Introduction
Laryngeal carcinoma (LC) is a common head and neck
malignancy and accounts for ~2.4% of newly diagnosed malignancies
worldwide every year (1,2). Surgery, chemotherapy and radiation
therapy are the current treatment modules for laryngeal cancer
(3–5). Although early-stage laryngeal cancer
can be effectively treated with surgery or radiotherapy, the 5-year
survival rate of patients with advanced LC is still below 60% even
after systematic surgery and post-surgical adjuvant radiotherapy or
chemotherapy (6). In addition,
surgery may result in complete or partial loss of swallowing and
vocal functions. Many patients have to maintain a tracheal cannula
on a long-term basis due to laryngeal stenosis after surgery, which
markedly impairs their quality of life (6,7).
Therefore, there is urgent need to develop novel approaches and
strategies for the treatment of these advanced LC patients.
The development and progression of cancer are
closely associated with thrombosis (8). Thrombin, the key terminal enzyme of
coagulation, enhances angiogenesis, stimulates the adhesion of
tumor cells to platelet and endothelium, and promotes the growth
and metastasis of tumor cells (9).
Previous in vitro studies have shown that exogenous thrombin
(1 U/ml) acting through its protease-activated receptor (PAR)-1 is
capable of enhancing tumor adhesion to platelets, endothelial
cells, fibronectin and von Willebrand factor (10). Moreover, exogenous thrombin was
shown to promote tumor growth as well as metastasis in experimental
animals (11). In addition, in a
chorioallantoic membrane (CAM) model, exogenous thrombin was
demonstrated to induce angiogenesis (12). These data from in vitro and
in vivo studies suggest that thrombin is a potential target
for the development of molecular-targeted cancer therapies.
The anticoagulant recombinant hirudin (rH) is a
highly potent and specific inhibitor of thrombin, and has
demonstrated antitumor effects in different types of tumors
including melanoma, lung and prostate cancer (9). In a transgenic TRAMP mouse model of
prostate cancer, inhibition of endogenous thrombin by hirudin
retarded the growth of spontaneous tumors (9). Administration of hirudin at the early
stage after tumor cell inoculation in experimental animals led to
apparent central necrosis of the tumor nodule and inhibition of
spontaneous metastases from the subcutaneously implanted tumors,
accompanied by the reduced number of tumor nodules in the lungs
(11). Moreover, administration of
rH followed by stealthy liposomal vinblastine resulted in enhanced
inhibition of the growth and metastasis of melanoma in vivo
(10). Thus, targeting thrombin by
rH is a promising strategy for the development of anticancer
therapeutic modalities, particularly for those tumor cells with
PAR-1 on the cell surface. However, whether rH exerts antitumor
effects on LCs has not yet been investigated.
In the present study, we evaluated the antitumor
effects of rH and explored the underlying mechanisms in LC cells.
We treated Hep-2 LC cells with various dosages of rH and analyzed
cell viability, adhesion, migration and invasion. We additionally
assessed angiogenesis and apoptosis, and determined the expression
levels of vascular endothelial growth factor receptor (VEGF-R),
focal adhesion kinase (FAK), B-cell CLL/lymphoma 2
(Bcl-2)-associated agonist of cell death (Bad) and Bcl-2 following
treatment of Hep-2 cells with rH.
Materials and methods
Cell culture and treatment
Hep-2 human laryngeal cancer cells were obtained
from Beinglay Biotech (Wuhan, China) and cultured at 37°C in a 5%
CO2 atmosphere in RPMI-1640 medium (Gibco, Grand Island,
NY, USA) containing 10% fetal bovine serum (FBS) (HyClone, Logan,
UT, USA), and antibiotics (100 IU/ml penicillin and 100 IU/ml
streptomycin). rH was purchased from Combination Botai
Biotechnology (Dalian, China) and used at final concentrations of
0.5, 1, 2 mg/ml or 25, 50, 100 μg/ml in the experiments. The drugs
were prepared in RPMI-1640 medium before addition to the cell
cultures. Cells treated with phosphate-buffered saline (PBS) were
used as the negative controls, and cells treated with doxorubicin
hydrochloride (DOX) (4 μg/ml) as the positive controls.
Cell proliferation assay
Hep-2 cells were seeded in 96-well plates at a
density of 104 cells/well in RPMI-1640 containing 10%
FBS for 24 h and then treated with various concentrations of rH
(0.5, 1 or 2 mg/ml) or DOX (4 μg/ml). Following treatment for 24 h,
10 μl/well of water-soluble tetrazolium salt (WST) (Beyotime,
Beijing, China) was added, and the plates were incubated for an
additional 4 h. The spectrometric absorbance at a wavelength of 570
nm was measured on a microplate reader. This experiment was
repeated in triplicate.
