Introduction
Acute lymphoblastic leukemia (ALL), which can be
divided into B-lineage ALL (B-ALL) and T-lineage ALL (T-ALL)
(1), is one of the most common
forms of pediatric malignancies originating from lymphoid
precursors (2). T-ALL accounts for
10 to 15% of pediatric cases and 25% of adult ALL cases (3). With current intensified multi-agent
chemotherapy protocols, the 5-year event-free survival (EFS) of
children with T-ALL has reached 70–75% (4). However, these therapies are highly
toxic. Moreover, relapsed patients often develop resistance to
chemotherapy and experience very poor prognosis (5). Therefore, the mechanisms that cause
relapses and chemo-resistance in T-ALL should be understood to
identify novel molecular targets and design effective
therapies.
T-ALL is an aggressive blood malignancy originating
from T-cell progenitors in the thymus. Genes encoding
transcriptional regulators of T-cell development and maturation are
potential targets of T-ALL therapy. The IKAROS family, an important
group of transcription factors in hematopoietic lineages, encodes a
group of zinc-finger DNA-binding proteins essential for normal
lymphocyte development (6–8). AIOLOS is an IKAROS family member that
was first described in committed lymphoid progenitors and was
strongly upregulated as these progenitors become restricted into T-
and B-lymphoid pathways (7).
Previous studies have shown that AIOLOS controls T and B lymphocyte
apoptosis by regulating Bcl-xL (9,10) and
also regulates cell death in T cells by controlling Bcl-2
expression and cellular localization (11). Deregulated AIOLOS expression has
been associated with leukemia and lymphoma in human patients
(12–15).
In the present study, a lentiviral system was used
to stably overexpress the AIOLOS gene in Jurkat cells, a T-ALL cell
line, and to examine apoptosis, cell cycle distribution and cell
chemosensitivity to etoposide in vitro. Our results
demonstrated that AIOLOS overexpression in Jurkat cells induced
cell apoptosis, arrested the cell cycle at the G0/G1 phase, and
synergistically increased the sensitivity of Jurkat cells to
etoposide by inhibiting NF-κB activity.
Materials and methods
Cell lines and cell culture
Two T-ALL cell lines (Jurkat and Molt-4) were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in standard culture medium
[RPMI-1640 containing 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (all from Gibco, Grand Island, NY, USA)] at
37°C in 5% CO2 in air. The cells were subcultured after
24–48 h with an initial concentration of 4×104 cells/ml
and were used at the logarithmic phase in all of the experiments.
Peripheral blood lymphocytes collected from consenting normal
healthy children were used as control cells. The experimental
design and protocols were approved by the Ethics Committee of Qilu
Hospital. Informed consent was obtained for all participants prior
to enrollment.
Lentiviral vector construction, virus
production and transfection
The lentiviral vectors pWPT-PURO-GFP-AIOLOS
(Lenti-AIOLOS) and pWPT-PURO-GFP (Lenti-Mock) were constructed and
identified as previously described (16). Viral concentrate was diluted in
Polybrene (5 μg/ml; Sigma, St. Louis, MO, USA) to infect Jurkat
cells at a multiplicity of infection (MOI) of 100. Successful
transduction was confirmed by visualizing enhanced green
fluorescent protein (EGFP; included in the pWPT-PURO-GFP vector)
after 4 days. The cells were maintained and allowed to grow for
another 3–5 days; the AIOLOS expression level was confirmed by
qRT-PCR and western blot analysis. Virus-infected cells were
selected with 8 μg/ml puromycin (Invitrogen, Carlsbad, CA, USA).
Antibiotic-resistant clones were pooled and used for subsequent
assays.
Jurkat cells were divided into three groups:
untransfected (UT) control, lentiviral vector control (Lenti-Mock)
and AIOLOS-transfected (Lenti-AIOLOS) groups.
Quantitative real-time
reverse-transcription polymerase chain reaction
Total RNA was extracted from Jurkat cells of the
three groups by using TRIzol reagent (Invitrogen). To perform
reverse transcription (RT), we synthesized first-strand cDNA from 5
μg of total RNA using the Omniscript cDNA synthesis kit (Qiagen,
Hamburg, Germany) according to the manufacturer’s instructions. PCR
was performed using 2 μl of 10-fold diluted cDNA.
cDNA samples were analyzed by qRT-PCR in an Applied
Biosystems 7500 PCR system (Applied Biosystems, Foster City, CA,
USA) with SYBR-Green I dye (Toyobo, Osaka, Japan). Primers
(Table I) were obtained from Bioasi
Co., Ltd., Shanghai, China. Data were analyzed using the 2−Δ
ΔCt method, where ΔCt = (Cttarget gene −
Ctβ-actin), to obtain the relative expression level.
Each sample was then normalized using β-actin expression. Results
are expressed as fold change relative to the cDNA of the UT group.
