Introduction
Several epidemiological studies and systematic
reviews have identified a lower cancer prevalence among patients
with type 2 diabetes mellitus who take metformin (1–3), and
recent in vitro and in vivo studies have shown that
metformin inhibits cancer cell proliferation (4,5).
Cytotoxic chemotherapy has long been used to treat cancer. Focus
has been placed on the use of metformin combined with other drugs
to improve treatment efficacy (4,5).
Although recent studies have assessed combination
treatment approaches with metformin in vitro (6–20), the
findings have been controversial. First, the concentrations of
metformin used in the majority of these studies were much higher
(0.5–20 mM) than the clinically relevant doses (4,5,21,22),
and it is therefore unclear whether those concentrations are
effective for cancer treatment (4,5).
Second, results from the aforementioned studies have also been
controversial, with some reports describing a synergistic or
additive effect of combined drug treatment (6–17,19,20),
while antagonistic effects were observed in tumor cells of the
brain, blood, soft tissue, and lung (with the exception of
adenocarcinomas) as well as adriamycin-resistant breast cancer
cells, where the majority of these cells were non-carcinoma or
specific types of carcinoma cells (10,12,14,16,18).
In addition, metformin was shown to accelerate the growth of
BRAFV600E-driven melanoma (23).
Thus, the anti-proliferative effects of metformin may be cancer
type-specific.
These contradictory findings may be due to
differences in methodology and quantification. Colorimetric assays
such as the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT) assay have been widely used in high-throughput drug
screens that are based on the assessment of cell proliferation or
viability (24,25). Several agents that can influence the
results of colorimetric assays have been recognized, such as the
production of reactive oxygen species by mitochondria (26), as well as drugs that induce G2/M
cell cycle arrest which can lead to an increase in cell size, which
in turn affect the results of the MTT assay (27). Thus, caution must be applied to the
interpretation of results from colorimetric assays.
Endometrial cancer patients may benefit from
combination drug treatment for the following reasons. First,
metformin improves resistance to insulin, which is believed to play
a role in the development of endometrial cancer (28–30).
Second, chemotherapy has been successfully used to treat
endometrial cancer (31,32). In two recent in vitro
studies, the efficacy of combination treatment on endometrial
cancer cell lines using colorimetric assays to analyze cell
proliferation was investigated (6,7).
However, experiments conducted in those studies used metformin
concentrations of 0.5 and 1.0 mM which, as explained above, may
lead to unreliable conclusions (6,7). We
therefore examined the effects of combined metformin and cisplatin
treatment in an endometrial cancer cell line using various assays
to measure cell proliferation and other parameters associated with
cancer progression.
Materials and methods
Cell culture
The Ishikawa endometrial cancer cell line was grown
in Dulbecco’s modified Eagle’s medium supplemented with 4.5 g/l
glucose, 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml
streptomycin, and 100 μg/ml kanamycin sulfate (all from Life
Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere
of 5% CO2 and 21% O2 for normoxic or 1%
O2 for hypoxic conditions. A range of concentrations was
assessed for cisplatin (0–5 μM) (Bristol-Myers-Squibb, New York,
NY, USA) and metformin (0–10 mM) (Sigma Aldrich, St. Louis, MO,
USA) in the cell proliferation assay. Subsequent experiments using
a combination of the two drugs were performed with 0–2 mM metformin
and 1 μM cisplatin.
Cell quantification
Cells were counted as an index of cell
proliferation. A total of 2×103 cells/well was seeded in
96-well plates and incubated for 24 h. The medium was replaced, and
metformin and/or cisplatin were added as indicated. After 48 h, the
cells were observed using a light microscope (Diaphot-TMD; Nikon,
Tokyo, Japan) and counted using a hemocytometer.
Colorimetric assay
The MTT assay was used to assess cell viability.
