mTOR
pathway.
mTOR knockdown by PPY in MCF-7 cells
To further elucidate the role of PPY in autophagy,
we used small interfering RNA (siRNA) conjugated with PPY to knock
down mTOR expression in MCF-7 cells. MCF-7 cells were transfected
with siRNA/Lipofectamine complexes using different PPY
concentrations (0, 125, 250, 500 ng/ml). Total protein was
harvested 3 days after siRNA treatment and western blot analysis
was used to assess mTOR expression. There was a significant
reduction in mTOR expression by siRNA when the PPY concentration
was 500 ng/ml (Fig. 7). In
addition, p70S6K and PDK protein levels were significantly
suppressed by mTOR siRNA treatment in vitro compared with
transfection of non-targeting siRNA controls.
Discussion
Cancer is caused by alterations in gene expression
and is one of the major causes of mortality worldwide (29), since all cancers acquire resistance
to long-term anticancer drug treatments. In the MCF-7 cells used in
this study, we found that a peptide isolated from Porphyra
yezoensis can target the mTOR signaling pathway, which has
emerged as a critical regulator of cell proliferation, growth and
translation (29). Recent studies
have shown that aberrant activation of mTOR is involved in many
cancers, including ovarian carcinoma, lung cancer, prostate cancer
and mantle cell lymphoma (30).
This study showed that PPY markedly decreased mTOR and p70S6K, and
high concentrations of PPY decreased PDK1. Upon activation, mTOR
and its downstream target p70S6K promoted cell growth by inducing
protein synthesis (41). These
results suggest that activation of mTOR plays an important role in
the pathogenesis of MCF-7 cells.
The process of metastasis has three major steps. The
first is the separation of cells from their original tissue; the
second is immune surveillance in the circulation; and the third is
the homing of cells to other tissues (31). In this study, we focused on
metastasis since we wanted to determine the link between p70S6K and
cell attachment proteins, such as TG2 and FAK, which were
previously reported to be involved in metastasis (31). PPY decreased activation of p70S6K in
MCF-7 cells and downregulated TG2, β-catenin and p-FAK proteins
(Fig. 2). Downregulation of p70S6K
also inhibited TG2 and β-catenin expression. These results
demonstrated that p70S6K is involved in the metastasis of MCF-7
cells.
Expression of the apoptosis regulating factors, p53
and Bcl-2/Bax, correlates with apoptosis of cancer cells, including
breast cancer (42). In the present
study, we examined the involvement of p53 and Bcl-2 family members
in PPY-induced apoptosis of MCF-7 cells. We found that the
expression of NF-κB and Bcl-2 were decreased in the PPY-treated
MCF-7 cells (Fig. 4). p53 modulates
Bcl-2 during apoptosis in two ways: by direct trans-repression of
Bcl-2 transcription and by transcription-independent, direct
binding to Bcl-2 (40). p53
released from the p53-Bcl-2 complex can directly induce
mitochondrial permeabilization and subsequent apoptosis (43). Importantly, these results are the
first to show that PPY can regulate apoptosis regulator gene
expression by downregulating NF-κB and upregulating p53 activity in
MCF-7 cells. Additionally, PPY enhanced the mTOR/p70S6K signaling
pathway in MCF-7 cells.
Autophagy is a catabolic process in which cells
respond to various stress stimuli, such as hypoxia, nutrients,
nutrient starvation and DNA damage (37). During this process, proteins or
organelles, sequestered by double-membrane structures, fuse with
lysosomes and are subsequently degraded by lysosomal hydrolases to
be recycled and sustain metabolism (44). As shown in Fig. 5, we observed increased expression of
autophagy-associated proteins LC3, Beclin-1, Atg5 and Atg7,
revealing that PPY induced autophagy accompanied by apoptosis in
MCF-7 cells. Collectively, these results indicate that autophagy
provides a protective mechanism against PPY-induced apoptosis.
mTOR plays a critical role in cell cycle regulation,
and rapamycin, a known inhibitor of mTOR (45), can inactivate mTOR specifically.
Because mTOR regulates cell proliferation, it has been investigated
extensively as a potent target for both anticancer and
anti-restenotic therapies (46).
Rapamycin and its analogues are reported to effectively prevent
cardiac and pulmonary fibrosis in vivo (47,48),
and mTOR promotes cell growth and proliferation by regulating
protein synthesis. It is therefore conceivable that mTOR knockdown
may also control or alter cell proliferation (49,50).