Data were also analyzed using Sequence Detection Software 1.4
(Applied Biosystems). Reported data are representative of at least
three independent experiments.
 | Table IPrimer sequences used for qRT-PCR. |
Table I
Primer sequences used for qRT-PCR.
| Gene | Primer sequence | Product length
(bp) |
|---|
| AIOLOS | F:
5′-GCCCTTCAAGTGTTTCACCAA-3′
R: 5′-GCCTTTCCAGCCAGACAAATAT-3′ | 90 |
| β-actin | F:
5′-GGACATCCGCAAAGACCTGTA-3′
R: 5′-GCATCCTGTCGGCAATGC-3′ | 80 |
| BCL-2 | F:
5′-GCTGGGAGAACAGGGTACGA-3′
R: 5′-CCTCTGCGACAGCTTATAATGGA-3′ | 80 |
| BAX | F:
5′-CTTGTTGCCCAGGCTTGAGT-3′
R: 5′-GCAGGAGAATCGCTTGAACCT-3′ | 81 |
| CCND3 | F:
5′-GAGGTGCAATCCTCTCCTCG-3′
R: 5′-TCACATACCTCCTCGTCAGGT-3′ | 87 |
| P21 | F:
5′-TGCCGAAGTCAGTTCCTTGT-3′
R: 5′-GTTCTGACATGGCGCCTCC-3′ | 83 |
| P27 | F:
5′-TCCGGCTAACTCTGAGGACA-3′
R: 5′-GAAGAATCGTCGGTTGCAGG-3′ | 81 |
| SKP2 | F:
5′-AGCTCTGCAAGTTTAATGCACG-3′
R: 5′-CTTGCTGGAATCCCATCCCC-3′ | 88 |
Protein extraction and western blot
analysis
Jurkat cells were harvested and washed twice with
cold phosphate-buffered saline (PBS). Total and nuclear protein
fractions were extracted using RIPA lysis buffer and nuclear and
cytoplasmic protein extraction kit (both from Beyotime Institute of
Biotechnology, Jiangsu, China), respectively, according to the
manufacturer’s protocols. Total AIOLOS and nuclear NF-κB expression
levels were analyzed. The proteins were quantified using the
Bradford protein assay kit (Beyotime Institute of Biotechnology).
Equal amounts of proteins were loaded in each well of 12% sodium
dodecyl sulfate-polyacrylamide gels and transferred to
polyvinylidene fluoride microporous membranes (Millipore, Bedford,
MA, USA). Membranes containing the transferred proteins were
blocked with PBS containing 0.1% Tween-20 (PBS-T) and 5% skim milk
for 1 h at room temperature. After three washes with PBS-T, the
membranes were incubated with antibodies against AIOLOS (1:1,000),
NF-κB (1:1,000), GAPDH (1:1,000) or lamin B (1:1,000) (all from
Abcam Inc., Cambridge, MA, USA) at 4°C overnight. After three
washes with PBS-T, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (1:1,000; Beyotime
Institute of Biotechnology) for 1 h at room temperature. After
three final washes in PBS-T and two in PBS, chemiluminescence was
detected using an ECL Plus immunoblotting detection system
(Beyotime Institute of Biotechnology).
Cell cycle and apoptosis assay
Jurkat cells were obtained 9 days after
transfection; cell cycle and apoptosis were detected using a Muse™
cell cycle reagent, Muse™ Annexin V and a dead cell kit (all from
Millipore) according to the manufacturer’s instructions. Assay
results were obtained using a Muse™ cell analyzer (Millipore). Cell
cycle results are expressed as the percentage of cells in each cell
cycle phase. Cell apoptosis results are expressed as the percentage
of apoptotic cells. Error bars represent standard errors of the
means (SEM).
Cytotoxicity assay
The effect of AIOLOS overexpression on the
sensitivity of the Jurkat cell line to etoposide (Sigma, St. Louis,
MO, USA) was evaluated using the CCK-8 (Beyotime Institute of
Biotechnology, Haimen, China) assay. The experiment was divided
into six groups: etoposide, Lenti-AIOLOS, Lenti-Mock, Lenti-AIOLOS
and etoposide, Lenti-Mock and etoposide and etoposide blank
control. The cells treated with only 0.9% NS were used as etoposide
blank controls. In brief, the cells were cultivated at a density of
2×104 cells/well in 96-well culture plates. At 96 h
after transfection, the cells were treated with various
concentrations of etoposide (0, 2.5, 10 and 40 μM). After 48 h of
culture, the cytotoxicity of the treatments was determined using
WST-8 dye (Beyotime Institute of Biotechnology) according to the
manufacturer’s instructions. The generated formazan was determined
using a Model 450 microplate reader (Bio-Rad Laboratories,
Richmond, CA, USA) at an optical density of 570 nm (OD570) to
determine cell viability. Survival rate (SR) was calculated using
the following equation: SR (%) = (A Test/A Control) × 100%, where A
is the absorbance value.
Statistical analysis
All of the experiments were performed at least
thrice. Prism 5.0 (GraphPad Software) was used for statistical
analysis. P-values were obtained from two-tailed tests and were
considered to indicate a statistically significant result at
P<0.05.