Cell adhesion to fibronectin
Fibronectin (BD Biosciences, San Jose, CA, USA) was
diluted to a final concentration of 100 μg/ml in PBS and 40 μl was
added to each well of 96-well plates at 4°C overnight. Hep-2 cells
were pretreated with various concentrations of rH (25, 50 or 100
μg/ml) or DOX (4 μg/ml) for 0.5 h. After treatment,
1×105 cells were added to each well of 96-well plates
coated with fibronectin and incubated for 2 h. The cells that did
not adhere to the fibronectin were removed. The bound cells were
fixed with 4% (w/v) paraformaldehyde and stained with crystal
violet, followed by gentle rinsing with PBS and drying. Afterwards,
1% SDS was added, followed by mixing. Spectrometric absorbance at a
wavelength of 540 nm was measured on a microplate reader. This
experiment was repeated in triplicate.
Cell migration and invasion assay
A Transwell assay was performed using polycarbonate
Transwell filters (Corning Costar, Cambridge, MA, USA). Briefly,
cells were suspended in culture medium containing various
concentrations of rH (25, 50 or 100 μg/ml) or DOX (4 μg/ml) and
plated to the upper chamber with the bottom filled with culture
medium containing basic fibroblast growth factor (bFGF) (Roche,
Basel, Switzerland) (3 ng/ml). After incubation for 24 h, the cells
on the upper side of the filters were removed mechanically, while
cells that migrated to the bottom side were fixed in 4% (w/v)
paraformaldehyde. The migrated cells were photographed and counted
using Image-Pro Plus. Three independent experiments were
performed.
For the invasion assay, modified polycarbonate
Transwell filters (Corning Costar) coated with Matrigel (Matrigel
basement membrane matrix; BD Biosciences) were used. The upper
surface of the filter was coated with 60 μl Matrigel (4 mg/ml) at
37°C for 30 min. Cells were suspended in culture medium containing
various concentrations of rH (25, 50 or 100 μ/ml) or DOX (4 μg/ml)
and added to the upper chamber. The bottom chambers were filled
with culture medium containing bFGF (3 ng/ml). After incubation for
16 h, cells on the upper surface of the filter were removed by
scraping, and the invaded cells on the bottom side were fixed in 4%
(w/v) paraformaldehyde. The invaded cells were photographed and
counted using Image-Pro Plus software. Three independent
experiments were performed.
Cell apoptosis assay
For detection of cell apoptosis, the Hep-2 cells
were stained with Hoechst 33324 (Beyotime). In brief, cells were
plated on coverslips and treated with various concentrations of rH
(0.5, 1 or 2 mg/ml) or DOX (4 μg/ml) for 24 h, followed by washing
in PBS (pH 7.4) and fixing with 4% (w/v) paraformaldehyde at room
temperature for 10 min. Following fixation, cells were washed three
times in PBS (pH 7.4) and stained with Hoechst 33324 according to
the manufacturer’s instructions. Stained nuclei were observed and
photographed under a fluorescence microscope. Cells undergoing
apoptosis demonstrated blue fluorescent nuclei (intact or
fragmented). Three independent experiments were performed.
Chick chorioallantoic membrane assay
The chicken chorioallantoic membrane (CAM) assay is
an established in vivo model for investigating the process
of new blood vessel formation and vessel responses to
anti-angiogenic agents (13). We
thereby applied the CAM assay to assess the effect of rH on
angiogenesis. All experiments were performed on day 6 of chick
embryo development, when CAM and its vasculature are
well-developed. A 1 cm2 window was made in the shell
under aseptic conditions. Next, a sterile methylcellulose disc was
added with 20 μl of recombinant human bFGF (10 ng/μl) (Roche) or
vehicle PBS and placed on the CAM. The window was sealed with
sterile scotch tape and the egg was kept in an incubator at 37°C
with 60% humidity for 24 h. Then, various concentrations of rH
(0.5, 1 or 2 mg/ml) or PBS was pipetted onto the methylcellulose
disc and the window was sealed again with sterile scotch tape. The
egg was incubated for an additional 24 h, observed and photographed
with a Nikon digital camera.
Western blot analysis
After the rH treatment, Hep-2 cells were washed with
ice-cold PBS (pH 7.4) and scraped into lysis buffer (KeyGen
Biotech, Nanjing, China). The lysate was collected by
centrifugation at 12,000 × g for 15 min at 4°C, and the supernatant
(total cell lysate) was stored at −80°C. Protein concentrations
were determined with a BCA protein assay reagent (Beyotime).
Proteins (60 μg) were separated using SDS-PAGE and transferred to a
PVDF membrane. Membranes were blocked with Tris-buffered saline
(TBS; 137 mM NaCl, 20 mM Tris-HCl, pH 7.5) containing 0.1% Tween-20
and 5% dried milk powder. Rabbit anti-mouse VEGF-R antibody
(diluted 1:1,000), rabbit anti-mouse FAK antibody (diluted 1:1,000)
(both from Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit
anti-mouse Bcl-2 antibody (diluted 1:1,000; Bioworld Technology,
USA) and rabbit anti-mouse Bad antibody (diluted 1:1,000;
Proteintech Group, Chicago, IL, USA) were used to detect the
corresponding proteins. Signals were developed with the enhanced
chemiluminescence detection system (Beyotime). Relative intensities
of the specific bands were quantified using Gel-Pro Analyser 4.0
software.