Transfection of mTOR siRNA in MCF-7 cells downregulated mTOR
expression, as monitored by western blotting. Knockdown of mTOR
occurred only when the PPY concentration was 500 ng/ml. Compared
with the non-targeting siRNA complexes, mTOR siRNA complexes
reduced mTOR protein levels in MCF-7 cells (Fig. 7), confirming the suppression of
targeted gene expression via RNA interference.
In conclusion, this study investigated the effect of
PPY on the inhibition of MCF-7 cell proliferation, as well as the
possible mechanism of growth inhibition. This study demonstrated
the apoptosis of PPY cells and we identified regulation of the mTOR
signaling pathway and autophagy in MCF-7 cells (Fig. 8).
Acknowledgements
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (2012R1A6A1028677).
References
|
1
|
American Cancer Society, Cancer Facts and
Figures. American Cancer Society; Atlanta, GA: 2009
|
|
2
|
He X, Wang Y, Zhu J, Orloff M and Eng C:
Resveratrol enhances the anti-tumor activity of the mTOR inhibitor
rapamycin in multiple breast cancer cell lines mainly by
suppressing rapamycin-induced AKT signaling. Cancer Lett.
301:168–176. 2011. View Article : Google Scholar
|
|
3
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pattingre S, Espert L, Biard-Piechaczyk M
and Codogno P: Regulation of macroautophagy by mTOR and Beclin1
complexes. Biochimie. 90:313–323. 2008. View Article : Google Scholar
|
|
5
|
Bärlund M, Forozan F, Kononen J, et al:
Detecting activation of ribosomal protein S6 kinase by
complementary DNA and tissue microarray analysis. J Natl Cancer
Inst. 92:1252–1259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekulic A, Hudson CC, Homme JL, et al: A
direct linkage between the phosphosphoinositide 3-kinase-AKT
signaling pathway and the mammalian target of rapamycin in
mitogen-stimulated and transformed cells. Cancer Res. 60:3504–3513.
2000.
|
|
7
|
Lmoki K, Li Y, Zhu T, Wu J and Guan KL:
TSC2 is phosphorylated and inhibited by AKT and suppresses mTOR
signaling. Nat Cell Biol. 4:648–657. 2002. View Article : Google Scholar
|
|
8
|
Chung J, Kuo CJ, Crabtree GR and Bieris J:
Rapamycin FKBP specifically blocks growth-dependent activation of
and signaling by the 70 kd S6 protein kinases. Cell. 69:1227–1236.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holland EC, Sonenberg N, Pandolfi PP and
Thomas G: Signaling control of mRNA translation in cancer
pathogenesis. Oncogene. 23:3138–3144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin XM, Oltvai ZN and Korsmeyer SJ: BH1
and BH2 domain of Bcl-2 are required for inhibition of apoptosis
and heterodimerization with Bax. Nature. 369:321–323. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reed JC: Bcl-2 family protein: regulators
of apoptosis and chemo-resistance in hematologic malignancies.
Semin Hematol. 34:9–19. 1997.PubMed/NCBI
|
|
14
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malaguarnera L: Implications of apoptosis
regulators in tumorigenesis. Cancer Metastasis Rev. 23:367–387.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khwaja A: Akt is more than just a Bad
kinase. Nature. 401:33–34. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCormick F: Cancer: survival pathways
meet their end. Nature. 428:267–269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: from innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steele RJ and Lane DP: p53 in cancer: a
paradigm for modern management of cancer. Surgeon. 3:197–205. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haldar S, Negrini M, Monne M, Sabbioni S
and Croce CM: Downregulation of Bcl-2 by p53 in breast cancer
cells. Cancer Res. 54:2095–2097. 1994.PubMed/NCBI
|
|
21
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bentires-Alj M, Dejardin E, Viatour P, Van
Lint C, Froesch B, Reed JC, Mervile MP and Bours V: Inhibition of
the NF-kappa B transcription factor increases Bax expression in
cancer cell lines. Oncogene. 20:2805–2813. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heckman CA, Mehew JW and Boxer LM:
NF-kappaB activates Bcl-2 expression in t(14;18) lymphoma cells.
Oncogene. 21:3898–3908. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
h and Troxel A: Promotion of tumorigenesis by heterozygous
disruption of the beclin1 autophagy gene. J Clin Invest.
112:1809–1820. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matshushita M, Suzuki NN, Obara K, Fujioka
Y, Ohsumi Y and Inagaki F: Structure of Atg5.Atg16, a complex
essential for autophagy. J Biol Chem. 282:6763–6772. 2007.
