Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer, and it is the third leading cause of cancer
mortality worldwide (1). Despite
advances in the detection and treatment of HCC, the mortality
remains high because the majority of HCC patients present at an
advanced stage or with metastasis for which most potentially
curative therapies have limited efficacy (2). Thus, understanding the mechanism
underlying carcinogenesis and metastatic formation and treating
invasion and metastasis as therapeutic targets are essential for
the management of liver malignancies.
Cancer stem cell (CSC) hypothesis assumes that a
small amount of cancer cells with stem-like characteristics,
including self-renewal and differentiation to a particular lineage
of mature cells (3), can initiate
and sustain tumor growth, drive relapse (4) and be responsible for early systemic
dissemination and metastasis formation (5). The side population (SP) phenotype is
thought to be enriched for stem-like cells in normal tissues and
various types of cancer. Previously, the identification and
isolation of SP cells in human HCC cell lines was reported and its
stem-like characteristics compared with main population (MP) cells,
including quiescence, elevated chemoresistance, increased
tumorigenicity, higher actin polymerization ability and increased
migration capacity towards the chemokine CXCL12 (6) were confirmed.
Signal transducer and activator of transcription 3
(STAT3) is a transcription factor that regulates various genes
involved in different biological and tumorigenic processes. In
response to cytokines or environmental factors, STAT3 becomes
phosphorylated on Tyr-705 and/or Ser-727, resulting in enhanced
transcriptional activity, making it a potent inducer of
proliferation, anchorage-independent growth, tumorigenesis,
invasion, metastasis and angiogenesis (7–9).
As a downstream regulator of the STAT3 signaling
pathway, STAT3 phosphorylation is also involved in the regulation
of microRNAs (10), which can
negatively regulate various protein expression levels at the
post-transcriptional level by translational inhibition and/or mRNA
degradation. It was reported that STAT3 bound to multiple sites in
miR-21 promoter and was necessary for miR-21 expression and
induction of transcription (11,12).
It has been widely demonstrated that miR-21 can function as an
oncogene and increase tumor cell migration and invasion by directly
targeting PTEN, RECK and programmed cell death 4 (PDCD4) (13,14). A
higher miR-21 expression in HCC SP cells compared with MP cells was
previously identified (15), as
well as the fact that repression of miR-21 inhibited SP cell
migration and invasion in vitro, possibly due to the
downregulation of the tumor suppressor PTEN, RECK or PDCD4.
Considering STAT3, miR-21 and its targets have been
widely explored as cancer-related targets for tumors including HCC,
the effects of STAT3 on the metastasis-related capacities of HCC SP
cells, and whether the effects were mediated through miR-21 and its
targets were determined. To the best of our knowledge, no
information is available concerning this issue. Thus, we conducted
the present study to determine the effects and potential mechanism
of STAT3 in the metastasis of HCC SP cells.
Materials and methods
Cell culture and cell sorting
The MHCC97H human HCC cell line was cultured in DMEM
medium supplemented with 10% fetal calf serum (Sigma Chemical Co.,
St. Louis, MO, USA) and 1% penicillin-streptomycin G (Invitrogen
Life Technologies, Carlsbad, CA, USA). In all experiments, the
cells were cultured at 37°C in a humidified 5% CO2/95%
air atmosphere. To identify and isolate the SP and MP fractions, we
used flow cytometric analysis as described previously (6). Briefly, the cells were preincubated at
37°C for 15 min and stained with Hoechst 33342 dye (Sigma Chemical
Co.) at 6 μg/ml. The cells were then incubated for 90 min at 37°C
alone or with 50 μM verapamil (Sigma Chemical Co.), and then
stained with 2 μg/ml of propidium iodide (BD Pharmingen, San Diego,
CA, USA) to label dead cells. The cells were then filtered through
a 40-μm strainer (BD Falcon) and maintained at 4°C before analysis
and sorting using a FACSAria flow cytometer (BD Falcon). Samples of
sorted cells were reanalyzed to examine the sorting purities.