Statistical analysis
Statistical analysis was performed on SPSS version
16.0 (version 16.1; SPSS, Inc., Chicago, IL, USA). All values are
expressed as means ± standard deviation (SD) of at least three
independent experiments. p-values were determined by the Student’s
t-test and one-way ANOVA, with p<0.05 considered to indicate a
statistically significant result.
Results
Treatment of Hep-2 cells with rH results
in loss of cell viability and apoptosis
To investigate the effects of rH on the cell
viability of human laryngeal cancer cells, Hep-2 cells were treated
with varying concentrations of rH (0.5, 1 or 2 mg/ml) in parallel
with a standard widely used clinical chemotherapy drug (DOX) for 24
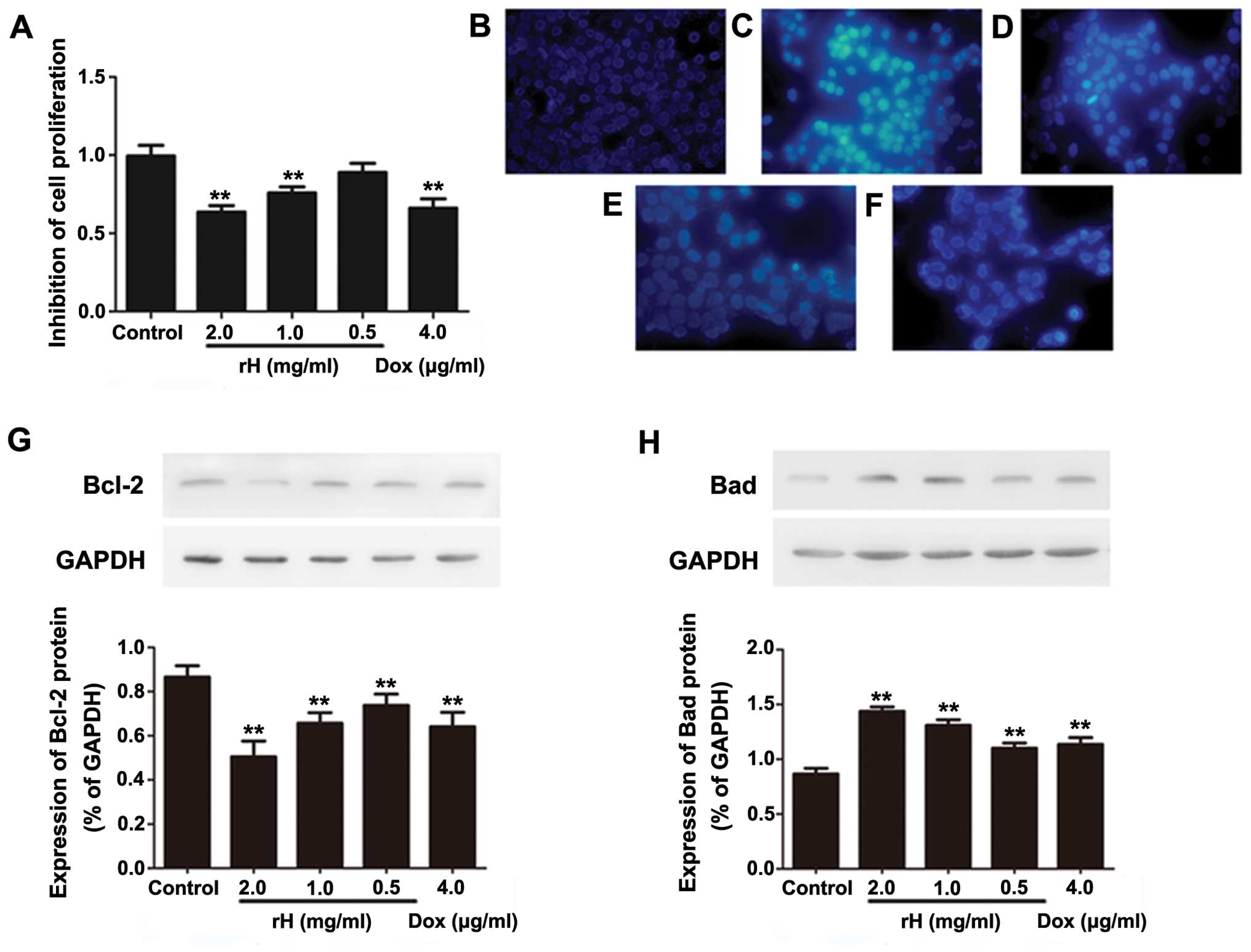
h, and subjected to a WST assay. As shown in Fig. 1A, compared to the negative control
group, treatment of cells with rH resulted in a significant
reduction in cell viability in a dose-dependent manner. The
inhibitory rate increased from 12.7 to 39.8% at 24 h as the
concentration of rH was escalated from 0.5 to 2 mg/ml. The
inhibitory effect of rH at 2 mg/ml was similar to that of DOX at a
concentration of 4 μg/ml. Thus, rH inhibits the cell viability of
Hep-2 human laryngeal cancer cells.
