Introduction
Squamous cell carcinoma of the head and neck (SCCHN)
accounts for over 90% of all head and neck cancer (1). The 5-year survival for patients is
only 30–40%, mainly due to lymphatic metastatic and local
recurrence (2). No significant
increase in the long-term survival rate has been achieved during
the past 30 years, despite substantial improvements in the surgical
management of radiotherapy and chemotherapy for SCCHN. The
mechanisms determining the directional migration and invasion of
SCCHN cells into specific organs remain to be elucidated.
Chemokines induce cytoskeleton rearrangement, firm
adhesion to endothelial cells, and directional migration by binding
to G-protein-coupled receptors (3).
Chemokines and their respective receptors were involved in the
metastasis of several types of cancer (4–8). The
metastatic SCCHN cells were shown to express chemokine receptor 7
(CCR7), which activated phosphoinositide-3 kinase (PI3K) Cdc42,
Pyk2, mTOR and NF-κB pathways to promote ‘homing’ into lymph nodes
and support their survival (9–12).
However, when these downstream molecules were inhibited, the effect
of CCR7 was not blocked completely, revealing the involvement of
other downstream signaling molecules in the process.
Attention has focused on the involvement of Rho
family GTPases and their downstream effectors in chemokine-elicited
migration (13–15). Important Rho effectors are the
serine/threonine kinase Rho-associated kinase (ROCK) (16). ROCKs are important Rho effectors
that enhance myosin light chain (MLC) and cofilin phosphorylation,
thus regulating actin-myosin contraction (17) and cell motility (18). Pyk2 is another mediator of chemokine
receptors. It has been reported that the Rho/Pyk2/cofilin pathway
controls chemotaxis and the migration speed of dendritic cells
(14).
In the present study we examined whether RhoA was
activated by CCR7, as well as the role and the molecular mechanisms
of the RhoA pathway in CCR7 regulating SCCHN metastasis. The
results showed that CCL19 induced activation of the
Rho/ROCK-Pyk2-cofilin pathway, whereas MLC was not involved in the
process. Since chemical inhibitors of this signal transduction
pathway are currently in use clinically (19), our results suggested that the ROCK
inhibitor Y-27632 may be useful for treating SCCHN patients.
Materials and methods
Cell line and human tumor samples
The PCI-37B metastatic SCCHN cell line, which
strongly expresses CCR7, was a kind gift from the University of
Pittsburgh (12). Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen,
Carlsbad, CA, USA), which contained 10% fetal bovine serum
(Gibco-BRL Corporation, Grand Island, NY, USA), 100 U/ml penicillin
and 100 U/ml streptomycin. When inhibitors were used, treatment at
the dose used did not affect cell viability or CCR7 expression.
SCCHN tissue specimens were obtained from 75
patients by biopsy prior to chemotherapy or radiotherapy at the
Department of Oral and Maxillofacial Surgery, School of
Stomatology, China Medical University. The term ‘metastatic’ in the
present study refers to patients with positive lymph nodes that
were recognized at initial presentation or later based on
histopathological diagnosis following neck dissection. The
classification of SCCHN, including primary tumors (T), regional
lymph nodes (N), distant metastasis (M) and stage grouping, was
determined according to the regulations of the Union for
International Cancer Control (UICC) for Head and Neck Cancer
[tumor-node-metastasis (TNM) classification, 1997]. Ten samples of
normal tissues adjacent to the benign tumor were chosen as
controls. The study protocol was approved by the Medical Ethics
Committee of the Affiliated Stomatological Hospital of China
Medical University and performed according to the declaration of
Helsinki. All the specimens were obtained with the consent of the
patients prior to surgery and in accordance with the Health
Insurance Portability. Written informed consent was obtained from
all individuals.
Reagents and antibodies
The CCR7 chemokine ligand, CCL19 (MIP-3β) was
purchased from R&D Systems (Minneapolis, MN, USA). Mouse
anti-CCR7 antibody was purchased from BD Biosciences (San Jose, CA,
USA). C3 exoenzyme (Rho inhibitor) was purchased from Cytoskeleton
Inc. (Denver, CO, USA) and Y-27632 (ROCK inhibitor) was purchased
from Sigma (Santa Clara, CA, USA). Tyrphostin A9 (Pyk2 inhibitor)
was purchased from Calbiochem (San Diego, CA, USA). Anti-RhoA,
anti-phospho-Pyk2 (Tyr402), anti-phospho-cofilin (Ser3) and
anti-phospho-myosin light chain (Ser19) were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Bio-Rad protein
assay dye reagent was purchased from Bio-Rad Laboratories
(Richmond, CA, USA). Enhanced chemiluminescence was purchased from
Amersham Pharmacia Biotechnology (Piscataway, NJ, USA).