Quantitative RT-PCR
Total RNA, including miRNAs, was isolated from
MCHH97H cells with TRIzol reagent (Invitrogen) according to the
manufacturer’s instructions. Expression of hsa-miR-21 was analyzed
with the miScript system (Qiagen, Valencia, CA, USA), which
consists of the miScript Reverse Transcription kit, miScript Primer
assays and miScript SYBR-Green PCR kit, according to the
manufacturer’s instructions. Small nuclear RNA U6 was used for
normalization. Quantitative RT-PCR (RT-qPCR) was run on the ABI
PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA,
USA). Reactions were run in triplicate. The ΔΔCt method was used
for relative quantification of the gene expression to determine
miR-21 expression levels.
Protein extraction and western
blotting
The cells were lysed in lysis buffer as previously
described (15) by incubating for
20 min at 4°C. The protein concentration was determined using the
Bio-Rad assay system (Bio-Rad Laboratories, Hercules, CA, USA).
Total proteins were fractionated using SDS-PAGE and transferred
onto nitrocellulose membranes. The membranes were blocked with 5%
non-fat dried milk or bovine serum albumin in 1X TBS buffer
containing 0.1% Tween-20 and then incubated with the appropriate
primary antibodies. Horseradish peroxidase-conjugated anti-rabbit
or anti-mouse IgG was used as the secondary antibody and the
protein bands were detected using the enhanced chemiluminescence
detection system (Amersham Pharmacia Biotech, Amersham, UK).
Quantification of the western blots was performed using laser
densitometry and the relative protein expression was then
normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
levels.
Apoptosis assays
JSI-124 (cucurbitacin-I, Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) was dissolved in DMSO. Cells were
treated with the appropriate volume of DMSO for the vehicle
control. Following treatment with different concentrations of
JSI-124 or DMSO, the cells were washed twice in PBS and resuspended
in 500 μl of 1X binding buffer prior to incubation with 5 μl of
Annexin V and 10 μl of PI. The cells were then analyzed by using
flow cytometry after incubation for 5–10 min in the dark. Early
apoptotic cells were stained with Annexin V alone whereas necrotic
and late apoptotic cells were stained with both Annexin V and
PI.
Actin polymerization
SP cells were resuspended and maintained at 37°C.
Human CXCL12 (Peprotech, Inc., Rocky Hill, NJ, USA) or PBS were
added to cell suspensions and aliquots were removed at the
indicated times and immediately fixed in 4% paraformaldehyde for 10
min. After washing, the samples were stained with FITC Phalloidin
(Molecular Probes, Eugene, OR, USA) stain F-actin and analyzed by
flow cytometry. The relative F-actin index was determined as the
ratio of the F-actin level of different cells treated with CXCL12
to cells treated with PBS.
Migration and invasion assays
Cell migration was analyzed with non-Matrigel-coated
Transwell cell culture chambers (Millipore, Billerica, MA, USA).
Cell invasion was analyzed with Matrigel-coated Transwell cell
culture chambers (Millipore). Cells treated with different reagents
(5×104 cells/well) were serum-starved for 24 h and
plated in the upper insert of a 24-well chamber in serum-free
medium. A medium with or without CXCL12 chemokine was added to the
well. After incubation for 24 h, the cells on the upper side of the
filters were mechanically removed by scrubbing with a cotton swab,
after which the membrane was fixed with 4% formaldehyde for 10 min
at room temperature and stained with 0.5% crystal violet for 10
min. Invasive or migrated cells were counted at a magnification of
×200 from 10 different fields of each filter.
Statistical analysis
Each experiment was repeated at least three times.
The data were summarized and presented as means ± SD. The
differences among means were statistically analyzed using a t-test.
Statistical analyses were performed using SPSS 13.0 software
(Chicago, IL, USA). P<0.05 was considered statistically
significant.
Results
MHCC97H cells contain stem-like SP
cells
Using flow cytometry, we identified and successfully
isolated SP and MP cell populations from MHCC97H cell lines. The SP
gate was defined as the region where cells were absent in the
presence of verapamil, an agent that blocks the efflux of Hoechst
33342. The SP cells accounted for 2.97±0.33 of the total cells in
the MHCC97H cell line. The purities of sorted SP and MP cells were
>97%. As previously reported (6), MHCC97H SP cells exhibited stem-like
characteristics including tumorigenic potential and chemoresistance
migration and invasion abilities, which may lead to relapse and
metastasis formation.