This colorimetric assay measures the absorbance of formazan
molecules that are produced by the reduction of the tetrazolium
salt by cellular enzymes. The protocol was performed according to
the manufacturer’s instructions. Briefly, cells were seeded in
96-well plates at 2×103 cells/well and incubated for 24
h. The medium was replaced, and metformin and/or cisplatin were
added as indicated. After 48 h, the chromogenic reagent was added
to the culture medium for 3 h. After removing the culture medium,
the formazan crystals were dissolved by adding isopropanol and
dimethyl sulfoxide, and absorbance at 570 nm was measured using an
automated microplate reader (Infinite 200; Tecan, Männedorf,
Switzerland).
Cell size measurement
Changes in cell size after treatment with metformin
or cisplatin were determined from images obtained by light
microscopy. A total of 2×104 cells/well was seeded in
12-well plates and incubated for 24 h, then treated with metformin
or cisplatin for 48 h. The cells were observed and imaged using a
light microscope (Diaphot-TMD; Nikon). The cell area was measured
from the images of 100 cells for each drug concentration using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Flow cytometry
The effect of drug treatment on the cell cycle was
determined by flow cytometry, using the CycleTEST PLUS DNA Reagent
kit (Becton-Dickinson, Franklin Lake, NJ, USA) according to the
manufacturer’s instructions. The kit enabled the isolation of cells
with propidium iodide-stained nuclei. Between 1×105 and
1×106 cells were seeded in a 6-cm dish and incubated for
24 h. The medium was replaced and combinations of metformin (0, 0.1
and 2.0 mM) and cisplatin (1 μM) were added. The cells were sorted
48 h later using a FACSCalibur flow cytometer
(Becton-Dickinson).
Caspase activity assay
Caspase activity was determined using the
Caspase-Glo 3/7 assay (Promega, Madison, WI, USA) 48 h after drug
treatment. This assay is based on the cleavage of a caspase-3/7
substrate, which produces a free aminoluciferin that is consumed by
luciferase and generates a luminescent signal proportional to
caspase activity. The protocol was performed according to the
manufacturer’s instructions. A total of 2×103 cells/well
was seeded in 96-well plates and incubated for 24 h. The medium was
then replaced and combinations of metformin (0–2 mM) and cisplatin
(1 μM) were added. After 48 h, the caspase-3/7 substrate was added
to the culture medium for 60 min, and luciferase activity was
measured using an automated microplate luminometer (Infinite 200;
Tecan). Cells in other wells were also counted with a hemocytometer
using a microscope. Luminescence values were divided by the total
number of counted cells to calculate the luminescence per cell.
Thymidine incorporation assay
The thymidine incorporation assay, which quantifies
[3H]thymidine uptake during DNA synthesis, was used to
measure cell proliferation. A total of 2×103 cells/well
was seeded in 96-well plates and incubated for 24 h. The culture
medium was replaced and metformin (0–10 mM) or cisplatin (0–5 μM)
was added for 48 h, or combinations of metformin (0–5 mM) and
cisplatin (0–5 μM) were added for 24 h, followed by
[3H]thymidine for 3 h. The experiment for combination
treatments was performed under normoxic and hypoxic conditions,
since many types of tumor cell are thought to tolerate hypoxia
in vivo.
Mitochondrial and nuclear staining
Mitochondria were stained with MitoTracker Red
CMXRos (Life Technologies) according to the manufacturer’s
instructions. A total of 4×104 cells/well was seeded in
6-well plates or glass-bottom dishes (for imaging) and incubated
for 24 h. The medium was replaced and metformin was added for 8 or
48 h. The staining solution was prepared at a concentration of 100
nM and added to the culture medium. After a 15-min incubation, the
cells were washed with PBS and fluorescence was observed by
confocal microscopy (FV10i-LIV; Olympus, Tokyo, Japan) and analyzed
by flow cytometry. Nuclei were stained with Hoechst 33342 (1 μg/ml)
(Dojindo, Kumamoto, Japan) for 20 min and then washed with PBS.
Measurement of lactate concentration
Lactate concentration in the cell culture medium was
used as a measure of mitochondrial function. A total of
2–2.5×104 cells/well was seeded in 12-well plates and
incubated for 24 h, then treated with metformin as indicated. The
medium was collected and lactate concentrations were measured 48 h
later. The cells were also counted with a hemocytometer using a
microscope. The lactate concentration was divided by the total
number of counted cells to calculate the lactate production per
cell.
