Introduction
Bladder cancer is a common malignant disease in the
USA and is the 4th most common cancer in men and the 10th in women
(1). Genetic factors, including
oncogenes, such as the epidermal growth factor receptor (EGFR) and
tumor-suppressor genes, are risk factors for the development of
bladder cancer. Bladder cancer highly expresses EGFR and/or HER2
(2,3). Transgene-driven overexpression of the
EGFR within the bladder enhances tumor progression in mice,
providing direct support for its importance in the biology of this
disease (4). EGFR is a cell-surface
receptor, belonging to the EGFR family of receptor tyrosine
kinases. The EGFR family comprises four members: EGFR (HER1,
ErbB1), ErbB2 (HER2), ErbB3 (HER3) and ErbB4 (HER4). The EGFR
family members have four ectodomains, a single transmembrane domain
and a cytoplasmic tail containing the active tyrosine kinase
domain. Following the kinase domain, a C-terminal tail contains
autophosphorylation sites that recruit signaling molecules
(5). The EGFR-associated signaling
pathway plays an important role in the development and progression
of cancers. It has become one of the most important targets for
anticancer drug discovery, and a large number of different
small-molecule and antibody-based EGFR antagonists have been tested
in clinical trials. Three small-molecule EGFR tyrosine kinase
inhibitors (TKIs) are in clinical use and include gefitinib
(ZD1839, Iressa®), erlotinib (Tarceva®) and
lapatinib (GW 572016, Tykerb®). All are based on a
4-anilinoquinazoline scaffold and target the ATP site to inhibit
receptor autophosphorylation causing suppression of signal
transduction. Gefitinib and erlotinib, selective EGFR inhibitors,
target the active form of the kinase and have been approved for
non-small cell lung cancer. Lapatinib, a dual inhibitor of EGFR and
HER2, preferentially targets the inactive conformation and has been
approved for HER2-positive breast cancer (6,7).
Although TKIs display a survival advantage in clinical trials, only
a minority of patients appear to respond to this approach.
Resistance to TKIs has become a major clinical issue. Thus,
research is needed to identify and validate predictive factors that
can be used to select patients with disease likely to respond to
TKIs. Recent data showed that the tumor response is not associated
with a higher proportion of EGFR-positive tumor cells or more
intensive EGFR staining in lung cancer (8). Therefore, the identification of
additional predictive markers is extremely important.
In the present study, we compared the signaling
pathway(s) induced by TKIs (gefitinib and lapatinib), in UM-UC-5
(drug-sensitive) and UM-UC-14 (drug-resistant) bladder cancer cell
lines and identified molecular markers as predictors of their
efficacy. Here, we report that HER3 and ERK1/2 phosphorylation was
extremely high in the UM-UC-5 cells and substantially suppressed by
TKIs. However, a phosphorylated mutant p53 was overexpressed in the
UM-UC-14 cells. These data suggest that increased activation of
HER3 and ERK1/2 may be related to the response to TKIs, and that
enhanced phosphorylation of mutant p53 may be linked with
resistance to TKIs.
Materials and methods
Reagents
Eagle’s minimum essential medium (MEM) was purchased
from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum (FBS) was
purchased from Gemini Bio-Products (Calabasas, CA, USA) and the
antibiotics (penicillin and streptomycin) were from Invitrogen. The
human phospho-RTK array kit, human phospho-MAPK array kit and human
phospho-kinase array kit were purchased from R&D Systems
(Minneapolis, MN, USA). The antibodies against phosphorylated HER3
(Tyr1289), phosphorylated ERKs (Tyr-202/Tyr-204) and phosphorylated
p53 (Ser15) were purchased from Cell Signaling Biotechnology
(Beverly, MA, USA). The protein assay kit was from Bio-Rad
(Hercules, CA, USA).
Cell culture
The bladder cancer UM-UC-5 and UM-UC-14 cells were
cultured in monolayers at 37°C in a 5% CO2 incubator in
MEM containing 10% FBS and penicillin/streptomycin.
Protein array
Each cell line was cultured to 90% confluency and
then starved 24 h in serum-free media. They were treated,
respectively, with 2.5 μM gefitinib or lapatinib in culture medium
containing 10% FBS for 24 h and then harvested. Cell samples were
disrupted and then proteins were extracted. The protein
concentration was determined using a dye-binding protein assay kit
(Bio-Rad) as described in the manufacturer’s manual. Following the
instructions provided with the protein arrays, cell lysates were
subjected to Proteome Profiler™ Arrays including tyrosine
phosphorylation of receptor tyrosine kinases (RTKs) (42 signals),
phosphorylation of mitogen-activated protein kinases (MAPKs) (21
signals) and phospho-kinase (46 signals) array analysis.