Results
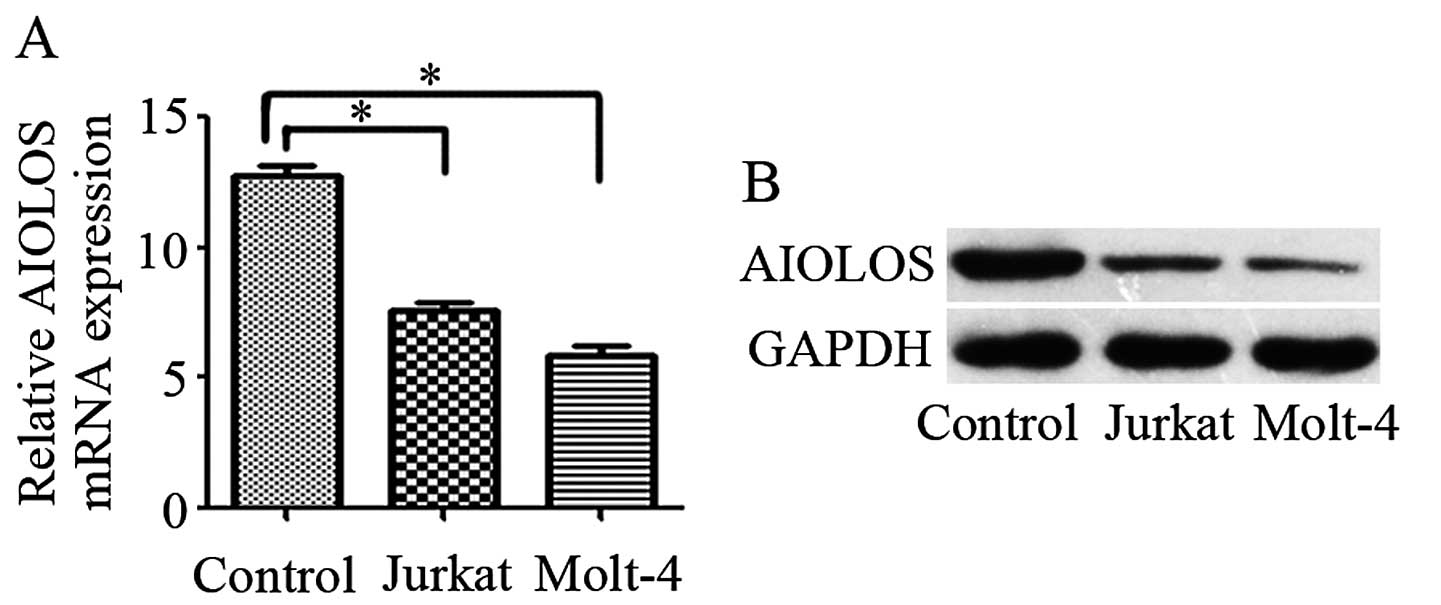
AIOLOS protein expression in the Jurkat
cells is lower than that in normal child peripheral blood
lymphocytes
To evaluate whether or not aberrant AIOLOS
expression is observed in T-ALL, we quantified AIOLOS expression
levels by qRT-PCR and western blot analysis in the T-ALL cell lines
and normal child peripheral blood lymphocytes. The mRNA levels of
AIOLOS were lower in both T-ALL cell lines than the levels in the
normal control (P<0.05; Fig.
1A). Western blot analysis results were consistent with those
of qRT-PCR (Fig. 1B). The Jurkat
cell line was chosen for a series of functional experiments.
AIOLOS is overexpressed by stable
transfection in Jurkat cells
The Jurkat cells were infected with the lentiviral
vector pWPT-PURO-GFP-AIOLOS. As a control sample, the Jurkat cells
were either infected with a lentiviral vector expressing GFP or
not. At 96 h after the Jurkat cells were infected, infection
efficiency was detected using a fluorescence microscope. More than
90% of the cells emitted bright green fluorescence, which
represented high infection efficiency (Fig. 2A–F). Cells were maintained and
allowed to grow for 3–5 days. The mRNA and protein expression
levels of AIOLOS in the Jurkat cells of the three groups were
determined by qRT-PCR and western blot analysis at 7 days after
infection. qRT-PCR results demonstrated that the mRNA expression
level of AIOLOS in the Jurkat cells of the Lenti-AIOLOS group was
markedly increased compared with that of the Lenti-Mock and the UT
group. This finding was consistent with the increase in AIOLOS
protein expression (Fig. 2G and H).
No significant difference between the cells of the Lenti-Mock and
the UT group was observed. These results revealed that the stable
transfection of pWPT-PURO-GFP-AIOLOS upregulated AIOLOS expression
in the Jurkat cells.