Immunohistochemical analysis
Immunohistochemical staining used conventional
horseradish peroxidase immunohistochemical staining methods.
Briefly, 5-μm sections of the specimens were deparaffinized and
hydrated with 0.6% H2O2 in methanol to
inhibit endogenous peroxidase. Antigen retrieval was performed and
slides were incubated with normal blocking serum for 10 min.
Sections were incubated with primary antibodies (1:100 dilution):
CCR7-specific monoclonal antibody, and rabbit anti-RhoA polyclonal
antibody overnight at 4°C. Immunodetection was performed using
peroxidase-labeled secondary antibody (R&D Systems) and
diaminobenzidine for visualization. The sections were
counterstained with hematoxylin (Sigma). Negative controls included
omission of the primary antibody. Cell morphology was analyzed by
microscopy (Nikon Eclipse 80i; Tokyo, Japan) at ×100–400
magnification. According to the percentage of positive tumor cells,
the cells were scored as negative (−), <10% or no staining; weak
positive (+), 11–50%; positive (++), 51–75%; or strongly positive
(+++), >75%.
Chemotaxis assay
Chemotaxis in response to chemokines was measured by
counting cells migrating through a polycarbonate filter (8-μm pore
size) in 24-well Transwell chambers in triplicate in DMEM with 0.5%
(w/v) BSA (Invitrogen). Cell suspensions (2×105
cells/200 μl) were placed in the top chamber of the filter.
Aliquots of chemokines were added to wells. After 24 h, the cells
in each lower well were counted under a light microscope in at
least five different fields (original magnification, ×200). Means ±
SD were recorded for each condition, and an index was calculated
based on the control involving random migration.
Matrigel invasion assay
Cell invasion was quantified in vitro using
matrigel-coated semipermeable, modified inserts with a pore size of
8-μm. Analysis of the invasion assay was performed as described in
the chemotaxis assay incubated with CCL19 for 36 h. An invasion
index was normalized to non-specific cell invasion (from
media-pulsed wells).
Scrape wound-healing assay
Migration of PCI-37B cells was measured using an
in vitro monolayer wound assay (20). Cells grown in 24-well plates in DMEM
to confluence were scraped with a pipette tip to create a cell-free
area. Wounded monolayers were washed with serum-free media. The
cells were pretreated with/without RhoA inhibitor C3 exoenzyme (50
ng/ml), Y-27632 ROCK inhibitor (10 M) and CCL19 (200 ng/ml) for 1
h. Wound closure was followed after 0 and 24 h. The wound-healing
effect was calculated as the percentage of the remaining cell-free
area compared to the initial wound area (arbitrarily set as 100%).
Monolayer wound assays included serum-free DMEM controls. The
wounds were observed by phase contrast microscopy (Nikon TE2000-S
Eclipse) at ×200 magnification and images captured were documented
with photography. Each experiment was performed in triplicate,
analyzing four or five scratches per well. The relative cell-free
area was calculated based on the control group.
RhoA pull-down assay
RhoA-GTP was measured with recombinant purified
glutathione-S-transferase rhotekin Rho-binding domain (GST-TRBD)
bound to glutathione beads and purified as previously described
(21). SCCHN cells were stimulated
with CCL19 in serum-free medium lysed with ice-cold RIPA buffer and
cleared by centrifugation. SCCHN cell lysates were incubated for 1
h at 4°C with GST or GST-TRBD coupled to glutathione-sepharose
beads. Beads were then washed and boiled in SDS sample buffer and
precipitates were analyzed with immunoblotting using an anti-RhoA
antibody and then developed using chemiluminescence.
Western blotting
Cells were harvested in a lysis buffer (10 mM
Tris-HCl, pH 7.6, 50 mM NaF, 1 mM NaV3O4, 1%
Triton X-100 and 1X protease inhibitor of protein tyrosine
phosphatases). Lysates were sonicated for 3 sec and centrifuged at
4°C at 12,000 rpm for 30 min. The supernatant was collected for
protein quantification using the Bio-Rad protein assay dye reagent.