Western blot analysis
After cells (1×106) were cultured in a
10-cm dish overnight, they were starved in serum-free medium for
another 24 h to eliminate the influence of FBS on the activation of
mitogen-activated protein kinases. The cells were then treated with
2.5 μM gefitinib or lapatinib for 24 h in culture medium containing
10% FBS. The harvested cells were disrupted, and the supernatant
fractions were boiled for 5 min. The protein concentration was
determined using a dye-binding protein assay kit (Bio-Rad). Lysate
proteins (50 μg) were subjected to 8–10% SDS-PAGE and
electrophoretically transferred to a polyvinylidene difluoride
membrane (GE Healthcare). After blotting, the membrane was
incubated with a specific primary antibody at 4°C overnight.
Protein bands were visualized using a chemiluminescence detection
kit (GE Healthcare) after hybridization with an AP-linked secondary
antibody.
Results
Phosphorylated HER3 is highly expressed
in the drug-sensitive bladder cancer cells and is substantially
decreased by TKIs
The EGFR family plays a central role in the
pathogenesis and progression of bladder cancer (9). Manifold actions for other growth
factor receptors, e.g., MET, have been reported in cancer (10). MET encodes a transmembrane tyrosine
kinase receptor for the hepatocyte growth factor (scatter factor),
and MET amplification has been correlated with resistance to TKIs
in lung cancer (10). We first
examined the relative level of tyrosine phosphorylation of RTKs in
both bladder cancer cell lines (UM-UC-5 and UM-UC-14) before and
after treatment with TKIs (gefitinib or lapatinib). The results
revealed that phosphorylated EGFR, HER3 and MET were overexpressed
in the sensitive cell line (UM-UC-5), yet were barely detectable in
the resistant cell line (UM-UC-14; Fig.
1A and B). Gefitinib and lapatinib markedly suppressed EGFR,
HER3 and MET phosphorylation in the UM-UC-5 cells, yet had no
effect in the UM-UC-14 cells. Based on these data, the most notable
difference between these two cell lines was the HER3
phosphorylation and the effectiveness of TKIs in suppressing this
signal in UM-UC-5 cells.
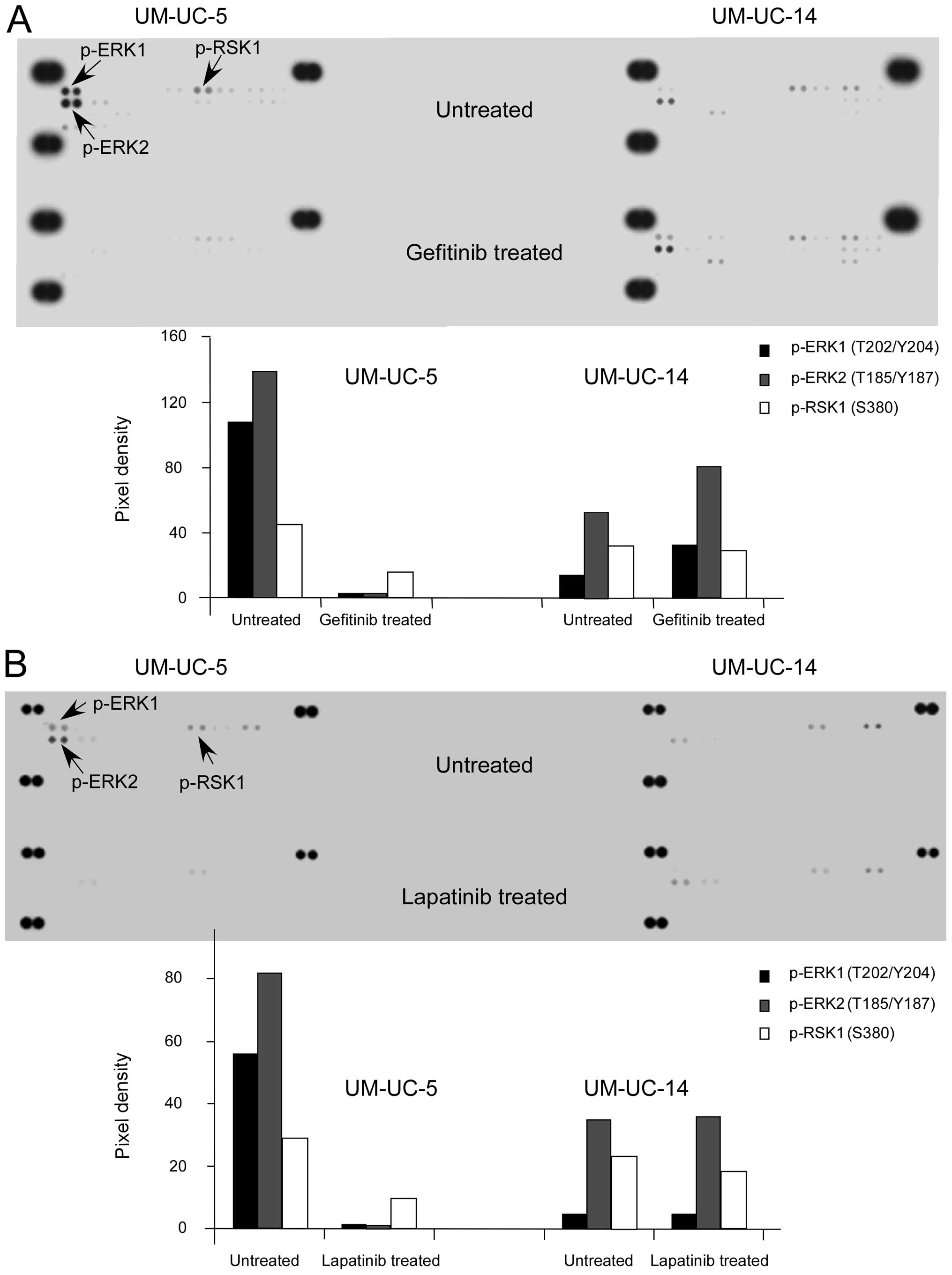
Phosphorylated ERK1/2 is overexpressed in
sensitive bladder cancer cells and is substantially inhibited by
TKIs
Next, we investigated the level of phosphorylation
of the MAPK pathways induced by gefitinib and lapatinib in the
UM-UC-5 and UM-UC-14 cell lines. The results revealed that the
level of ERK1/2 phosphorylation was higher in the UM-UC-5 cell line
when compared with that in the UM-UC-14 cells (Fig. 2A and B). The TKIs markedly