Statistical analysis
Data are shown as the mean ± standard error. Data
were analyzed with SPSS ver. 21.0 (SPSS Inc., Chicago, IL, USA)
using the Student’s t-test, except for flow cytometry data for
which the Mann-Whitney U test was used. P<0.05 was considered
statistically significant.
Results
Cell proliferation and cell viability
assays yield discrepant results
Cell proliferation was assessed by cell counts as
well as colorimetric and thymidine incorporation assays in Ishikawa
cells treated for 48 h with metformin or cisplatin. Each drug alone
inhibited cell proliferation in a dose-dependent manner, as
determined by cell counts (Fig. 1).
However, significant discrepancies were observed between these
results and those of the colorimetric and thymidine incorporation
assays. For instance, at 10 mM metformin, the proliferative
fraction as measured by thymidine incorporation and the MTT assay
was 46% lower and 23% higher, respectively, than the value obtained
from the cell counts (Fig. 1A).
Similarly, at a cisplatin concentration of 0.5 μM, the
proliferative fraction as measured by thymidine incorporation was
~43% lower than the value obtained by the cell counts, while the
fraction determined using the MTT assay was almost 53% higher
(Fig. 1B). A similar trend was
observed for higher concentrations of cisplatin. As results from
the colorimetric assay were consistently higher than values
obtained from the cell counts, the assay was not used further in
this study to assess cell proliferation.
Cisplatin induces the enlargement of
endometrial cancer cells
Cell size was measured to determine the efficacy of
each drug. Treatment with cisplatin at concentrations of 0.5 and 2
μM led to an increase in cell size compared to the control cells,
while treatment with metformin at concentrations of 2 and 10 mM led
to a decrease in cell size compared to the control cells (Table I).
 | Table ICell size (area) in Ishikawa cells
treated with MET or CDDP. |
Table I
Cell size (area) in Ishikawa cells
treated with MET or CDDP.
| Drug treatment | Cell area
(ratio) |
|---|
| Control | 1.00±0.06 |
| CDDP 0.5 μMa | 1.30±0.10 |
| CDDP 2 μMa | 2.33±0.15 |
| MET 2 mMa | 0.84±0.04 |
| MET 10 mMa | 0.63±0.03 |
Metformin potentiates the anticancer
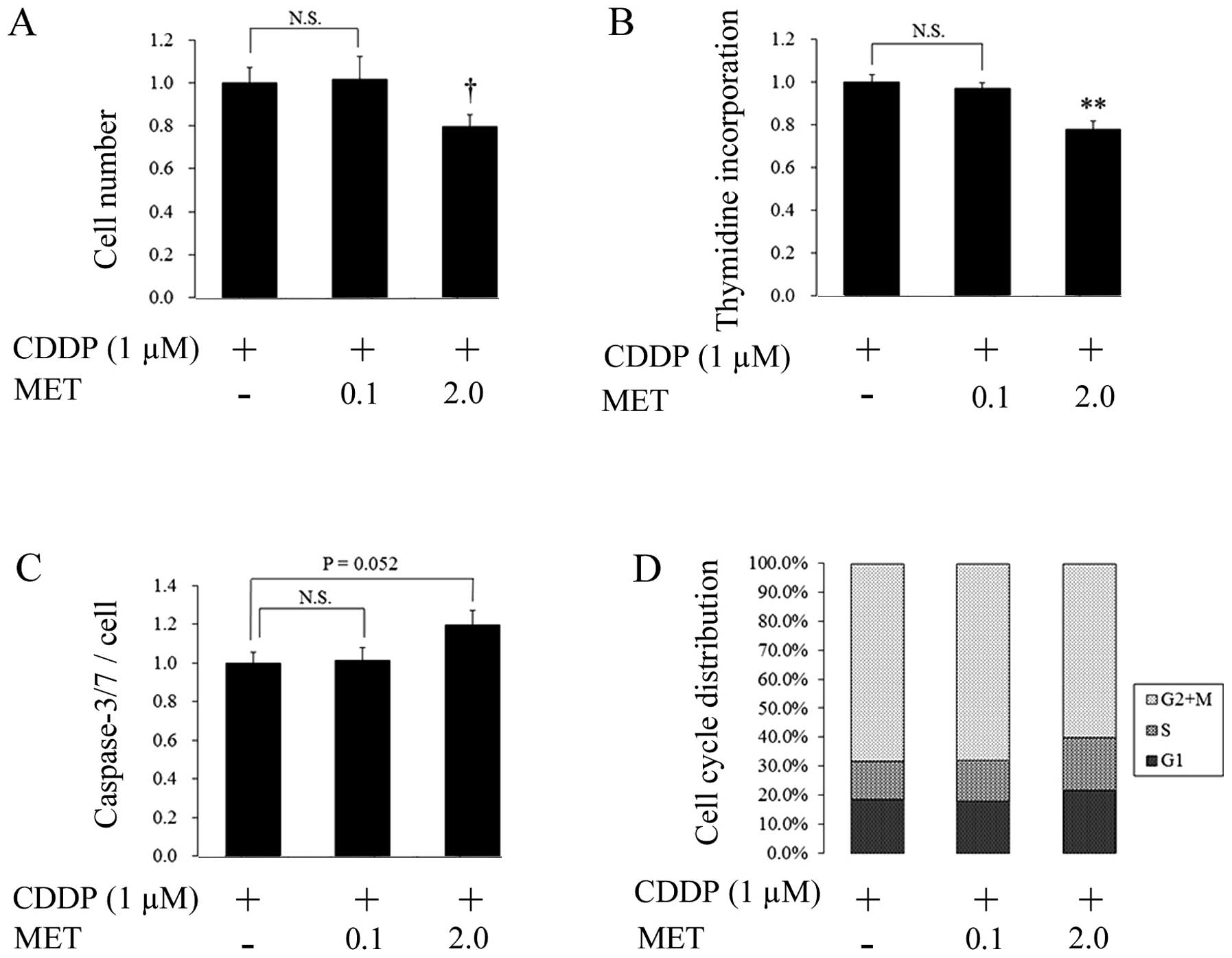
effects of cisplatin in endometrial cancer cells
The combined treatment of metformin and cisplatin
had additive, dose-dependent, anticancer effects compared to the
cells treated with cisplatin alone (Fig. 2). For instance, 0.1 mM metformin + 1