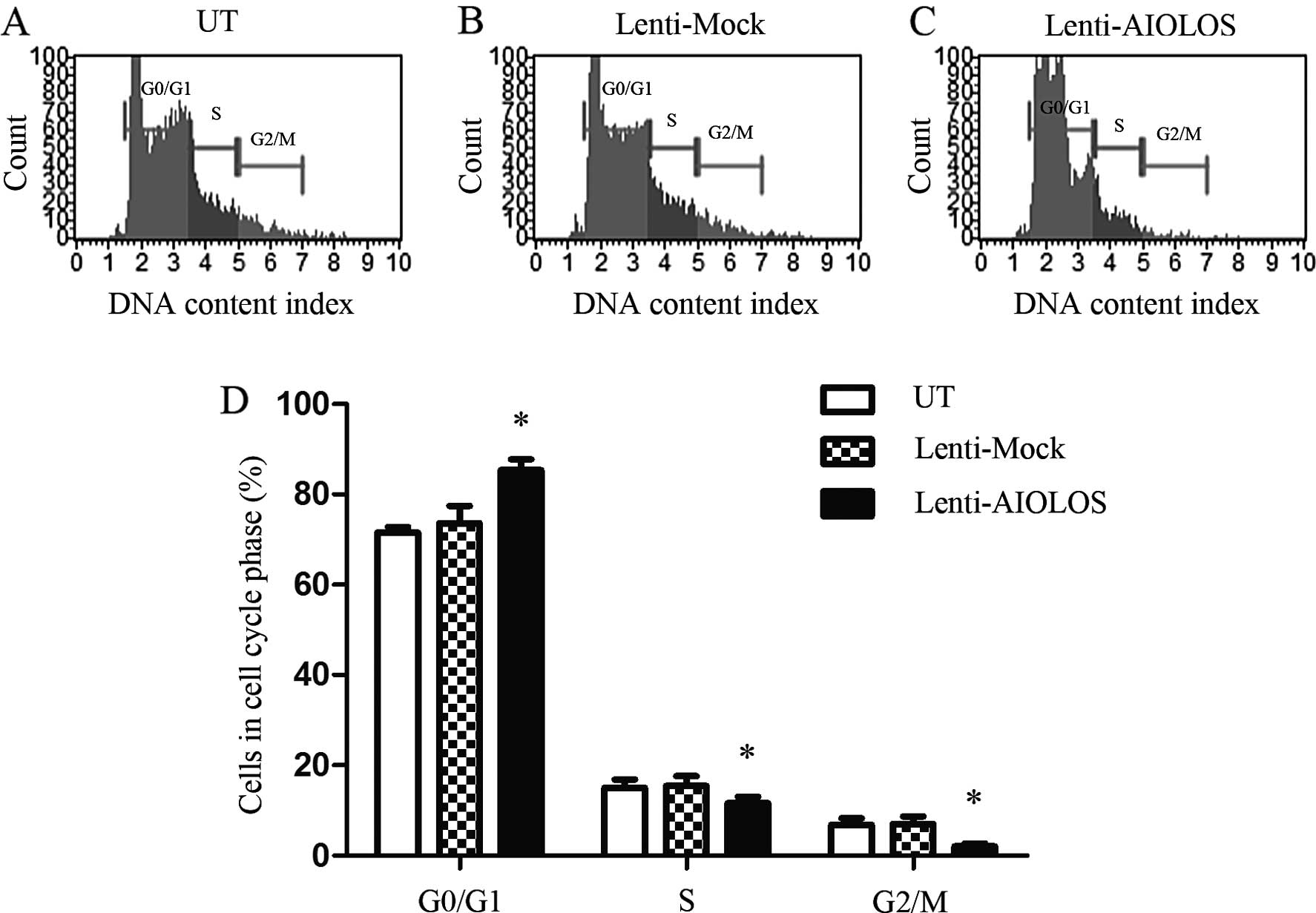
AIOLOS overexpression arrests the cell
cycle in the G1 phase in Jurkat cells
The cell cycle distribution of the Jurkat cells in
the Lenti-AIOLOS, Lenti-Mock and UT groups was characterized by
fluorescence-activated cell sorting (FACS) analysis 9 days after
transfection. The percentage of Jurkat cells in the G0/G1 phase
increased from 71.5 (UT) to 85.4% (Lenti-AIOLOS; P<0.05;
Fig. 3), and the percentage of
S-phase cells was decreased from 15.1 (UT) to 11.6% (Lenti-AIOLOS;
P<0.05). The difference between Jurkat cells of the Lenti-AIOLOS
and the UT group in the G2/M phase was significant (2.0 vs. 6.8%;
P<0.05). No significant difference between the Lenti-Mock and
the UT Jurkat cells was observed (P>0.05). These data revealed
that the upregulation of AIOLOS expression arrested Jurkat cells at
the G0/G1 phase.
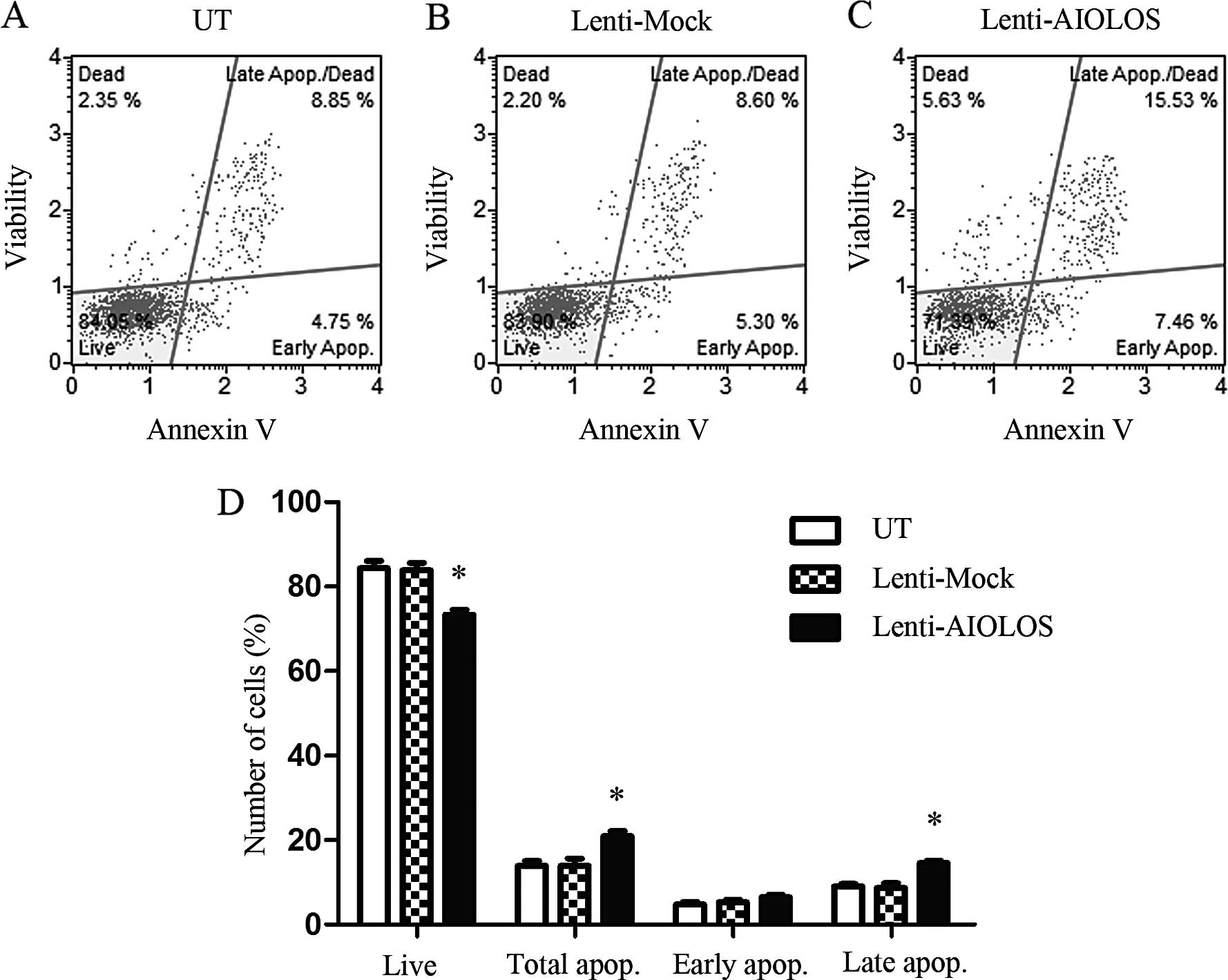
AIOLOS overexpression induces apoptosis
in Jurkat cells
To determine whether or not AIOLOS overexpression
results in apoptosis in the Jurkat cells, we used the Muse™ Annexin
V and the dead cell kit and determined the changes in cell
apoptosis on day 9. The percentage of total apoptotic cells was
significantly increased in the AIOLOS-transfected Jurkat cells
(21.93%) compared with the percentage in the Lenti-Mock (13.35%) or
UT group (13.30%; P<0.05; Fig.