suppressed ERK1/2 phosphorylation in the UM-UC-5 cells, yet had no
effect on the UM-UC-14 cells. Phosphorylated RSK was also decreased
in the sensitive cells following drug treatment. However, the most
important event was that phosphorylation of ERK1/2, which is
upstream of RSK and was highly expressed in the sensitive cells,
was substantially decreased by the TKIs.
TKI-resistant bladder cancer cells
overexpress a mutant phosphorylated p53
The activation of the EGFR turns on several
different signaling pathways, including MAPKs, PI3K/Akt, STAT and
SRC/FAK (11). We found that the
phosphorylation of GSK3, AMPK, Akt and c-Jun was higher in the
UM-UC-5 cells and was suppressed by TKIs, whereas these proteins
were not affected in the UM-UC-14 cells (Fig. 3A and B). Notably, phosphorylated p53
was markedly overexpressed in the untreated resistant cells
compared with that in the sensitive cells (Fig. 3A and B). As for the other signals,
no difference was observed in either cell line.
In order to confirm the protein array data, we
performed western blot analysis to examine the phosphorylation of
HER3, ERK1/2 and p53 in these two cell lines both before and after
TKI treatments. Western blot results also showed that
phosphorylated HER3 and ERK1/2 were overexpressed in the UM-UC-5
cells and were strongly suppressed following gefitinib or lapatinib
treatment and that phosphorylated p53 was highly expressed in the
UM-UC-14 cells (Fig. 4).
Discussion
EGFR mutation and amplification and dysfunction of
other members of the EGFR family and downstream of EGFR signaling
are likely involved in the response of lung cancer to TKI treatment
(12). Previous findings from a
phase II study of erlotinib in patients with NSCLC did not show a
correlation between the degree of EGFR expression and response to
therapy, and suggest that alternative markers (indicative of
activation of the receptor or the pathway, or both) are more likely
to be predictive of sensitivity to EGFR inhibition (8). EGFR and/or HER2 are reported to be
deregulated in bladder cancer, yet mutations within the EGFR and
expression of EGFR vIII are rare events in bladder cancer (13). Recent findings revealed that
although EGFR levels appear to roughly correspond with
responsiveness, they cannot be used alone to identify bladder
cancers that will be sensitive to TKIs (4). EGFR and/or HER2 are associated with
poor prognosis (14), whereas HER3
and/or HER4 are related to a better outcome for bladder cancer
patients (15). Expression of EGFR
and HER2 alone does not appear to be associated with survival.