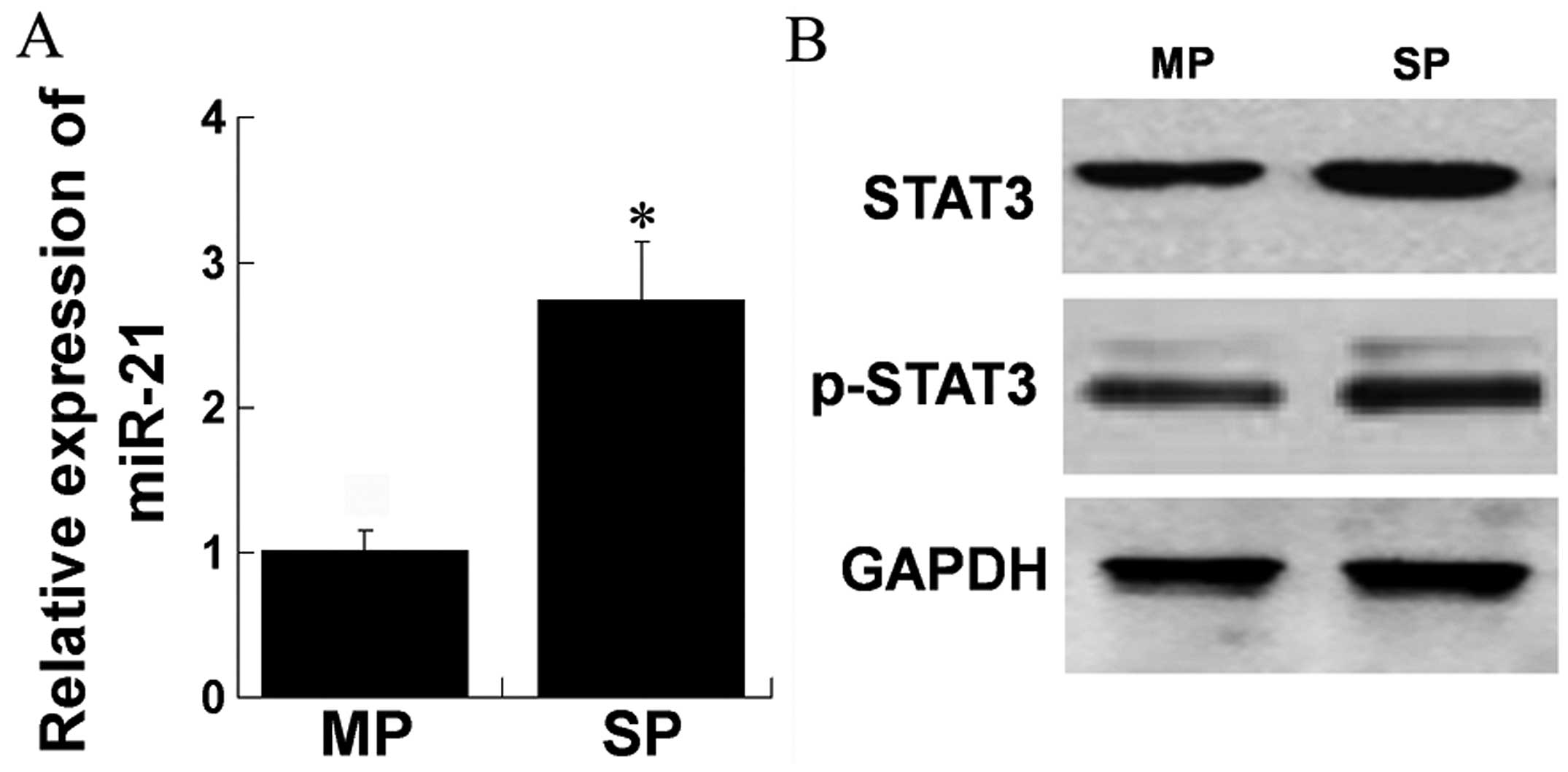
STAT3, phospho-STAT3 and miR-21 are
overexpressed in HCC SP cells
STAT3 and miR-21 are known to be overexpressed in
carcinogenesis and metastasis. We assessed the expression levels of
STAT3, phospho-STAT3 and miR-21 in SP cells as compared to MP
cells, respectively. RT-qPCR was performed and a higher expression
of miR-21 was observed in SP compared to MP cells (Fig. 1A). Western blot results showed that
the levels of STAT3 and phospho-STAT3 were higher in SP compared to
MP cells (Fig. 1B). These data
indicated that STAT3, phospho-STAT3 and miR-21 were overexpressed
in SP cells of MHCC97H cell line.