4). In particular, the difference between AIOLOS-transfected
Jurkat and UT Jurkat cells in regards to the percentage of early
apoptotic cells was minimal (6.46 vs. 4.81%; P>0.05). The
difference between the cell groups in regards to the percentage of
late apoptotic cells was significant (14.55 vs. 9.05%; P<0.05).
These data revealed that AIOLOS overexpression suppressed cell
apoptosis in the Jurkat cells.
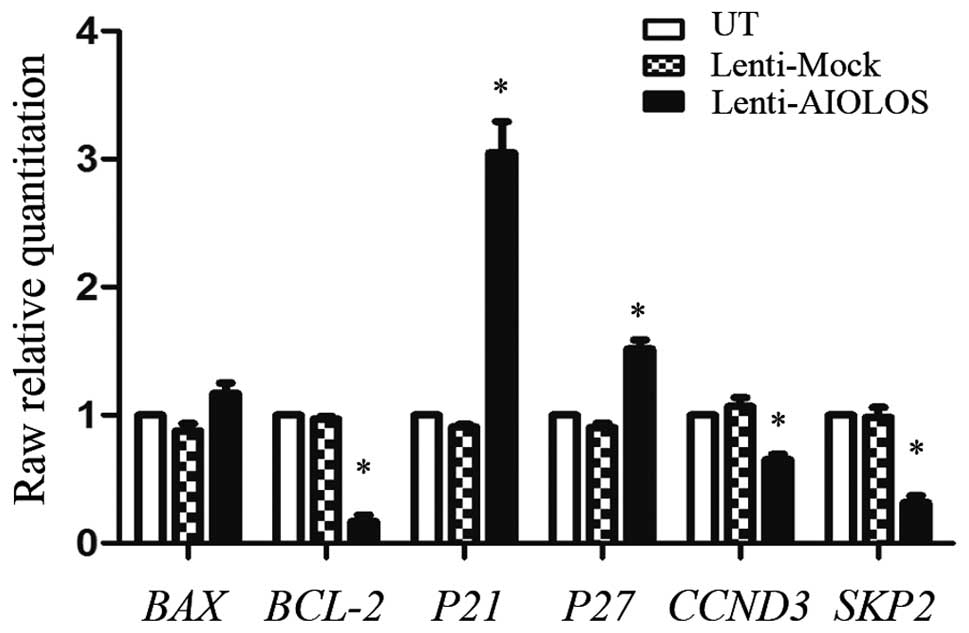
AIOLOS affects the expression levels of
apoptosis- and cell cycle-related genes in Jurkat cells
To investigate the mechanism by which AIOLOS blocks
the cell cycle and promotes the apoptosis of Jurkat cells, we
examined the expression of genes associated with apoptosis and the
cell cycle in response to AIOLOS overexpression by performing
qRT-PCR (Fig. 5). The mRNA
expression levels of P21 and P27 were significantly
increased in the AIOLOS-transfected Jurkat cells compared with
these levels in the UT and Lenti-Mock Jurkat cells (Fig. 5); this result was consistent with
that of the cell cycle assay. In addition, CCND3, one of the known
cell cycle-related genes and SKP2, a typical representative
of cell cycle negative regulators, were downregulated in the
AIOLOS-transfected Jurkat cells. Furthermore, BCL-2
expression in the Lenti-AIOLOS group was significantly decreased
(P<0.05). No distinct changes were detected in BAX
(P>0.05).