To ascertain the mechanism of the loss of cell
viability following rH treatment, we detected the effect of rH on
the apoptosis of Hep-2 cells. We first used Hoechst 33324 staining
to observe the change of morphology after rH treatment. As shown in
Fig. 1B–F, cells exposed to rH for
24 h showed apparent cell shrinkage, chromatin compaction and
nuclear fragmentation, all of which are typical apoptotic
morphological changes. Similar morphological alterations were
observed in the Hep-2 cells treated with DOX (Fig. 1F). In contrast, no obvious apoptosis
was observed in the negative control cells. These results indicated
that induction of cell apoptosis is one of the mechanisms by which
rH inhibits the proliferation of Hep-2 cells.
To further support the above conclusion, we treated
Hep-2 cells with rH and DOX, and determined the expression of the
apoptosis-associated proteins Bad and Bcl-2 by western blot
analysis. One of the cytotoxic effects of DOX is the induction of
apoptosis (14). As expected, DOX
decreased the anti-apoptotic protein Bcl-2 (Fig. 1G), while it increased the
pro-apoptotic protein Bad (Fig.
1H). Similarly, rH dose-dependently decreased Bcl-2 (Fig. 1G), yet increased Bad (Fig. 1H). These data suggest that rH
inhibits cell viability and induces apoptosis in Hep-2 cells, at
least partly, by regulating the levels of pro-apoptotic and
anti-apoptotic proteins.
rH inhibits the adhesion, migration and
matrix invasion of Hep-2 cells
The adhesion of cancer cells to the extracellular
matrix and cell surface molecules is a key step during metastasis
in vivo (15). We next aimed
to ascertain whether rH promotes the adhesion of Hep-2 cells to the
extracellular matrix and cell surface molecules by testing the
adhesion of Hep-2 cells to fibronectin. rH (50–100 μg/ml)
significantly inhibited the adhesion of Hep-2 cells to fibronectin,
while 25 μg/ml did not (Fig. 2).
The reduction in cell adhesion capabilities following rH treatment
at concentrations of 25 to 100 μg/ml was increased from 13.4 to
46.5%. The inhibitory effect of rH at 100 μg/ml was approximately
equal to that of DOX at 4 μg/ml.
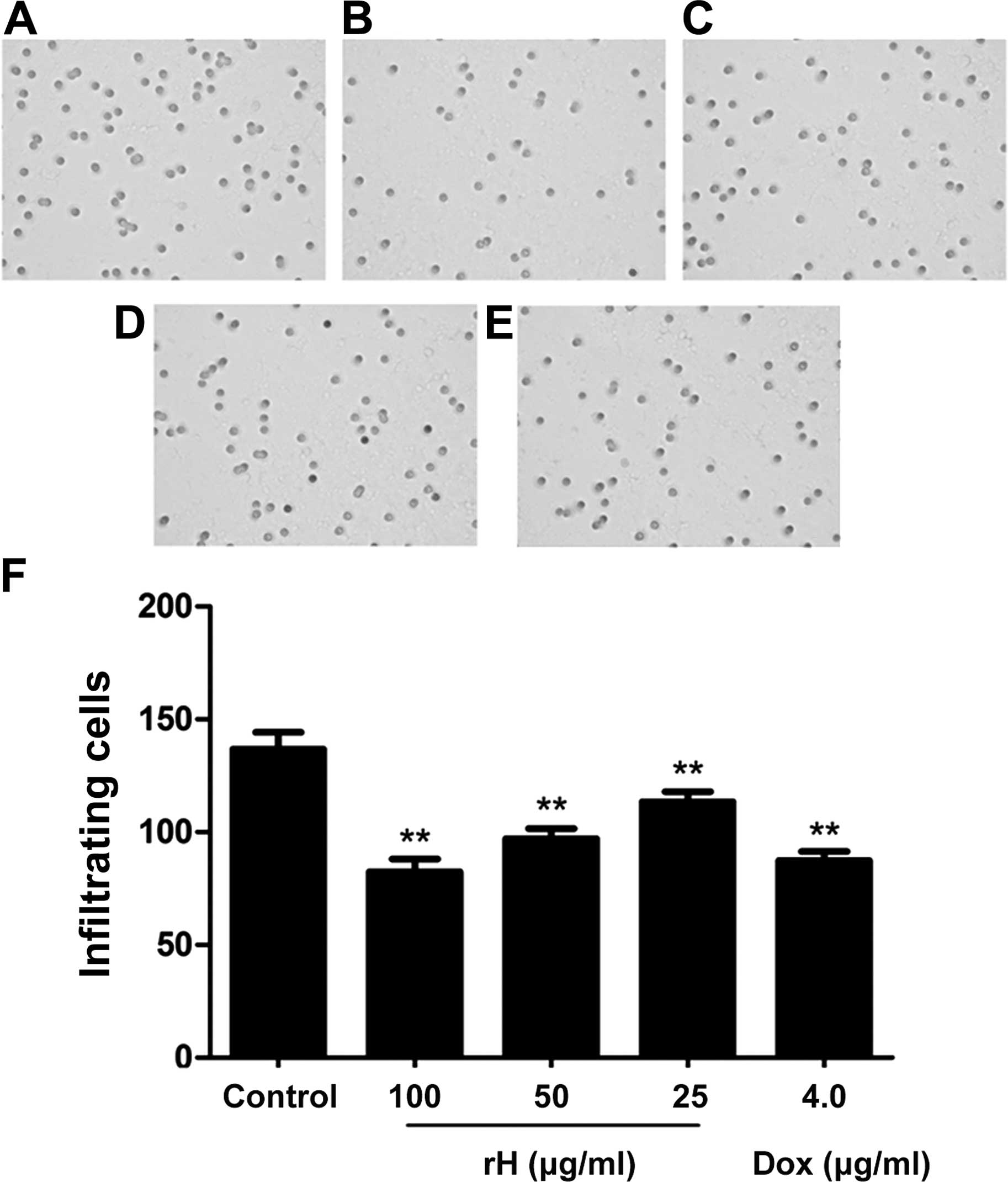
We further assessed the in vitro migration
and invasion of Hep-2 cells following treatment with rH using the
Boyden chamber model. Results from the migration assay showed that