Inhibition of STAT3-regulated
metastasis-related capacities of MHCC97H SP cells
Accumulating evidence indicated that STAT3 plays a
key role in the maintenance of stem-like cancer cells associated
with tumor recurrence, metastasis and chemo-resistance (16). As the effects of STAT3 on HCC SP
cells were to be determined, we used JSI-124 to inhibit STAT3 and
observed the role of STAT3 on MHCC97H SP cell metastatic
capacity.
STAT3 inhibition induced by JSI-124 was evaluated by
western blotting. JSI-124 was dissolved in DMSO. The cells were
treated with the appropriate volume of PBS or DMSO for the vehicle
control. The results showed that phospho-STAT3 protein levels were
downregulated significantly when treated with different
concentrations of JSI-124 (50 and 100 nM). The protein levels
decreased with the increase of JSI-124 concentrations (Fig. 2A). In order to exclude the
possibility that the downregulation of phospho-STAT3 was caused by
cell apoptosis, we evaluated JSI-124-treated cells by Annexin V/PI
analysis. The results did not indicate significant cell apoptosis
in either concentration (Fig.
2B).
Actin polymerization was required by cell
polarization. Our previous data indicated that MHCC97H SP cells
contained higher actin polymerization levels than MP cells when
treated with CXCL12. In the present study, we determined whether
STAT3 affected actin polymerization of SP cells. As shown in
Fig. 3, we treated MHCC97H SP cells
with PBS, DMSO and 50 or 100 nM JSI-124, respectively, and then
detected the F-actin levels of different cells after 3, 5 and 10
min stimulation with CXCL12. Actin polymerization levels of cells
treated with PBS or DMSO were similar to each other. Actin
polymerization levels of cells treated with 50 or 100 nM JSI-124
were significantly lower than cells treated with PBS at the studied
time points (P<0.05, P<0.01). This result suggested that the
higher actin polymerization level of SP cells treated with CXCL12
was reduced by STAT3 inhibitor JSI-124 (Fig. 3A).
To examine the possible differences in chemotaxis
towards CXCL12 between SP cells treated with different reagents, we
performed a Transwell-based migration assay. The results indicated
that SP cells showed higher migration ability towards CXCL12 rather
than PBS (P<0.05). However, when treated with 50 or 100 nM
JSI-124, the chemotaxis towards CXCL12 decreased significantly
(P<0.01) (Fig. 3B).
We previously reported MHCC97H SP cells exhibited
higher abilities of migration and invasion than MP cells and may be
important in HCC metastasis. We wondered whether STAT3 affect the
metastasis capacities of SP cells. Migration and invasion cell
numbers were counted through the Transwell system. The results
showed that 50 and 100 nM JSI-124 decreased the SP cell abilities
of migration and invasion (P<0.05) (Fig. 3C).
Inhibition of STAT3 regulated the
expression levels of miR-21 and its targets in MHCC97H SP
cells
It was reported that STAT3 binds to multiple sites
in the miR-21 promoter and was required for the induction of
transcription in normal or cancer cells (9,17,18).
Our data indicated STAT3 phosphorylation affected
metastasis-related capacities in vitro of HCC SP cells. We
also improved the repression of miR-21-inhibited SP cell migration
and invasion in vitro due to upregulation of its target
tumor-suppressor genes, i.e., PTEN, RECK or
PDCD4. However, whether the effects of phospho-STAT3 on
metastasis-related capacities of MHCC97H SP cells were mediated by
miR-21 and its targets remained to be determined. We measured the
expression of miR-21 in cells treated with PBS (Basal), DMSO, and
50 or 100 nM JSI-124 through RT-qPCR. It was observed that 50 and
100 nM JSI-124 repressed the expression of miR-21 (P<0.05,
compared with Basal) (Fig. 4A).
Western blot results showed that the levels of PTEN, RECK and PDCD4
were increased with the increasing concentrations of JSI-124
(Fig. 4B).