View Article : Google Scholar
|
|
26
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Induction of apoptosis by a peptide from Porphyra yezoensis:
regulation of the insulin-like growth factor I receptor signaling
pathway in MCF-7 cells. Int J Oncol. 45:1011–1016. 2014.PubMed/NCBI
|
|
27
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: variations on theme. Oncogene. 27:5497–5510.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Sakon M, Nagano H, Hiraoka N, et al:
Akt2 expression correlates with prognosis of human hepatocellular
carcinoma. Oncol Rep. 11:25–32. 2004.
|
|
29
|
Faivre S, Kroener G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dann SG and Thomas G: The amino acid
sensitive TOR pathway from yeast to mammals. FEBS Lett.
580:2821–2829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akar U, Ozpolat B, Mehta K, et al:
Targeting p70S6k prevented lung metastasis in a breast cancer
xenograft model. Mol Cancer Ther. 9:1180–1187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fesus L and Szondy Z: Transglutaminase 2
in the balance of cell death and survival. FEBS Lett.
579:3297–3302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herman JF, Mangala LS and Mehta K:
Implications of increased tissue transglutaminase (TG2) expression
in drug-resistant breast cancer (MCF-7) cells. Oncogene.
25:3049–3058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fesus L and Piacentini M: Transglutaminase
2: an enigmatic enezyme with diverse function. Trends Biochem Sci.
27:534–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Malek R, Borrowicz KK, Jargiełło M and
Czuczwar SJ: Role of nuclear factor kappaB in the in the central
nervous system. Pharmacological Rep. 59:25–33. 2007.
|
|
36
|
Jurgensmeier JM, Xie Z, Deveraux Q,
Ellerby L, Bredesen D and Reed JC: Bax directly induced release of
cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA.
95:4997–5002. 1998. View Article : Google Scholar
|
|
37
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar
|
|
39
|
Zhang DM, Liu JS, Deng LJ, et al:
Arenobufagin, a natural bufadienolide from toad venom, induces
apoptosis and autophagy in human hepatocellular carcinoma cells
through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis.
34:1331–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eskelinen FL: Maturation of autophagic
vacuoles in Mammalian cells. Autophagy. 1:1–10. 2005. View Article : Google Scholar
|
|
41
|
Jing Ji and Zheng PS: Activation of mTOR
signaling pathway contributes to survival of cervical cancer cells.
Gynecol Oncol. 117:103–108. 2010. View Article : Google Scholar
|
|
42
|
Cho MY, Park SY, Park S, Lee YR, Han GD
and Kim JA: Geranyl derivative of phloroacetophenone induces cancer
cell-specific apoptosis through Bax-mediated mitochondrial pathway
in MCF-7 human breast cancer cells. Biol Pharm Bull. 35:98–104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee KB, Byun HJ, Park SH, Park CY, Lee SH
and Rho SB: CYR61 controls p53 and NF-κB expression through
PI3K/AKT/mTOR pathways in carboplatin-induced ovarian cancer cells.
Cancer Lett. 315:86–95. 2012. View Article : Google Scholar
|
|
44
|
Klionsky DJ: The molecular machinery of
autophagy: unanswered questions. J Cell Sci. 118:7–18. 2005.
View Article : Google Scholar
|
|
45
|
Dumont FJ and Su Q: Mechanism of action of
the immunosuppressant rapamycin. Life Sci. 58:373–395. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Windecker S, Roffi M and Meier B:
Sirolimus eluting stent: a new era in interventional cardiology?
Curr Pharm Des. 9:1077–1094. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao XM, Wong G, Wang B, et al: Inhibition
of mTOR reduces chronic pressure overload cardiac hypertrophy and
fibrosis. J Hypertens. 24:1663–1670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Simler NR, Howell DC, Marshall RP, et al:
The rapamycin analogue SDZ RAD attenuates bleomycin-induced
pulmonary fibrosis in rat. Eur Respir J. 19:1124–1127. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Achenbach TV, Barrow RK and Heerneier K:
Oligonucleotide-based knockdown technologies: antisense versus RNA
interference. Chembiochem. 4:928–935. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Burnet PE, Barrow RK, Cohen NA, Snyder SH
and Sabatini DM: RAFT1 phosphorylation of the translational
regulators p70 S6 kinase and 4E-BP1. Proc Nat Acad Sci USA.
95:1432–1437. 1998. View Article : Google Scholar
|