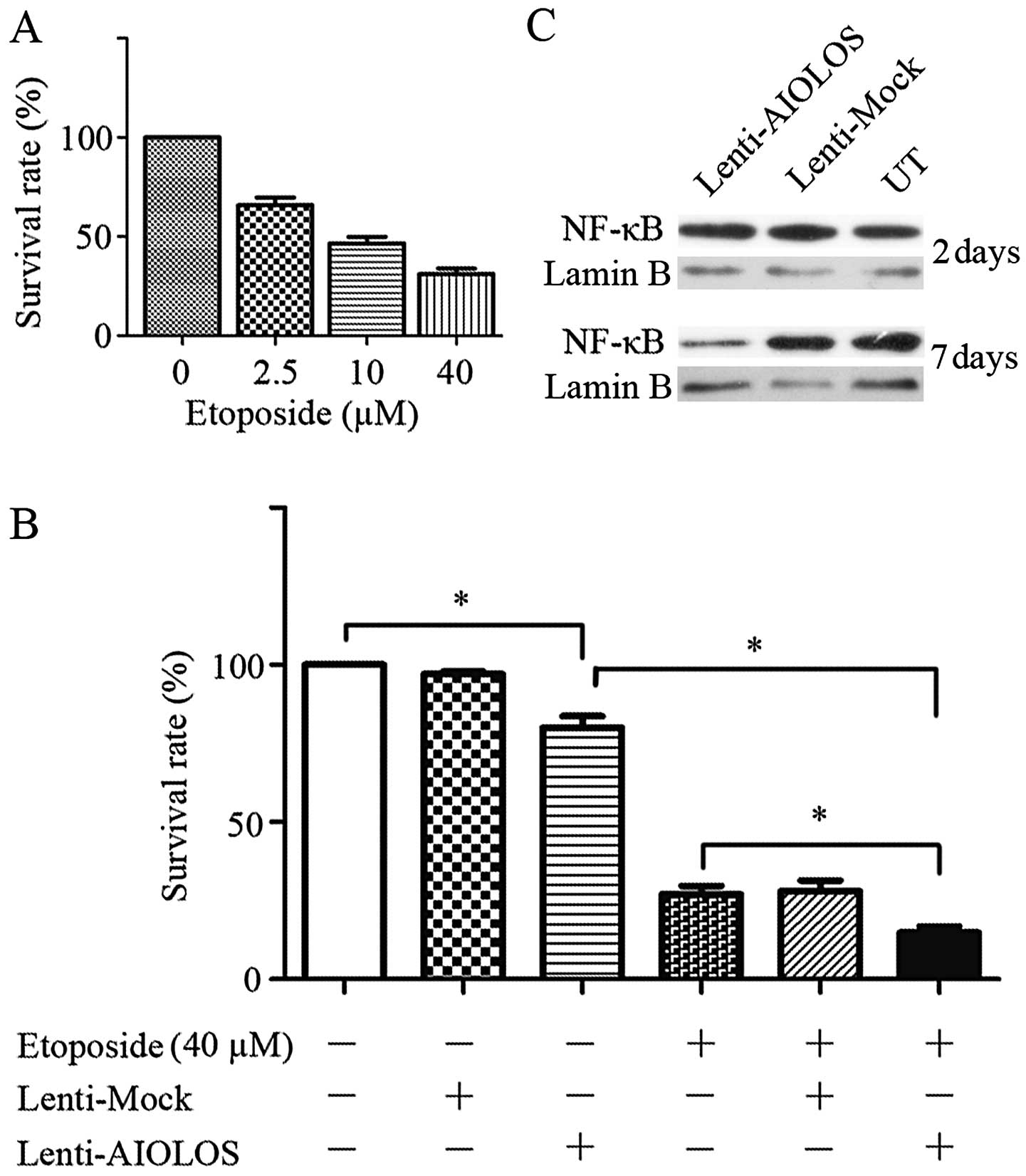
AIOLOS overexpression synergistically
increases the sensitivity of Jurkat cells to etoposide by
inhibiting NF-κB activity
To analyze whether or not increased AIOLOS
expression can enhance the sensitivity of Jurkat cells to
etoposide, we performed a combined treatment of Lenti-AIOLOS and
etoposide. Single treatment with etoposide induced cytotoxicity in
a dose-dependent manner (Fig. 6A).
Thus, 40 μM etoposide was chosen for further experiments. The
results showed that Lenti-AIOLOS alone significantly lowered the
cell SR to 80.07% when compared with the blank control (P<0.05).
As shown in Fig. 6B, combined
therapy further reduced the cell SR compared with Lenti-AIOLOS or
etoposide monotreatment (P<0.05). Lenti-Mock affected the
chemosensitivity of the cells compared with etoposide alone, yet
this effect was not significant (P>0.05). To explore the
mechanism of the observed synergistic cytotoxic effects between
Lenti-AIOLOS and etoposide, we investigated NF-κB expression since
this transcription factor is involved in several pathways and
broadly regulates targets in cancer. Fig. 6C shows that NF-κB expression
decreased as AIOLOS was overexpressed in the Jurkat cells.