μM cisplatin had no effect on cell numbers, but a higher
concentration of metformin (2 mM) with cisplatin reduced
proliferation by 20% (Fig. 2A).
Similarly, following the treatment of cells with 2 mM metformin + 1
μM cisplatin, thymidine incorporation was decreased by a similar
amount (Fig. 2B). Caspase activity
and G1/S arrest also increased slightly, although these differences
were not statistically significant (P=0.052 and 0.083,
respectively) (Fig. 2C and D).
Anticancer effects of combined metformin
and cisplatin treatment are attenuated under hypoxic
conditions
Metformin has been reported to inhibit complex I of
the mitochondrial respiratory chain (33,34),
suggesting that the anticancer effects of combined metformin and
cisplatin treatment could be compromised under hypoxic conditions.
Notably, while thymidine incorporation was reduced in cells treated
with 2 or 5 mM metformin and various concentrations of cisplatin
(0–5 μM) relative to cells treated with cisplatin alone under
normoxic conditions (Fig. 3Aa and
Ba), these differences were attenuated when cells were
subjected to hypoxia (Fig. 3Ab and
Bb).
Metformin treatment inhibits
mitochondrial function
To examine the effects of metformin on mitochondria,
the cells treated with metformin for 48 h were stained with
MitoTracker dye. After drug treatment, the mitochondria were more
compact, and connections between them were less apparent (Fig. 4A). The intensity of staining
decreased with higher concentrations of metformin (Fig. 4B). To exclude the effects of cell
shrinkage that were observed after a 48-h exposure to metformin,
the cells were stained and examined by flow cytometry after 8 h of
drug treatment (Fig. 4C). Following
metformin treatment, the fraction of cells with low levels of
fluorescence was greater than that for the control cells,
indicating that the decreased signal intensity in mitochondria
induced by the drug was not due to reductions in cell size. Lactate
concentrations in the culture medium were then measured, since
glycolysis is stimulated by the impairment of mitochondrial
function by metformin (33,34). Lactate levels showed a
dose-dependent increase following treatment with metformin
(Fig. 4D). Taken together, these
results indicate that cisplatin exerts anticancer effects in
endometrial cancer cells that are potentiated by metformin under
normoxic conditions, but during hypoxia, the additive effects of
this drug combination are attenuated, which may be linked to the
action of metformin on mitochondria.