the number of cells that migrated to the lower side of the membrane
was significantly reduced in the rH-treated groups in a
dose-dependent manner, as compared to the untreated cells (Fig. 3A–D). Treatment of the cells with 25,
50 or 100 μg/ml rH for 24 h inhibited cell migration by 15.5, 24.9
and 43.1%, respectively (Fig. 3F).
Treatment of Hep-2 cells with 4 μg/ml of DOX led to a 36.0%
reduction in cell migration (Fig. 3E
and F).
Movement of cells through Matrigel-coated Boyden
chambers mimics the early steps of tumor invasion (16). We thus applied a Matrigel assay to
test whether rH affects the invasion of Hep-2 cells and found that
rH significantly decreased bFGF-induced cell invasion through the
Matrigel in a dose-dependent manner (Fig. 4A–D and F). Relative to the untreated
control, treatment of Hep-2 cells with 25, 50 or 100 μg/ml rH for
24 h inhibited the number of cells invading the lower chamber by
17.1, 29.0 and 39.8%, respectively. The inhibitory effect of 100
μg/ml of rH was approximately equal to that of 4 μg/ml DOX
(Fig. 4E and F). These results
demonstrated that rH significantly decreased the migratory and
invasive capabilities of the Hep-2 cells.
rH inhibits angiogenesis in vivo
Rapidly proliferating tumor cells rely on sustained
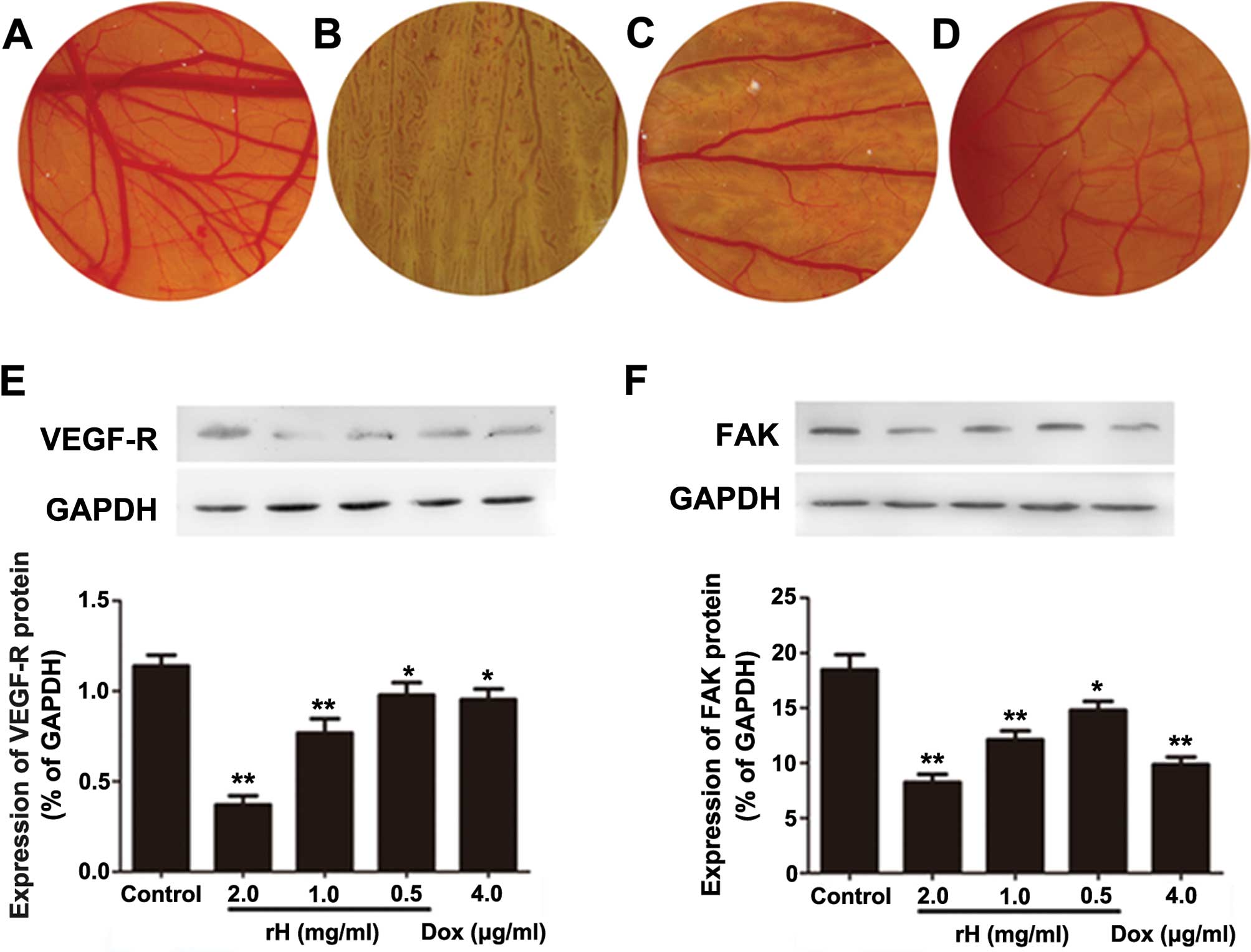
angiogenesis, a hallmark of cancer cells (17). To determine whether rH suppresses
microvessel formation in vivo, we performed a CAM assay
using day 6 fertilized eggs. PBS-treated CAMs showed normal
vascularization with primary, secondary and tertiary vessels and
dendritic branching (Fig. 5A). In
sharp contrast, rH-disc implanted CAMs demonstrated significantly
reduced formation of new microvessels in a dose-dependent manner
(Fig. 5B–D). rH significantly
decreased the vessel area, vessel length and number of dendrites.
Impressively, the addition of rH at 2 mg/ml totally blocked the
microvessel formation (Fig. 5B–D).
These results indicate that rH possesses the capacity for
anti-angiogenesis.
rH suppresses the protein levels of
VEGF-R and FAK
VEGF and its corresponding receptor (VEGF-R) are
important regulators of tumor angiogenesis. Moreover, VEGF-Rs are
not only expressed on endothelia, yet also on different types of
solid tumor cells and leukemic cells (18–20).