JSI-124 inhibited phospho-STAT3, while miR-21 and
its target gene expression were increased. We determined whether
STAT3 was able to regulate PTEN, RECK and PDCD4 via miR-21. MHCC97H
SP cells were treated with PBS (Basal), and 100 nM JSI-124 with or
without transfection with miR-21 mimics. Transfection with miR-21
mimics led to upregulated miR-21 expression, whereas the increased
protein expression of PTEN, RECK and PDCD4 was markedly
reduced.
The effects of phospho-STAT3 on migration
and invasion of SP cells
We also examined whether inhibition of phospho-STAT3
is involced in the migration and invasion of MHCC97H SP cells via
miR-21 and its targets. Western blotting indicated that 100 nM
JSI-124 did not induce SP cell migration or invasion when miR-21
expression was upregulated (Fig.
5A). In addition, cell migration and invasion were increased by
silencing PTEN, RECK or PDCD4 and these increases were reduced by
JSI-124 (all P<0.05) (Fig. 5B).
These results indicated that the metastatic effect of phospho-STAT3
is partly mediated through miR-21 and its negative regulation of
PTEN, RECK and PDCD4 expression.
Discussion
Accumulating evidence indicated that stem-like
cancer cells are the source of malignant phenotypes in many solid
tumors. It has also been suggested that stem-like HCC cells play a
critical role in initiating and sustaining HCC primary tumors and
in facilitating HCC metastasis and recurrence (24,25).
SP cells which efflux the DNA-binding dye Hoechst 33342 out of the
cell membrane through an ATP-binding cassette (ABC) transporter was
first reported in the analysis of hematopoietic stem cells and
expanded to various fields. Our previous results (6) showed that SP cells were critical for
HCC meta stasis because of higher migration and invasion abilities
compared to MP cells. Therefore, we determined the potential
mechanism involved in HCC SP cell regulation of HCC metastasis.
Abnormal STAT3 signaling, particularly the
constitutive activation of STAT3 is important in development and
carcinogenesis, since it critically regulates the transcription of
multiple key genes involved in cell proliferation, differentiation,
apoptosis, angiogenesis, immune response and metastasis (19,20).
Activation of STAT3 has been shown to upregulate the expression
levels of miRNAs, including miR-21 (21). miR-21 has been shown to be
overexpressed in a variety of malignancies, and linked to cell
metastasis through its targets PTEN, RECK and PDCD4. PTEN is a
phosphoinositide phosphatase acting as a tumor suppressor through
Akt and ERK signaling pathways associated with cell survival,
proliferation, differentiation, cell migration and invasion. RECK,
a membrane-anchored glycoprotein, is a molecular marker for cancer
prognosis and controller of cell metastatic capacity (22). Low levels of RECK are often
associated with increased invasiveness and poor prognosis (23,24).
PDCD4 is a tumor suppressor that inhibits metastasis in human
cancer cells (25). It was reported
that PDCD4 suppressed the expression and/or activity of the
invasion-related proteins such as AKT (26), MAP4K1 (27), and increased the release of
metastasis-suppressor proteins such as E-cadherin (28) and TIMP2 (29). We already partly verified that
miR-21 regulated MHCC97H SP cell metastasis through its targets
PTEN, PDCD4 and RECK. However, whether STAT3 functions as an
upstream mediator to regulate the effects of miR-21 and its targets
on metastasis in MHCC97H SP cells remained to be determined.
To confirm the speculation, we first investigated
the expression level of STAT3, phospho-STAT3 and miR-21 of MHCC97H
SP and MP cells. A higher expression of STAT3, phospho-STAT3 and
miR-21 was observed in SP compared to MP cells. As the critical
role of SP cells in HCC malignant phenotypes and the function of
STAT3 in HCC survival, proliferation, invasion and angiogenesis
(30,31) have been previously proven, we
examined the effects of STAT3 on metastasis-related capacities of
MHCC97H SP cells.
JSI-124, a chemical compound belonging to the
cucurbitacin family, was initally identified as a potent
phospho-STAT3 inhibitor in multiple cancer cell lines (18). Inhibition of STAT3 activity was
attributed to a disruption in STAT3 DNA-binding activity and gene
expression. Thus, we treated MHCC97H SP cells with JSI-124 to
observe the effects of STAT3 on SP cell metastatic capacity.