However, high expression of EGFR along with high expression of HER3
and/or HER4 was found to correspond to a better prognosis compared
with high expression of EGFR together with low expression of HER3
and/or HER4 (16). Therefore, the
prognostic significance of EGFR and/or HER2 overexpression is
modulated by expression of HER3 and/or HER4, indicating the
complexity of interactions between the different EGFRs. The present
study demonstrated that the phosphorylated levels of EGFR and
particularly, HER3, were high whereas phosphorylation of HER2 and
HER4 was not observed in the TKI-sensitive bladder cancer cells.
Following TKI treatment, phosphorylation of EGFR and HER3 was
markedly decreased in the sensitive cells and no change was
observed in the resistant cells. Additional evidence suggests that
the functions of HER3 may be important in the prediction of
sensitivity to TKIs (17). For
example, in a comparison of lung cancer cell lines that are
sensitive or resistant to gefitinib, the best marker of sensitivity
to gefitinib was found to be the sensitivity of HER3 signaling
(18). In agreement with previous
data, an increased activation of HER3 was also found to be related
to the response of bladder cancer to TKIs.
EGFR and HER2 are classically coupled to the
Ras/Raf/MEK/ERK-dependent pathway, whereas HER3 is a potent
activator of PI3K/Akt (19).
Studies have shown that a majority of bladder cancers express
activated Ras, and that this oncogenic activation is an important
tumorigenic factor (20–23). Phosphorylated ERK is the key
downstream target of the Ras/Raf/MEK/ERK signaling pathway, and
deregulation of this pathway occurs in approximately one-third of
all human cancers (24).
Furthermore, phosphorylated ERK1/2 may be a potential predictive
marker of sensitivity to sorafenib in hepatocellular carcinoma.
Sorafenib was reported to inhibit ERK1/2 phosphorylation and was
dependent on the degree of basal expression level of phosphorylated
ERK1/2 (25). We report a similar
finding in that TKI-sensitive bladder cancer cells exhibited a high
expression level of phosphorylated ERK1/2 and the elevated
phosphorylation was suppressed by TKIs. Hence, phosphorylated
ERK1/2 may be an important biomarker for the prediction of
sensitivity to TKIs in bladder cancer.
Bladder cancers exhibit frequent alterations in the
tumor-suppressor gene p53, and mutant p53 expression demonstrates a
strong association with disease grade and stage, as well as with
survival (26). Aberrations of p53
are usually associated with drug resistance. Recent data showed
that human urothelial cells with loss of p53 function displayed
reduced sensitivity to TKIs (27)
and p53 is required for maximal sensitivity to gefitinib-induced
apoptosis in non-small cell lung cancer (28). One point mutation (codon 135,
cysteine to serine) was identified in the UM-UC-14 cells (data not
shown). Our data showed that phosphorylated mutant p53 was highly
expressed in the resistant bladder cancer cells. p53 cysteine
substitution at C135 was found to decrease human p53 activity in
wild-type yeast (29), thus
silencing of mutant p53 had no effect on the sensitivity to TKIs
(data not shown). These results suggest that overexpressed
phosphorylated p53 may be a marker of resistance to TKIs in bladder
cancer.
In conclusion, phosphorylated HER3, ERK1/2 and p53
may be used as biomarkers to determine bladder cancer sensitivity
to TKIs. A combination of these markers may be more likely to
predict sensitivity to TKIs.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81201710 and 81272434),
the Guangdong Natural Science Foundation (S2012010008259), and the
Guangdong Medical College research grant (XG 1101 and STIF
201105).
References
|
1
|
van Rhijn BW, Burger M, Lotan Y, et al:
Recurrence and progression of disease in non-muscle-invasive
bladder cancer: from epidemiology to treatment strategy. Eur Urol.
56:430–442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McHugh LA, Sayan AE, Mejlvang J, et al:
Lapatinib, a dual inhibitor of ErbB-1/-2 receptors, enhances
effects of combination chemotherapy in bladder cancer cells. Int J
Oncol. 34:1155–1163. 2009.PubMed/NCBI
|
|
3
|
Wang X, Zhang S, MacLennan GT, et al:
Epidermal growth factor receptor protein expression and gene
amplification in small cell carcinoma of the urinary bladder. Clin
Cancer Res. 13:953–957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shrader M, Pino MS, Brown G, et al:
Molecular correlates of gefitinib responsiveness in human bladder
cancer cells. Mol Cancer Ther. 6:277–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson LN: Protein kinase inhibitors:
contributions from structure to clinical compounds. Q Rev Biophys.