FAK is a cytoplasmic tyrosine kinase that plays a fundamental role
in integrin and growth factor-mediated signaling, and plays
critical roles in cell migration and proliferation, processes vital
for angiogenesis (21). The
increased expression of FAK in cancer cells has been suggested to
play a role in the tumor angiogenic switch to promote aggressive
tumor progression and metastasis (22). To assess the mechanism of the
inhibition of angiogenesis and explore the possible molecules
involved in rH-induced reduction of migration and invasion in Hep-2
cells, we determined the expression of VEGF-R and FAK by western
blot analysis. Compared to PBS, DOX apparently reduced the protein
levels of VEGF-R and FAK. Exposure of Hep-2 cells to rH for 24 h
resulted in decreased expression levels of VEGF-R and FAK in a
dose-dependent manner (Fig. 5E and
F), suggesting that rH reduces adhesion, migration, invasion
and angiogenesis of cancer cells through suppressing VEGF-R and FAK
expression.
Discussion
In the present study, we found that rH
dose-dependently suppressed the viability, adhesion, migration and
invasion of Hep-2 human laryngeal carcinoma (LC) cells. Treatment
of Hep-2 cells with rH resulted in reduced cell viability
accompanied by increased expression of Bad and decreased expression
of Bcl-2. In addition, we discovered that rH inhibited angiogenesis
and decreased the expression of VEGF-R and FAK.
Thrombin promotes tumor cell growth, angiogenesis
and metastasis by stimulating the adhesion of tumor cells to
platelets and endothelium (23).
These functions of thrombin mainly depend on the cell surface
receptor of thrombin, the protease-activated receptor (PAR).
Binding of thrombin to PAR-1 results in activation of PAR-1, which
in turn induces a series of physiological reactions and hence
promotes the growth of tumor cells in vivo (23,24).
rH is a highly specific and potent inhibitor of thrombin and has
shown inhibitory effects against tumor growth and metastasis in
experimental tumor models. In the present study, we evaluated the
antitumor effects of rH in human laryngeal cancer with Hep-2 cells,
which express PAR-1 on their cell surface (25). Our results demonstrated that rH
inhibited cell viability, adhesion, migration and invasion, and
induced apoptosis in Hep-2 cells, suggesting that rH is a potential
therapeutic agent for LC.