Discussion
The results of the present study have demonstrated
that metformin potentiates, without antagonizing, the anticancer
effects of cisplatin in endometrial cancer cells under normoxic
conditions. Cells treated with a combination of the two drugs had
greater reductions in cell proliferation, as assessed by cell
counts and thymidine incorporation, than cells treated with
cisplatin alone (Fig. 2).
Significantly, these additive effects were not observed at
metformin concentrations of 0.1 mM. Thus, a combination treatment
regimen of metformin and cisplatin may not be useful for
endometrial cancer patients, since the lowest concentration of
metformin tested in this study was several times higher than the
clinically prescribed doses for the treatment of diabetes, which
resulted in plasma metformin concentrations of 0.6±0.5 mg/l
(22).
This discrepancy in dosing between in vitro
studies and clinical observations is typically overlooked (5). Metformin uptake has been observed in
many tissues (21), and this
potential for accumulation in organs may necessitate concentrations
that are higher than those indicated by plasma levels for effective
cancer therapy (4). Other studies
have shown that metformin does not accumulate in the plasma nor in
any tissues other than the intestine, even with repeated
administration (35). However,
these results are from animal studies, and there are little data
available on metformin levels in human organs (36).
Mitochondrial uncoupling proteins have been shown to
prevent tumor growth in breast cancer cells (37). In Ishikawa cells, metformin
treatment led to decreased staining with MitoTracker dye (Fig. 4), which is used as a measure of
mitochondrial integrity (38).
Thus, metformin likely inhibits cancer cell proliferation by
inducing mitochondrial dysfunction. The present study also found
that the additive effects of metformin in combination with
cisplatin were attenuated under hypoxic conditions (Fig. 3). Metformin inhibits complex I of
the respiratory chain in mitochondria (33,34),
which may not be relevant under low O2 conditions since
this complex is involved in oxidative phosphorylation. Taken
together, these results suggest that metformin has limited utility
for endometrial cancer treatment, since tumor cells often exist in
hypoxic microenvironments in which adaptive mechanisms enable them
to thrive.
The first indications that metformin can be
clinically beneficial for cancer patients were from epidemiological
studies (1), in which cancer
incidence and cancer-related mortality were found to be reduced in
diabetic patients taking metformin (2,3). Many
patients with endometrial cancer have concurrent diabetes and
insulin resistance (28–30), and obesity and insulin resistance
are risk factors for endometrial cancer (39). Furthermore, in a retrospective
analysis of neoadjuvant chemotherapy for breast cancer, metformin
significantly increased the frequency of pathological complete
response in diabetic compared to non-diabetic patients (40). Although in vitro studies have
examined the direct effects of metformin on cancer cells, results
of recent studies suggest that indirect effects may be important in
tumor growth inhibition in vivo (5), for instance by lowering blood glucose
and insulin levels (41). Thus, it
is possible that metformin can be used effectively to treat
endometrial cancer through combination treatments consisting of,
for instance, the continuous administration of metformin and cyclic
administration of cisplatin.
In conclusion, metformin potentiated the anticancer
effects of cisplatin under normoxic conditions in vitro.
Although these effects were attenuated under hypoxia, the reasons
for which are to be the focus of future studies, there was no
evidence of antagonism of cisplatin by metformin. Thus, the results
offer the potential for a combination chemotherapeutic strategy
that can be applied to endometrial cancer treatment.
Acknowledgements
This study was supported by the Japan Society for
the Promotion of Science KAKENHI Grant-in-Aid for Young Scientists
(B) (grant no. 24791686).