42:1–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McHugh LA, Kriajevska M, Mellon JK and
Griffiths TR: Combined treatment of bladder cancer cell lines with
lapatinib and varying chemotherapy regimens - evidence of
schedule-dependent synergy. Urology. 69:390–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shrader M1, Pino MS, Lashinger L, et al:
Gefitinib reverses TRAIL resistance in human bladder cancer cell
lines via inhibition of AKT-mediated X-linked inhibitor of
apoptosis protein expression. Cancer Res. 67:1430–1435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dancey JE and Freidlin B: Targeting
epidermal growth factor receptor - are we missing the mark? Lancet.
362:62–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Hausmann M, Dietmaier W, et al:
Expression of growth factor receptors and targeting of EGFR in
cholangiocarcinoma cell lines. BMC Cancer. 10:3022010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelman JA, Zejnullahu K, Mitsudomi T, et
al: MET amplification leads to gefitinib resistance in lung cancer
by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laurent-Puig P, Lievre A and Blons H:
Mutations and response to epidermal growth factor receptor
inhibitors. Clin Cancer Res. 15:1133–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shigematsu H and Gazdar AF: Somatic
mutations of epidermal growth factor receptor signaling pathway in
lung cancers. Int J Cancer. 118:257–262. 2006. View Article : Google Scholar
|
|
13
|
Villares GJ, Zigler M, Blehm K, et al:
Targeting EGFR in bladder cancer. World J Urol. 25:573–579. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Memon AA, Sorensen SB and Nexo E: The
epidermal growth factor family has a dual role in deciding the fate
of cancer cells. Scand J Clin Lab Invest. 66:623–630. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Memon AA, Sorensen BS, Melgard P, et al:
Expression of HER3, HER4 and their ligand heregulin-4 is associated
with better survival in bladder cancer patients. Br J Cancer.
91:2034–2041. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Memon AA, Sorensen BS, Meldgaard P, et al:
The relation between survival and expression of HER1 and HER2
depends on the expression of HER3 and HER4: a study in bladder
cancer patients. Br J Cancer. 94:1703–1709. 2006.PubMed/NCBI
|
|
17
|
Amin DN, Campbell MR and Moasser MM: The
role of HER3, the unpretentious member of the HER family, in cancer
biology and cancer therapeutics. Semin Cell Dev Biol. 21:944–950.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engelman JA, Jänne PA, Mermel C, et al:
ErbB-3 mediates phosphoinositide 3-kinase activity in
gefitinib-sensitive non-small cell lung cancer cell lines. Proc
Natl Acad Sci USA. 102:3788–3793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koutras AK, Fountzilas G, Kalogeras KT, et
al: The upgraded role of HER3 and HER4 receptors in breast cancer.
Crit Rev Oncol Hematol. 74:73–78. 2010. View Article : Google Scholar
|
|
20
|
Fontana D, Bellina M, Scoffone C, et al:
Evaluation of c-ras oncogene product (p21) in superficial bladder
cancer. Eur Urol. 29:470–476. 1996.PubMed/NCBI
|
|
21
|
Przybojewska B, Jagiello A and Jalmuzna P:
H-RAS, K-RAS, and N-RAS gene activation in human bladder cancers.
Cancer Genet Cytogenet. 121:73–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rose A, Grandoch M, vom Dorp F, et al:
Stimulatory effects of the multi-kinase inhibitor sorafenib on
human bladder cancer cells. Br J Pharmacol. 160:1690–1698. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vageli D, Kiaris H, Delakas D, Anezinis P,
Cranidis A and Spandidos DA: Transcriptional activation of H-ras,
K-ras and N-ras proto-oncogenes in human bladder tumors. Cancer
Lett. 107:241–247. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Zhou X, Shen H, et al:
Phosphorylated ERK is a potential predictor of sensitivity to
sorafenib when treating hepatocellular carcinoma: evidence from an
in vitro study. BMC Med. 7:412009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Proctor I, Stoeber K and Williams GH:
Biomarkers in bladder cancer. Histopathology. 57:1–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
MacLaine NJ, Wood MD, Holder JC, et al:
Sensitivity of normal, paramalignant, and malignant human
urothelial cells to inhibitors of the epidermal growth factor
receptor signaling pathway. Mol Cancer Res. 6:53–63. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rho JK, Choi YJ, Ryoo BY, et al: p53
enhances gefitinib-induced growth inhibition and apoptosis by
regulation of Fas in non-small cell lung cancer. Cancer Res.
67:1163–1169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stoner CS, Pearson GD, Koç A, et al:
Effect of thioredoxin deletion and p53 cysteine replacement on
human p53 activity in wild-type and thioredoxin reductase null
yeast. Biochemistry. 48:9156–9169. 2009. View Article : Google Scholar : PubMed/NCBI
|