Apoptosis is a tightly regulated process that
involves at least one of the caspase-dependent signaling pathways,
i.e., the cell death receptor pathway or the mitochondrial pathway
(26). Among the numerous factors
known to modulate apoptosis in cancer cells, the proteins of the
Bcl-2 family are considered to be the main regulators. Bcl-2 is an
anti-apoptotic protein (26),
whereas Bad is a crucial pro-apoptotic and tumor-suppressor protein
(27). The ability to induce
cellular apoptosis is an important property of many anticancer
drugs (28). Our results showed
that rH suppressed cell viability and induced apoptosis in Hep-2
cells in a dose-dependent manner. Similarly, rH not only
downregulated Bcl-2, yet also upregulated Bad in Hep-2 cells in a
dose-dependent manner compared to PBS. These results indicate that
rH induces apoptosis of cancer cells via regulating the expression
of key regulators in the apoptosis process.
Sustained angiogenesis, and enhanced invasion and
metastasis, are two hallmarks of cancer cells (29). LC is a type of solid tumor with a
high potential for metastasis and invasion (15). It is now widely accepted that most
patients with solid tumors die from metastasis, yet not the growth
of the primary tumors (15). Thus,
targeting angiogenesis and metastasis is one of the most rational
and promising strategies for the development of anticancer drugs.
We observed that rH inhibited the adhesion, migration and invasion
of cultured Hep-2 cells, and suppressed angiogenesis in a CAM
model. At the molecular level, the expression levels of VEGF-R and
FAK were inhibited by rH in a dose-dependent manner. A previous
study indicated that FAK plays a pivotal role in cancer cell
survival, migration, invasion and angiogenesis (30). The VEGF/VEGF-R pathway not only
regulates neoangiogenesis, yet also influences the matrix-related
migratory activity by interaction with focal adhesion kinase
(p125FAK) and proline-rich tyrosine kinase β (PYK2/CAK β) (31). Therefore, rH suppresses the
adhesion, migration and invasion of Hep-2 cells partly by reducing
the expression of VEGF-R and FAK.
A previous study suggested that rH had no impact on
adhesion to extracellular matrix (ECM) proteins, migration and
invasion of in vitro cultured human A375 melanoma cells
(10). However, our data clearly
showed that rH inhibited Hep-2 cell adhesion to fibronectin,
migration and invasion in a dose-dependent manner. Such
discrepancies may result from differences in cell models or other
experimental methods. For example, in a study by Guo et al,
melanoma cells were treated with rH for only 30 min (10), while Hep-2 cells were treated with
rH for 24 h during the migration and invasion assays in our
experiments.
In conclusion, rH inhibited cell viability,
adhesion, migration and invasion, and induced apoptosis in Hep-2
cells. The underlying mechanisms may be associated with the
regulation of the expression of key regulators involved in these
processes, such as VEGF-R, FAK, Bcl-2 and Bad. Our data may
accelerate the development of rH as a novel therapeutic agent for
the treatment of cancers with a deregulated thrombin/PAR-1
signaling pathway, including LC.
Acknowledgements
This study was supported by the Science and
Technology Plan Projects of Dalian (no. 2010E15SF180). We would
like to thank Professor Li Lv and Professor Jihong Yao, and the
students of Feng Yu, Xiaohan Zhai and Hu Yan, Department of
Pharmacology, Dalian Medical University, for their technical
assistance.
Abbreviations:
|
LC
|
laryngeal carcinoma
|
|
rH
|
recombinant hirudin
|
|
DOX
|
doxorubicin hydrochloride
|
|
Bcl-2
|
B-cell CLL/lymphoma 2
|
|
Bad
|
Bcl-2-associated agonist of cell
death
|
|
VEGF-R
|
vascular endothelial growth factor
receptor
|
|
FAK
|
focal adhesion kinase
|
|
CAM assay
|
chick chorioallantoic membrane
assay
|
References
|
1
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papadas TA, Alexopoulos EC, Mallis A,
Jelastopulu E, Mastronikolis NS and Goumas P: Survival after
laryngectomy: a review of 133 patients with laryngeal carcinoma.
Eur Arch Otorhinolaryngol. 267:1095–1101. 2010. View Article : Google Scholar
|
|
3
|
Moubayed SP, Bélair M, Saliba J, et al:
Prognostic value of cartilage sclerosis in laryngeal cancer treated
with primary radiation therapy. Otolaryngol Head Neck Surg.
147:57–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taki S, Homma A, Suzuki F, et al: Combined
modality therapy for laryngeal cancer with superselective
intra-arterial cisplatin infusion and concomitant radiotherapy. Int
J Clin Oncol. 17:441–446. 2012. View Article : Google Scholar
|
|
5
|
Agra IM, Ferlito A, Takes RP, et al:
Diagnosis and treatment of recurrent laryngeal cancer following
initial nonsurgical therapy. Head Neck. 34:727–735. 2012.
View Article : Google Scholar
|
|
6
|
Chai LP, Wang ZF, Liang WY, et al: In
vitro and in vivo effect of 5-FC combined gene therapy with TNF-α
and CD suicide gene on human laryngeal carcinoma cell line Hep-2.