Protein (50 mg) was size-fractionated through a 10% SDS-PAGE gel
and transferred onto nitrocellulose filters which were blocked (1%
non-fat dry milk, 0.1% Triton X-100, 150 mM NaCl, 50 mM Tris, pH
7.5) and incubated with primary antibody (1:1,000 dilution).
Nitrocellulose filters were incubated with horseradish
peroxidase-conjugated secondary antibodies and bands were
visualized with enhanced chemiluminescence and quantified by
scanning densitometry using ImageJ software.
Statistical analysis
Experiments were run in triplicate and repeated at
least three times. Numerical data were expressed as means ±
standard deviation (SD). Correlations were analyzed using the
Spearman’s and χ2 tests. Statistical differences between
two groups were evaluated using an unpaired Student’s t-test.
P<0.05 was considered to indicate a statistically significant
result. Statistical analyses were performed with SPSS 13.0
software.
Results
CCR7 and RhoA are overexpressed and CCR7
and RhoA expression is significantly positively correlated in
SCCHN
Immunohistochemistry was utilized to examine CCR7
and RhoA expression in SCCHN tumor tissues, metastatic and normal
lymph nodes and oral mucosal tissues. CCR7 and RhoA were evident in
the cell membrane and cytoplasm, and were mainly expressed in the
stromal surroundings of tumors and metastatic lymph node cells.
Stained cells were few or absent in normal lymph nodes and oral
mucosal tissues (Fig. 1). CCR7 and
RhoA expression was significantly correlated with cervical
lymph-node metastasis and SCCHN clinical stages (P<0.05;
Tables I and II). Spearman’s test indicated a
significant positive correlation between the expression of CCR7 and
RhoA (P<0.05; Table III).
 | Table ICorrelation between CCR7 expression
and SCCHN clinicopathological characteristics. |
Table I
Correlation between CCR7 expression
and SCCHN clinicopathological characteristics.
| Characteristics | Cases | +-+++ | + (%) | χ2 |
|---|
Age (years)
≥60/<60 | 39/36 | 29/25 | 74.4/69.4 | 0.224 |
| Male/female | 42/33 | 29/25 | 69.0/75.8 | 0.413 |
| Tumor size |
| T1, T2 | 24 | 16 | 66.7 | 0.498 |
| T3, T4 | 51 | 38 | 74.5 | 0.595 |
| High diff | 48 | 36 | 75 | |
| Low diff | 27 | 18 | 66.7 | |
| Clinical stage |
| I, II | 20 | 10 | 50 | 6.548a |
| III, IV | 55 | 44 | 80 | |
| Nodal
metastases |
| Yes | 30 | 27 | 90 | 8.036a |
| No | 45 | 27 | 60 | |
 | Table IICorrelation between RhoA expression
and SCCHN clinicopathological characteristics. |
Table II
Correlation between RhoA expression
and SCCHN clinicopathological characteristics.
|
Characteristics | Cases | +-+++ | + (%) | χ2 |
|---|
Age
(years)
≥60/<60 | 39/36 | 22/24 | 56.4/66.7 | 0.830 |
| Male/female | 42/33 | 29/17 | 69.0/51.5 | 2.395 |
| Tumor size |
| T1, T2 | 24 | 13 | 54.2 | 0.764 |
| T3, T4 | 51 | 33 | 64.7 | 0.077 |
| High diff | 48 | 30 | 62.5 | |
| Low diff | 27 | 16 | 59.3 | |
| Clinical stage |
| I, II | 20 | 6 | 30 | 11.29a |
| III, IV | 55 | 40 | 72.7 | |
| Nodal
metastases |
| Yes | 30 | 24 | 80 | 7.346a |
| No | 45 | 22 | 48.9 | |
 | Table IIICorrelations between the CCR7 and
RhoA expression in SCCHN primary tumor. |
Table III
Correlations between the CCR7 and
RhoA expression in SCCHN primary tumor.
| RhoA | |
|---|
|
| |
|---|
| CCR7 | +-+++ | - | Total |
|---|
| +-+++ | 36 | 18 | 54 |
| - | 10 | 11 | 21 |
| Total | 46 | 29 | 75 |
CCL19 induces RhoA GTPase activation in
PCI-37B
Since CCR7 regulates actin organization (22) and the latter can be controlled by
small GTPase Rho (16), we
investigated whether CCR7 induces the activation of RhoA in an
SCCHN cell line (PCI-37B). We measured activated RhoA (GTP-bound
form) with Rho-binding domain (RBD) pull-down assays using the Rho
effector protein, Rhotekin (23).