Discussion
As a member of the IKAROS family of zinc-finger
proteins, the AIOLOS transcription factor, encoded by the IKZF3
gene, is necessary to control lymphocyte differentiation,
proliferation and maturation. Thus, the T-ALL cell line Jurkat was
chosen for a series of functional studies to explore the function
of AIOLOS in the pathogenesis of T-ALL. To mimic the isoforms and
cellular localizations of AIOLOS in T-cells, we constructed a
plasmid pWPT-PURO-GFP-AIOLOS containing the entire AIOLOS coding
sequence and performed lentiviral-mediated transduction in Jurkat
cells to create a stable transfection cell line. qRT-PCR and
western blot analysis revealed that Lenti-AIOLOS treatment caused a
constant increase in AIOLOS expression at the mRNA and protein
levels for 4 days. These results revealed that the Jurkat cells
were successfully transduced with the lentivirus, and AIOLOS was
successfully overexpressed in Jurkat cells.
Cell cycle assay results indicated that AIOLOS
overexpression arrested the cell cycle of the Lenti-AIOLOS cells at
the G0/G1 phase. To explore the potential mechanisms of AIOLOS in
the Jurkat cell cycle, we analyzed the expression of cell
cycle-related genes, including P21, P27, CCND3
and SKP2 by qRT-PCR. Skp2 functions as an oncoprotein,
participates in many aspects of cancer progression by inducing p27
and p21 degradation (17,18), and establishes a crosstalk with
other major signaling pathways (19–21). A
previous study reported that activation of the JAK2/STAT3 pathway
enhanced leukemogenesis (22). In
addition, JAK2/STAT3 pathway inhibition was found to upregulate p27
and p21 expression (23).
Consistent with these results, our findings showed that AIOLOS
overexpression upregulated p27 and p21 expression and downregulated
Skp2. This result revealed that AIOLOS may interact with the
Skp2/p27/p21 pathway via JAK2/STAT3 signaling in Jurkat leukemia
cells.
AIOLOS reportedly controls T-cell death by
regulating the expression and localization of the anti-apoptotic
molecule Bcl-2 (11), suggesting
the possibility that apoptotic cell death evasion is a common
mechanism by which IKAROS family proteins participate in
leukemogenesis. In the present study, AIOLOS overexpression in
Jurkat cells induced cell apoptosis. Considering that Bcl-2 family
proteins play a critical role as promoters or inhibitors in the
regulation of apoptosis (24), we
investigated whether or not the disruption of apoptosis-related
genes BCL-2 and BAX contributes to apoptosis
induction of Jurkat cells by AIOLOS overexpression. Although no
distinct changes were found in BAX, BCL-2 was
downregulated, resulting in a low BCL-2/BAX ratio, which may
be a possible reason for the increased apoptosis in the Jurkat
cells. However, further research is required to explore the
complete mechanism.
Cell cycle and apoptosis assay results indicated
that AIOLOS overexpression may play a critical role in drug
resistance of leukemia cells. CCK-8 assay results showed that
Lenti-AIOLOS pretreatment synergistically increased the cytotoxic
effect of etoposide. This finding revealed that AIOLOS
overexpression could sensitize leukemia cells in response to
etoposide. Etoposide is a DNA topoisomerase II inhibitor commonly
used to treat several malignancies, including leukemia. The
exposure of leukemia cells to etoposide initiates both signaling
pathways of apoptosis by activating multiple caspases (25,26).
T-ALL is associated with NF-κB pathway activation, an important
regulator of cell survival, proliferation and differentiation
(27). Moreover, NF-κB expression
was found to decrease after AIOLOS was overexpressed in B-ALL
(16). Our initial hypothesis was
that the synergism of AIOLOS on the effects of etoposide was
probably related to the inhibition of basal NF-κB activity. To test
this hypothesis, we performed immunoblotting experiments for
nuclear NF-κB activity. As expected, the results of such
experiments clearly indicated that AIOLOS overexpression indeed
inhibited NF-κB activity in Jurkat cells. Therefore, AIOLOS
overexpression may sensitize leukemia cells to etoposide by
inhibiting NF-κB activity. Further studies should be conducted to
verify this conclusion.
In summary, the present study is the first to
explore the function of the transcription factor AIOLOS in regards
to the biological behaviors of a human T-ALL cell line. The present
study provides the basis for further research on the pathogenesis
of T-ALL. Our results revealed that the upregulation of AIOLOS
expression in Jurkat cells induced cell apoptosis and arrested the
cell cycle at the G0/G1 phase. In addition, AIOLOS overexpression
synergistically increased the sensitivity of Jurkat cells to
etoposide by inhibiting NF-κB activity. However, the mechanism by
which AIOLOS interacts with other regulators remains poorly
understood. These potential genetic interactions should be
characterized in future studies.
Acknowledgements
The present study was supported by Grants of the
Shandong Province Natural Science Foundation (ZR2011HM007 and
2013GSF11812), the Innovation Fund Project of Shandong University
(2014QY003-11), and the Scientific Research Fund of Shenzhen
(JCYJ20140418115449178).