References
|
1
|
Evans JM, Donnelly LA, Emslie-Smith AM, et
al: Metformin and reduced risk of cancer in diabetic patients. BMJ.
330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Decensi A, Puntoni M, Goodwin P, et al:
Metformin and cancer risk in diabetic patients: a systematic review
and meta-analysis. Cancer Prev Res (Phila). 3:1451–1461. 2010.
View Article : Google Scholar
|
|
3
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: a
systematic review and meta-analysis. PLoS One. 7:e334112012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin-Castillo B, Vazquez-Martin A,
Oliveras-Ferraros C and Menendez JA: Metformin and cancer: doses,
mechanisms and the dandelion and hormetic phenomena. Cell Cycle.
9:1057–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quinn BJ, Kitagawa H, Memmott RM, et al:
Repositioning metformin for cancer prevention and treatment. Trends
Endocrinol Metab. 24:469–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanna RK, Zhou C, Malloy KM, et al:
Metformin potentiates the effects of paclitaxel in endometrial
cancer cells through inhibition of cell proliferation and
modulation of the mTOR pathway. Gynecol Oncol. 125:458–469. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong L, Zhou Q, Zhang Z, et al: Metformin
sensitizes endometrial cancer cells to chemotherapy by repressing
glyoxalase I expression. J Obstet Gynaecol Res. 38:1077–1085. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shank JJ, Yang K, Ghannam J, et al:
Metformin targets ovarian cancer stem cells in vitro and in vivo.
Gynecol Oncol. 127:390–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Scholz C, Zang C, et al: Metformin
and the mTOR inhibitor everolimus (RAD001) sensitize breast cancer
cells to the cytotoxic effect of chemotherapeutic drugs in vitro.
Anticancer Res. 32:1627–1637. 2012.PubMed/NCBI
|
|
10
|
Ashinuma H, Takiguchi Y, Kitazono S, et
al: Antiproliferative action of metformin in human lung cancer cell
lines. Oncol Rep. 28:8–14. 2012.PubMed/NCBI
|
|
11
|
Rattan R, Graham RP, Maguire JL, et al:
Metformin suppresses ovarian cancer growth and metastasis with
enhancement of cisplatin cytotoxicity in vivo. Neoplasia.
13:483–491. 2011.PubMed/NCBI
|
|
12
|
Kim HG, Hien TT, Han EH, et al: Metformin
inhibits P-glycoprotein expression via the NF-κB pathway and CRE
transcriptional activity through AMPK activation. Br J Pharmacol.
162:1096–1108. 2011. View Article : Google Scholar :
|
|
13
|
Rocha GZ, Dias MM, Ropelle ER, et al:
Metformin amplifies chemotherapy-induced AMPK activation and
antitumoral growth. Clin Cancer Res. 17:3993–4005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nilsson S, Huelsenbeck J and Fritz G:
Mevalonate pathway inhibitors affect anticancer drug-induced cell
death and DNA damage response of human sarcoma cells. Cancer Lett.
304:60–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janjetovic K, Vucicevic L, Misirkic M, et
al: Metformin reduces cisplatin-mediated apoptotic death of cancer
cells through AMPK-independent activation of Akt. Eur J Pharmacol.
651:41–50. 2011. View Article : Google Scholar
|
|
17
|
Yasmeen A, Beauchamp MC, Piura E, et al:
Induction of apoptosis by metformin in epithelial ovarian cancer:
involvement of the Bcl-2 family proteins. Gynecol Oncol.
121:492–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harhaji-Trajkovic L, Vilimanovich U,
Kravic-Stevovic T, et al: AMPK-mediated autophagy inhibits
apoptosis in cisplatintreated tumour cells. J Cell Mol Med.
13:3644–3654. 2009. View Article : Google Scholar
|
|
19
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gotlieb WH, Saumet J, Beauchamp MC, et al:
In vitro metformin anti-neoplastic activity in epithelial ovarian
cancer. Gynecol Oncol. 110:246–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graham GG, Punt J, Arora M, et al:
Clinical pharmacokinetics of metformin. Clin Pharmacokinet.