Limited active bound RhoA was observed in non-stimulated cells, and
this was increased after CCL19 stimulation. Although total RhoA
remained unchanged, activated RhoA increased significantly 10 min
after CCL19 treatment compared with the controls (P<0.05),
whereas CCR7mAb, a C3-exo-enzyme, partly inhibited the
CCL19-induced activation of RhoA compared with the
CCL19-stimulation group (P<0.05; Fig. 2).
RhoA/ROCK regulates CCR7-dependent
migration and invasion
Using chemotaxis and matrigel invasion assay we
analyzed the role of RhoA in PCI-37B migration and invasiveness in
response to CCL19. The presence of CCL19 in the lower chamber
stimulated a >1.5-fold increase in chemotaxis and invasion of
PCI-37B across the filter membrane while C3 and Y-27632 blocked
this activity (P<0.05; Figs. 3
and 4). In addition, we evaluated
the migration ability of PCI-37B induced by CCR7 using a scrape
wound-healing assay. The width of the wound, which was
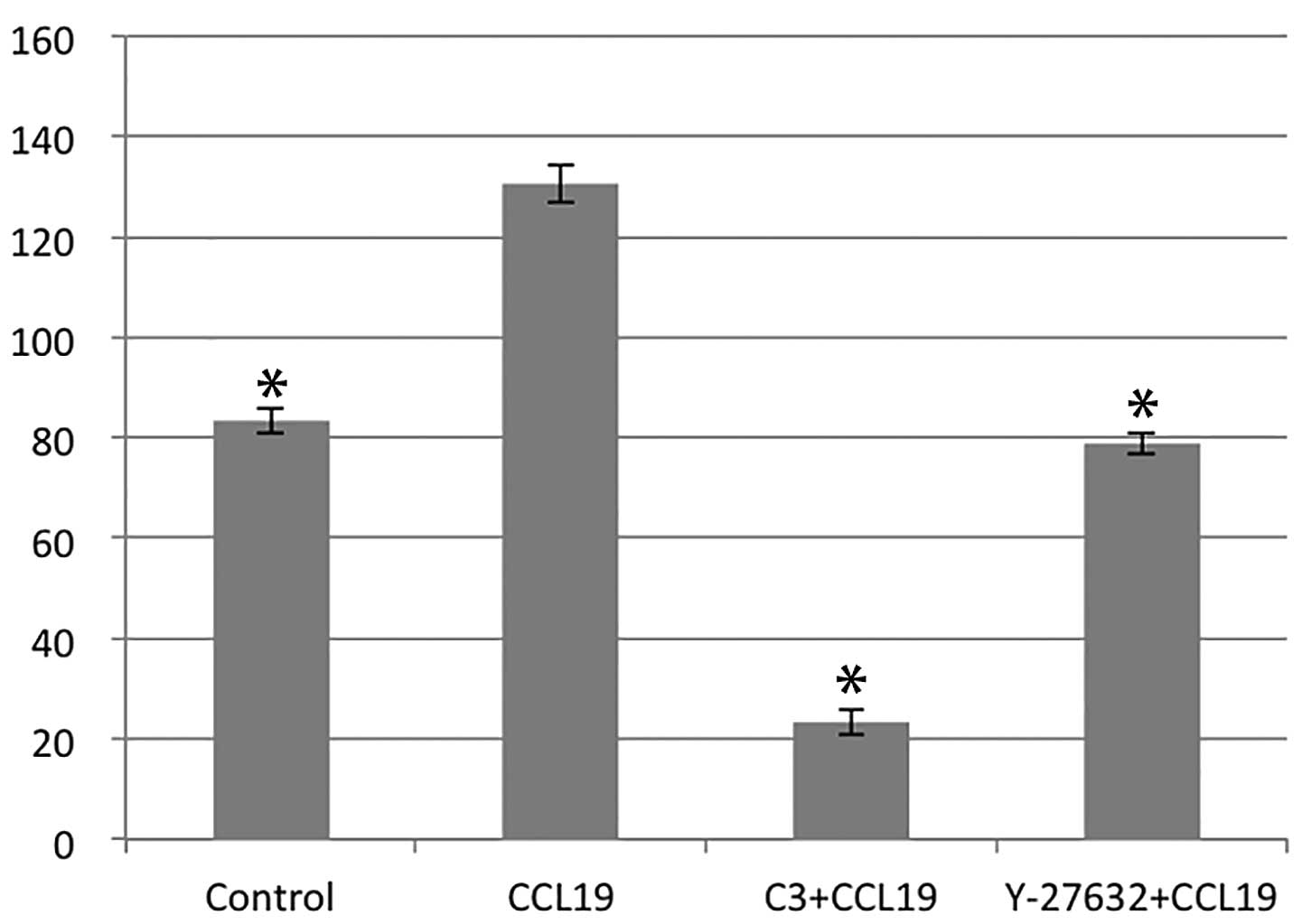
significantly reduced after 24 h in the presence of CCL19, and this
was blocked by C3 and Y-27632 (P<0.05; Fig. 5). Data show that RhoA/ROCK has a
role in the invasiveness of PCI-37B mediated by CCR7.