Relatively low concentrations of JSI-124 were selected to avoid
induction of apparent apoptosis. Western blot and Annexin V/PI
analysis show that 50 or 100 nM JSI-124 did not inhibit STAT3 but
phospho-STAT3 effectively, without inducing significant apoptosis.
Although SP cells contain a higher metastatic capacity, the present
data show that when phospho-STAT3 was inhibited in SP cells,
F-actin polymerization, chemotaxis towards CXCL12, migration and
invasion cells were weakened, which reduced SP cell metastasis.
miR-21 has been reported to be regulated by an
upstream promoter/enhancer containing STAT3 binding sites in
malignant disease (11,12). Although we have already confirmed
that phospho-STAT3 regulated SP cell chemotaxis, migration and
invasion, we determined whether STAT3 regulations were mediated by
miR-21 and its targets in MHCC97H SP cells. miR-21 was notably
decreased and the protein levels of miR-21 targets PTEN, RECK and
PDCD4 were increased when phospho-STAT3 was inhibited. To confirm
the relationship between phospho-STAT3 and miR-21, we developed the
‘rescue’ method. miR-21 expression was upregulated to ‘rescue’ the
inhibitory effect of JSI-124. A marked down-regulation was
reflected in PTEN, RECK or PDCD4 protein expression. Moreover,
Transwell analyses demonstrated that higher migration and invasion
abilities gained by silencing the PTEN, RECK or PDCD4 expression
could be counteracted by inhibition of phospho-STAT 3 to some
extent. These findings further indicated that STAT3 and
phospho-STAT3 acted as an upstream regulator of miR-21 in MHCC97H
SP cells, as it regulated SP cell metastasis-related capacities
through targets of miR-21, PTEN, RECK and PDCD4.
Taken together, the findings provided evidence that
STAT3 is crucial in MHCC97H SP cell metastasis via miR-21 and its
targets. Consequently, suppression of miR-21 by inhibition of STAT3
may be a novel approach for preventing HCC metastasis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81101619/H1607), the
National Natural Science Foundation of Shaanxi Province, China (no.
2012JQ4032), and the National S&T Major Project for Infectious
Diseases of China (no. 2012ZX10002-017).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lau WY and Lai EC: Hepatocellular
carcinoma: current management and recent advances. Hepatobiliary
Pancreat Dis Int. 7:237–257. 2008.PubMed/NCBI
|
|
3
|
Bjerkvig R, Tysnes BB, Aboody KS, et al:
Opinion: the origin of the cancer stem cell: current controversies
and new insights. Nat Rev Cancer. 5:899–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumors: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simeone DM: Pancreatic cancer stem cells:
implications for the treatment of pancreatic cancer. Clin Cancer
Res. 14:5646–5648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang N, Li R, Tao KS, et al:
Characterization of a stem-like population in hepatocellular
carcinoma MHCC97 cells. Oncol Rep. 23:827–831. 2010.PubMed/NCBI
|
|
7
|
Chen J, Wang J, Lin L, et al: Inhibition
of STAT3 signaling pathway by nitidine chloride suppressed the
angiogenesis and growth of human gastric cancer. Mol Cancer Ther.
11:277–287. 2012. View Article : Google Scholar
|
|
8
|
Ho PL, Lay EJ, Jian W, et al: Stat3
activation in urothelial stem cells leads to direct progression to
invasive bladder cancer. Cancer Res. 72:3135–3142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen B, Liu J, Chang Q, et al: JNK and
STAT3 signaling pathways converge on Akt-mediated phosphorylation
of EZH2 in bronchial epithelial cells induced by arsenic. Cell
Cycle. 12:112–121. 2013. View
Article : Google Scholar :
|
|
10
|
Cao Q, Li YY, He WF, et al: Interplay
between microRNAs and the STAT3 signaling pathway in human cancers.
Physiol Genomics. 45:1206–1214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iliopoulos D, Jaeger SA, Hirsch HA, et al:
STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are
part of the epigenetic switch linking inflammation to cancer. Mol
Cell. 39:493–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Ren Y, Liu A, et al: STAT3
inhibitor WP1066 attenuates miRNA-21 to suppress human oral
squamous cell carcinoma growth in vitro and in vivo. Oncol Rep.