50:81–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lalau JD, Lacroix C, Compagnon P, et al:
Role of metformin accumulation in metformin-associated lactic
acidosis. Diabetes Care. 18:779–784. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin MJ, Hayward R, Viros A and Marais
R: Metformin accelerates the growth of BRAF V600E-driven melanoma
by upregulating VEGF-A. Cancer Discov. 2:344–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berridge MV, Herst PM and Tan AS:
Tetrazolium dyes as tools in cell biology: new insights into their
cellular reduction. Biotechnol Annu Rev. 11:127–152. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishiyama M, Miyazono Y, Sasamoto K, et al:
A highly water-soluble disulfonated tetrazolium salt as a
chromogenic indicator for NADH as well as cell viability. Talanta.
44:1299–1305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collier AC and Pritsos CA: The
mitochondrial uncoupler dicumarol disrupts the MTT assay. Biochem
Pharmacol. 66:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pagliacci MC, Spinozzi F, Migliorati G, et
al: Genistein inhibits tumour cell growth in vitro but enhances
mitochondrial reduction of tetrazolium salts: a further pitfall in
the use of the MTT assay for evaluating cell growth and survival.
Eur J Cancer. 29A:1573–1577. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burzawa JK, Schmeler KM, Soliman PT, et
al: Prospective evaluation of insulin resistance among endometrial
cancer patients. Am J Obstet Gynecol. 204:355.e1–7. 2011.
View Article : Google Scholar
|
|
29
|
Soliman PT, Wu D, Tortolero-Luna G, et al:
Association between adiponectin, insulin resistance, and
endometrial cancer. Cancer. 106:2376–2381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mu N, Zhu Y, Wang Y, et al: Insulin
resistance: a significant risk factor of endometrial cancer.
Gynecol Oncol. 125:751–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johnson N, Bryant A, Miles T, et al:
Adjuvant chemotherapy for endometrial cancer after hysterectomy.
Cochrane Database Syst Rev. 10:CD0031752011.PubMed/NCBI
|
|
32
|
Vale CL, Tierney J, Bull SJ and Symonds
PR: Chemotherapy for advanced, recurrent or metastatic endometrial
carcinoma. Cochrane Database Syst Rev. 8:CD0039152012.PubMed/NCBI
|
|
33
|
El-Mir MY, Nogueira V, Fontaine E, et al:
Dimethylbiguanide inhibits cell respiration via an indirect effect
targeted on the respiratory chain complex I. J Biol Chem.
275:223–228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owen MR, Doran E and Halestrap AP:
Evidence that metformin exerts its anti-diabetic effects through
inhibition of complex 1 of the mitochondrial respiratory chain.
Biochem J. 348:607–614. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilcock C and Bailey CJ: Accumulation of
metformin by tissues of the normal and diabetic mouse. Xenobiotica.
24:49–57. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cantrell LF, Nelson CL, Gary RD and
McIntyre IM: Fatal metformin intoxication with markedly elevated
blood and liver concentrations. J Anal Toxicol. 36:657–659. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sanchez-Alvarez R, Martinez-Outschoorn UE,
Lamb R, et al: Mitochondrial dysfunction in breast cancer cells
prevents tumor growth: understanding chemoprevention with
metformin. Cell Cycle. 12:172–182. 2013. View Article : Google Scholar :
|
|
38
|
Poot M, Zhang YZ, Krämer JA, et al:
Analysis of mitochondrial morphology and function with novel
fixable fluorescent stains. J Histochem Cytochem. 44:1363–1372.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Renehan AG, Tyson M, Egger M, et al:
Body-mass index and incidence of cancer: a systematic review and
meta-analysis of prospective observational studies. Lancet.
371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiralerspong S, Palla SL, Giordano SH, et
al: Metformin and pathologic complete responses to neoadjuvant
chemotherapy in diabetic patients with breast cancer. J Clin Oncol.
27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goodwin PJ, Pritchard KI, Ennis M, et al:
Insulin-lowering effects of metformin in women with early breast
cancer. Clin Breast Cancer. 8:501–505. 2008. View Article : Google Scholar : PubMed/NCBI
|