PLoS One. 8:e611362013. View Article : Google Scholar
|
|
7
|
Trzcieniecka-Green A, Bargiel-Matusiewicz
K and Borczyk J: Quality of life of patients after laryngectomy. J
Physiol Pharmacol. 58(Suppl 5): S699–S704. 2007.
|
|
8
|
Yoda Y and Abe T: Fibrinopeptide A (FPA)
level and fibrinogen kinetics in patients with malignant disease.
Thromb Haemost. 46:706–709. 1981.PubMed/NCBI
|
|
9
|
Green D and Karpatkin S: Role of thrombin
as a tumor growth factor. Cell Cycle. 9:656–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo RR, Liu Y, Lu WL, et al: A recombinant
peptide, hirudin, potentiates the inhibitory effects of stealthy
liposomal vinblastine on the growth and metastasis of melanoma.
Biol Pharm Bull. 31:696–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu L, Lee M, Campbell W, Perez-Soler R and
Karpatkin S: Role of endogenous thrombin in tumor implantation,
seeding, and spontaneous metastasis. Blood. 104:2746–2751. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caunt M, Huang YQ, Brooks PC and Karpatkin
S: Thrombin induces neoangiogenesis in the chick chorioallantoic
membrane. J Thromb Haemost. 1:2097–2102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Scanlon CS, Banerjee R, et al: The
histone methyl-transferase EZH2 mediates tumor progression on the
chick chorioallantoic membrane assay, a novel model of head and
neck squamous cell carcinoma. Transl Oncol. 6:273–281. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mackler NJ and Pienta KJ: Drug insight:
Use of docetaxel in prostate and urothelial cancers. Nat Clin Pract
Urol. 2:92–100. 2005. View Article : Google Scholar
|
|
15
|
Zhang H, Yang D, Wang H, et al:
Metastasis-associated gene 1 promotes invasion and migration
potential of laryngeal squamous cell carcinoma cells. Oncol Lett.
7:399–404. 2014.PubMed/NCBI
|
|
16
|
Ren J, Zhu D, Liu M, Sun Y and Tian L:
Downregulation of miR-21 modulates Ras expression to promote
apoptosis and suppress invasion of Laryngeal squamous cell
carcinoma. Eur J Cancer. 46:3409–3416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo S, Colbert LS, Fuller M, Zhang Y and
Gonzalez-Perez RR: Vascular endothelial growth factor receptor-2 in
breast cancer. Biochim Biophys Acta. 1806:108–121. 2010.PubMed/NCBI
|
|
19
|
Dias S, Choy M, Alitalo K and Rafii S:
Vascular endothelial growth factor (VEGF)-C signaling through FLT-4
(VEGFR-3) mediates leukemic cell proliferation, survival, and
resistance to chemotherapy. Blood. 99:2179–2184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dias S, Hattori K, Zhu Z, et al: Autocrine
stimulation of VEGFR-2 activates human leukemic cell growth and
migration. J Clin Invest. 106:511–521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tavora B, Batista S, Reynolds LE, et al:
Endothelial FAK is required for tumour angiogenesis. EMBO Mol Med.
2:516–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gabarra-Niecko V, Schaller MD and Dunty
JM: FAK regulates biological processes important for the
pathogenesis of cancer. Cancer Metastasis Rev. 22:359–374. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Even-Ram S, Uziely B, Cohen P, et al:
Thrombin receptor over-expression in malignant and physiological
invasion processes. Nat Med. 4:909–914. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi X, Gangadharan B, Brass LF, Ruf W and
Mueller BM: Protease-activated receptors (PAR1 and PAR2) contribute
to tumor cell motility and metastasis. Mol Cancer Res. 2:395–402.
2004.PubMed/NCBI
|
|
25
|
Kaufmann R, Schafberg H, Rudroff C and
Nowak G: Thrombin receptor activation results in calcium signaling
and protein kinase C-dependent stimulation of DNA synthesis in
HEp-2g laryngeal carcinoma cells. Cancer. 80:2068–2074. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Yang Y, Liang Z, et al:
Silybin-mediated inhibition of Notch signaling exerts antitumor
activity in human hepatocellular carcinoma cells. PLoS One.
8:e836992013. View Article : Google Scholar
|
|
27
|
Jaganathan R, Ravinayagam V, Panchanadham
S and Palanivelu S: Potential therapeutic role of Tridham in human
hepatocellular carcinoma cell line through induction of p53
independent apoptosis. BMC Complement Altern Med. 13:3232013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nguyen KC, Willmore WG and Tayabali AF:
Cadmium telluride quantum dots cause oxidative stress leading to
extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2
cells. Toxicology. 306:114–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thanapprapasr D, Hu W, Sood AK and Coleman
RL: Moving beyond VEGF for anti-angiogenesis strategies in
gynecologic cancer. Curr Pharm Des. 18:2713–2719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ludwig HC, Akhavan-Shigari R, Rausch S, et
al: Expression of focal adhesion kinase (p125 FAK) and proline-rich
tyrosine kinase 2 (PYK2/CAKb) in cerebral metastases, correlation
with VEGF-R-, ecNOS III-labelling and morphometric data. Anticancer
Res. 20:1419–1424. 2000.PubMed/NCBI
|