RhoA/ROCK mediates the activation of Pyk2
and cofilin induced by CCL19
Western blotting indicated that CCL19-pretreated
PCI-37B cells had an increased expression of p-Pyk2 (Fig. 6) and significantly decreased
expression of p-cofilin (Fig. 7),
which was reversed by CCR7mAb, suggesting CCR7-induced activation
of Pyk2 and cofilin. As expected, CCL19-dependent Pyk2
phosphorylation and cofilin dephosphorylation was abrogated in the
presence of RhoA, ROCK and Pyk2 inhibitors, suggesting that
RhoA/ROCK may mediate the activation of Pyk2 and cofilin induced by
CCL19. The results showed that MLC protein was not changed after
CCL19 stimulation, indicating that CCR7 has no effect on MLC
(Fig. 8).
Discussion
A critical problem in SCCHN therapy is metastasis,
particularly to lymph nodes, lung, liver and bone. Metastasis is
based on chemotaxis and the migratory ability of tumor cells. CCR7
induces chemotaxis and invasion of SCCHN cells via the activation
of several signaling pathways (9–11,24–26).
However, how migration is influenced by the signaling pathway
remains poorly understood. RhoA is also involved in regulating the
migration and invasion of diverse tumor types. Riol-Blanco et
al revealed that CCR7 activates the Rho/Pyk2/cofilin signaling
pathway, which controls chemotaxis and the migratory speed of
dendritic cells (14). Other
results also support a role for Rho/ROCK signaling in the
regulation of CCR7-dependent chronic lymphocytic leukemia migration
(15). However, no reports are
available to explain the role of RhoA in SCCHN. Our experiments
indicate that RhoA and CCR7 are highly expressed in SCCHN and
metastatic lymph-node cells. RhoA and CCR7 expression was
significantly correlated with cervical lymph-node metastasis and
SCCHN clinical stage. A Spearman’s test indicated a significant
positive correlation between expression of CCR7 and RhoA.
Therefore, RhoA may be necessary for the development and metastasis
of SCCHN, while CCR7 may regulate cell migration and survival via
the RhoA pathway in SCCHN.
RhoA is upregulated in various human tumor types.
RhoA stimulates cell cycle progression and cytokinesis, as well as
cell migration (27) through its
activity as a molecular switch cycling between an inactive
guanosine diphosphate (GDP)-bound and an active guanosine
triphosphate (GTP)-bound form (28,29). A
RhoA pull-down assay indicated that CCL19 (CCR7 ligand) was able to
induce RhoA activation 1.5-folds more compared with controls in
PCI-37B cell lines. The specific inhibitor of RhoA (C3 exoenzyme)
decreased RhoA-GTP to one-third of baseline and CCR7mAb decreased
RhoA-GTP significantly, however, the total RhoA did not change. The
results show that RhoA may participate in the downstream pathway of
CCR7 in SCCHN metastasis. Chemotaxis and Matrigel invasion, and
scrape wound-healing assay data confirmed that CCL19-induced cell
migration and invasion was >1.5-times greater than that of the
controls and this was blocked with C3 exoenzyme. These data also
confirm that RhoA may be an important downstream effector of the
CCR7 pathway in SCCHN metastasis.