31:2173–2180. 2014.PubMed/NCBI
|
|
13
|
Liu C, Yu J, Yu S, et al: MicroRNA-21 acts
as an oncomir through multiple targets in human hepatocellular
carcinoma. J Hepatol. 53:98–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Gao X, Wei F, Zhang X, et al:
Diagnostic and prognostic value of circulating miR-21 for cancer: a
systematic review and meta-analysis. Gene. 533:389–397. 2014.
View Article : Google Scholar
|
|
15
|
Zhou L, Yang ZX, Song WJ, et al:
MicroRNA-21 regulates the migration and invasion of a stem-like
population in hepatocellular carcinoma. Int J Oncol. 43:661–669.
2013.PubMed/NCBI
|
|
16
|
Marotta LL, Almendro V, Marusyk A, et al:
The JAK2/STAT3 signaling pathway is required for growth of
CD44+CD24− stem cell-like breast cancer cells
in human tumors. J Clin Invest. 121:2723–2735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourguignon LY, Earle C, Wong G, Spevak CC
and Krueger K: Stem cell marker (Nanog) and Stat-3 signaling
promote microRNA-21 expression and chemoresistance in
hyaluronan/CD44-activated head and neck squamous cell carcinoma
cells. Oncogene. 31:149–160. 2012. View Article : Google Scholar
|
|
18
|
Sawant DV, Wu H, Kaplan MH, et al: The
Bcl6 target gene microRNA-21 promotes Th2 differentiation by a T
cell intrinsic pathway. Mol Immunol. 54:435–442. 2012. View Article : Google Scholar
|
|
19
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Löffler D, Brocke-Heidrich K, Pfeifer G,
et al: Interleukin-6 dependent survival of multiple myeloma cells
involves the Stat3-mediated induction of microRNA-21 through a
highly conserved enhancer. Blood. 110:1330–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi C, Sheng Z, Horan TP, et al:
Regulation of matrix metalloproteinase-9 and inhibition of tumor
invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad
Sci USA. 95:13221–13226. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kotzsch M, Farthmann J, Meye A, et al:
Prognostic relevance of uPAR-del4/5 and TIMP-3 mRNA expression
levels in breast cancer. Eur J Cancer. 41:2760–2768. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takenaka K, Ishikawa S, Kawano Y, et al:
Expression of a novel matrix metalloproteinase regulator, RECK and
its clinical significance in resected non-small cell lung cancer.
Eur J Cancer. 40:1617–1623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allgayer H: Pdcd4, a colon cancer
prognostic that is regulated by a microRNA. Crit Rev Oncol Hematol.
73:185–191. 2010. View Article : Google Scholar
|
|
26
|
Lankat-Buttgereit B and Goke R: The tumour
suppressor Pdcd4: recent advances in the elucidation of function
and regulation. Biol Cell. 101:309–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang HS, Matthews CP, Clair T, et al:
Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated
protein kinase kinase kinase kinase 1 expression to suppress colon
carcinoma cell invasion. Mol Cell Biol. 26:1297–1306. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Sun Z and Yang HS: Downregulation
of tumor suppressor Pdcd4 promotes invasion and activates both
beta-catenin/Tcf and AP-1-dependent transcription in colon
carcinoma cells. Oncogene. 27:1527–1535. 2008. View Article : Google Scholar
|
|
29
|
Nieves-Alicea R, Colburn NH, Simeone AM,
et al: Programmed cell death 4 inhibits breast cancer cell invasion
by increasing tissue inhibitor of metalloproteinase-2 expression.
Breast Cancer Res Treat. 114:203–209. 2009. View Article : Google Scholar :
|
|
30
|
Rajendran P, Li F, Shanmugam MK, et al:
Celastrol suppresses growth and induces apoptosis of human
hepatocellular carcinoma through the modulation of STAT3/JAK2
signaling cascade in vitro and in vivo. Cancer Prev Res. 5:631–643.
2012. View Article : Google Scholar
|
|
31
|
Meydan N, Grunberger T, Dadi H, et al:
Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor.
Nature. 13:645–648. 1996. View
Article : Google Scholar
|