References
|
1
|
Chiaretti S and Foà R: T-cell acute
lymphoblastic leukemia. Haematologica. 94:160–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrando AA, Neuberg DS, Staunton J, Loh
ML, Huard C, Raimondi SC, Behm FG, Pui CH, Downing JR, Gilliland
DG, Lander ES, Golub TR and Look AT: Gene expression signatures
define novel oncogenic pathways in T cell acute lymphoblastic
leukemia. Cancer Cell. 1:75–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pui CH and Evans WE: Treatment of acute
lymphoblastic leukemia. N Engl J Med. 354:166–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhojwani D and Pui CH: Relapsed childhood
acute lymphoblastic leukaemia. Lancet Oncol. 14:e205–e217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelley CM, Ikeda T, Koipally J, Avitahl N,
Wu L, Georgopoulos K and Morgan BA: Helios, a novel dimerization
partner of Ikaros expressed in the earliest hematopoietic
progenitors. Curr Biol. 8:508–515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgan B, Sun L, Avitahl N, Andrikopoulos
K, Ikeda T, Gonzales E, Wu P, Neben S and Georgopoulos K: Aiolos, a
lymphoid restricted transcription factor that interacts with Ikaros
to regulate lymphocyte differentiation. EMBO J. 16:2004–2013. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Georgopoulos K, Winandy S and Avitahl N:
The role of the Ikaros gene in lymphocyte development and
homeostasis. Ann Rev Immunol. 15:155–176. 1997. View Article : Google Scholar
|
|
9
|
Narvi E, Nera KP, Terho P, Mustonen L,
Granberg J and Lassila O: Aiolos controls gene conversion and cell
death in DT40 B cells. Scand J Immunol. 65:503–513. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rebollo A, Ayllón V, Fleischer A, Martínez
CA and Zaballos A: The association of Aiolos transcription factor
and Bcl-xL is involved in the control of apoptosis. J
Immunol. 167:6366–6373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romero F, Martínez-A C, Camonis J and
Rebollo A: Aiolos transcription factor controls cell death in T
cells by regulating Bcl-2 expression and its cellular localization.
EMBO J. 18:3419–3430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakase K, Ishimaru F, Avitahl N, Dansako
H, Matsuo K, Fujii K, Sezaki N, Nakayama H, Yano T, Fukuda S,
Imajoh K, Takeuchi M, Miyata A, Hara M, Yasukawa M, Takahashi I,
Taguchi H, Matsue K, Nakao S, Niho Y, Takenaka K, Shinagawa K,
Ikeda K, Niiya K and Harada M: Dominant negative isoform of the
Ikaros gene in patients with adult B-cell acute lymphoblastic
leukemia. Cancer Res. 60:4062–4065. 2000.PubMed/NCBI
|
|
13
|
Nückel H, Frey UH, Sellmann L, Collins CH,
Duhrsen U and Siffert W: The IKZF3 (Aiolos) transcription factor is
highly upregulated and inversely correlated with clinical
progression in chronic lymphocytic leukaemia. Br J Haematol.
144:268–270. 2009. View Article : Google Scholar
|
|
14
|
Billot K, Soeur J, Chereau F, Arrouss I,
Merle-Beral H, Huang ME, Mazier D, Baud V and Rebollo A:
Deregulation of Aiolos expression in chronic lymphocytic leukemia
is associated with epigenetic modifications. Blood. 117:1917–1927.
2011. View Article : Google Scholar
|
|
15
|
Antica M, Cicin-Sain L, Kapitanovic S,
Matulic M, Dzebro S and Dominis M: Aberrant Ikaros, Aiolos, and
Helios expression in Hodgkin and non-Hodgkin lymphoma. Blood.
111:3296–3297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhuang Y, Li D, Fu J, Shi Q, Lu Y and Ju
X: Overexpression of AIOLOS inhibits cell proliferation and
suppresses apoptosis in Nalm-6 cells. Oncol Rep. 31:1183–1190.
2014.PubMed/NCBI
|
|
17
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and β-TrCP: tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hershko DD: Oncogenic properties and
prognostic implications of the ubiquitin ligase Skp2 in cancer.
Cancer. 112:1415–1424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Fukushima H, Inuzuka H, Wan L, Liu
P, Gao D, Sarkar FH and Wei W: Skp2 is a promising therapeutic
target in breast cancer. Front Oncol. 1:pii: 18702. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitagawa M, Lee SH and McCormick F: Skp2
suppresses p53-dependent apoptosis by inhibiting p300. Mol Cell.
29:217–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin HK, Wang G, Chen Z, Teruya-Feldstein
J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer
S, Tempst P and Pandolfi PP: Phosphorylation-dependent regulation
of cytosolic localization and oncogenic function of Skp2 by
Akt/PKB. Nat Cell Biol. 11:420–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stella S, Tirrò E, Conte E, Stagno F, Di
Raimondo F, Manzella L and Vigneri P: Suppression of survivin
induced by a BCR-ABL/JAK2/STAT3 pathway sensitizes
imatinib-resistant CML cells to different cytotoxic drugs. Mol
Cancer Ther. 12:1085–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,
2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces
tumor cell invasion in colorectal cancer cells. Neoplasia.
10:287–297. 2008.PubMed/NCBI
|
|
24
|
Kirkin V, Joos S and Zörnig M: The role of
Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martins LM, Mesner PW, Kottke TJ, Basi GS,
Sinha S, Tung JS, Svingen PA, Madden BJ, Takahashi A, McCormick DJ,
Earnshaw WC and Kaufmann SH: Comparison of caspase activation and
subcellular localization in HL-60 and K562 cells undergoing
etoposide-induced apoptosis. Blood. 90:4283–4296. 1997.PubMed/NCBI
|
|
26
|
Montecucco A and Biamonti G: Cellular
response to etoposide treatment. Cancer Lett. 252:9–18. 2007.
View Article : Google Scholar
|
|
27
|
Karin M and Greten FR: NF-κB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|