Rho GTPases participate in various signal
transduction pathways via the activation of multiple downstream
effector proteins. An important downstream effector is ROCK
(27). Studies indicate that ROCK
is a downstream effector mediating Rho tumor growth potential.
ROCKs phosphorylate and activate LIMKs, which in turn phosphorylate
and inactivate cofilin, an actin-regulatory protein (27,30)
that regulates the migration and invasion of cancer cells (31). ROCKs also regulate actomyosin
contractility in cells by increasing the phosphorylation of MLC,
which in turn stimulates myosin II to interact with and move along
actin filaments (17).
Tyrosine kinase Pyk2 is involved in signaling from
chemokine receptors in different cells (14). Our recent results suggest that CCR7
promotes tumor metastasis by sequential activation of Pyk2 and
cofilin followed by rearrangement of F-actin in SCCHN cells
(25). However, whether RhoA
participated in that pathway is unknown. Western blot results
indicate that CCR7 induced the activation of Pyk2 and cofilin.
Furthermore, CCL19-dependent Pyk2 phosphorylation and cofilin
dephosphorylation was blocked by the RhoA and ROCK inhibitors,
suggesting that RhoA/ROCK may mediate the activation of cofilin and
Pyk2 induced by CCL19 in SCCHN cells. MLC protein was not changed
following CCL19 stimulation, suggesting that CCR7 has no effect on
MLC.
In conclusion, CCR7 promotes tumor migration and
invasiveness via the RhoA/ROCK pathway in metastatic SCCHN. Direct
inhibition of ROCK, a downstream molecule of Rho GTPase, using a
pharmacological inhibitor (Y-27632) or a molecular approach
(dominant-negative expression vector) can produce substantial
therapeutic effects suggesting that direct targeting of the
RhoA/ROCK pathway, alone or in combination with other targets, may
be promising as a chemotherapeutic target (32). Fasudil (Y-27632), a potent and
selective ROCK inhibitor, is relatively safe and effective for
treating cardiovascular disease including cerebral and coronary
vasospasm, angina, hypertension and heart failure. No serious
adverse side-effects have been reported thus far (33). Future studies are needed to identify
the effectiveness and safety of ROCK inhibitors, specifically for
treatment of SCCHN. We found that ROCK inhibition may offer a novel
therapeutic strategy for the total management of SCCHN as well as
inhibiting metastases.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81372877), the National
Young Scholars Science Foundation of China (no. 81102058), the
Foundation of Education Bureau of Liaoning Province (nos. 2009A755
and L2014317), the Public Welfare Fund Project for Science of
Liaoning Province (no. 2011002001), the Natural Science Foundation
of Liaoning Province (no. 2014021096), and the Excellent Talent
Fund Project of Higher Education of Liaoning Province
(LJQ2014087).
References
|
1
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ben-Baruch A: Site-specific metastasis
formation: chemokines as regulators of tumor cell adhesion,
motility and invasion. Cell Adh Migr. 3:328–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen G, Chen SM, Wang X, Ding XF, Ding J
and Meng LH: Inhibition of chemokine (CXC motif) ligand
12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated
cell migration by targeting mammalian target of rapamycin (mTOR)
pathway in human gastric carcinoma cells. J Biol Chem.
287:12132–12141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nannuru KC, Sharma B, Varney ML and Singh
RK: Role of chemokine receptor CXCR2 expression in mammary tumor
growth, angiogenesis and metastasis. J Carcinog. 10:402011.
View Article : Google Scholar
|
|
7
|
Hao M, Zheng J, Hou K, et al: Role of
chemokine receptor CXCR7 in bladder cancer progression. Biochem
Pharmacol. 84:204–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YS, Choi I, Ning Y, et al:
Interleukin-8 and its receptor CXCR2 in the tumour microenvironment
promote colon cancer growth, progression and metastasis. Br J
Cancer. 106:1833–1841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu FY, Zhao ZJ, Li P, et al: NF-κB
participates in chemokine receptor 7-mediated cell survival in
metastatic squamous cell carcinoma of the head and neck. Oncol Rep.
25:383–391. 2011.
|
|
10
|
Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH and
Sun CF: Mammalian target of rapamycin (mTOR) is involved in the
survival of cells mediated by chemokine receptor 7 through PI3K/Akt
in metastatic squamous cell carcinoma of the head and neck. Br J
Oral Maxillofac Surg. 48:291–296. 2010. View Article : Google Scholar
|
|
11
|
Zhao ZJ, Liu FY, Li P, Ding X, Zong ZH and
Sun CF: CCL19-induced chemokine receptor 7 activates the
phosphoinositide-3 kinase-mediated invasive pathway through Cdc42
in metastatic squamous cell carcinoma of the head and neck. Oncol
Rep. 25:729–737. 2011.
|
|
12
|
Wang J, Xi L, Hunt JL, et al: Expression
pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell
carcinoma of the head and neck identifies a novel metastatic
phenotype. Cancer Res. 64:1861–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bardi G, Niggli V and Loetscher P: Rho
kinase is required for CCR7-mediated polarization and chemotaxis of
T lymphocytes. FEBS Lett. 542:79–83. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riol-Blanco L, Sánchez-Sánchez N, Torres
A, et al: The chemokine receptor CCR7 activates in dendritic cells
two signaling modules that independently regulate chemotaxis and
migratory speed. J Immunol. 174:4070–4080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cuesta-Mateos C, López-Giral S,
Alfonso-Pérez M, et al: Analysis of migratory and prosurvival
pathways induced by the homeostatic chemokines CCL19 and CCL21 in
B-cell chronic lymphocytic leukemia. Exp Hematol. 38:756.e4–764.e4.
2010. View Article : Google Scholar
|
|
16
|
Bishop AL and Hall A: Rho GTPases and
their effector proteins. Biochem J. 348:241–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ono S: Regulation of actin filament
dynamics by actin depolymerizing factor/cofilin and
actin-interacting protein 1: new blades for twisted filaments.
Biochemistry. 42:13363–13370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olson MF: Applications for ROCK kinase
inhibition. Curr Opin Cell Biol. 20:242–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Freitas VM, Rangel M, Bisson LF, Jaeger RG
and Machado-Santelli GM: The geodiamolide H, derived from Brazilian
sponge Geodia corticostylifera, regulates actin cytoskeleton,
migration and invasion of breast cancer cells cultured in
three-dimensional environment. J Cell Physiol. 216:583–594. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren XD and Schwartz MA: Determination of
GTP loading on Rho. Methods Enzymol. 325:264–272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pellegrin S and Mellor H: Rho GTPase
activation assays. Current Protocols in Cell Biology. Bonifacino
Juan S, et al: 38. John Wiley & Sons, Inc; Hoboken, NJ: pp.
14.8.1–14.8.19. 2008
|
|
24
|
Wang J, Zhang X, Thomas SM, et al:
Chemokine receptor 7 activates phosphoinositide-3 kinase-mediated
invasive and prosurvival pathways in head and neck cancer cells
independent of EGFR. Oncogene. 24:5897–5904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Liu F, Xu Z, Guo N, Zheng X and
Sun C: Chemokine receptor 7 via proline-rich tyrosine kinase-2
upregulates the chemotaxis and migration ability of squamous cell
carcinoma of the head and neck. Oncol Rep. 28:1659–1664.
2012.PubMed/NCBI
|
|
26
|
Kakinuma T and Hwang ST: Chemokines,
chemokine receptors, and cancer metastasis. J Leukoc Biol.
79:639–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ridley AJ: RhoA, RhoB and RhoC have
different roles in cancer cell migration. J Microsc. 251:242–249.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Routhier A, Astuccio M, Lahey D, et al:
Pharmacological inhibition of Rho-kinase signaling with Y-27632
blocks melanoma tumor growth. Oncol Rep. 23:861–867.
2010.PubMed/NCBI
|
|
30
|
Bernard O: Lim kinases, regulators of
actin dynamics. Int J Biochem Cell Biol. 39:1071–1076. 2007.
View Article : Google Scholar
|
|
31
|
Ghosh M, Song X, Mouneimne G, Sidani M,
Lawrence DS and Condeelis JS: Cofilin promotes actin polymerization
and defines the direction of cell motility. Science. 304:743–746.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rattan R, Giri S, Singh AK and Singh I:
Rho/ROCK pathway as a target of tumor therapy. J Neurosci Res.
83:243–255. 2006. View Article : Google Scholar
|
|
33
|
Shimokawa H and Rashid M: Development of
Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol
Sci. 28:296–302. 2007. View Article : Google Scholar : PubMed/NCBI
|