Introduction
Osteosarcoma is the most frequent primary malignant
bone tumor in young adolescents and children (1). Currently, patients with osteosarcoma
are evaluated and treated using multidisciplinary treatments
including surgical resection of the lesions, radiotherapy, adjuvant
chemotherapy and neoadjuvant chemotherapy (1). Combination of a variety of
chemotherapeutic drugs is usually recommended during the process of
chemotherapy. However, traditional chemotherapeutic drugs result in
greater renal, cardiac and auditory dysfunction toxicity, and
30–40% of patients with osteosarcoma still have a poor response
(2). Therefore, drug development is
important for the treatment of osteosarcoma.
Niclosamide, a type of salicylamide derivative, is
an anti-helminthic drug that has been used for approximately 50
years for tapeworm infections (3,4). In
the past few years, several groups have independently found that
niclosamide is a potential anticancer drug. In 2011, Sack et
al (5) screened 1,280 types of
pharmaceutically active compounds using high-throughput screening
technology and found niclosamide was able to inhibit colon cancer
metastasis. Recently, Wieland et al (6) found that niclosamide is cytotoxic,
inhibits cell migration and enhances the effects of
chemotherapeutic drugs in glioma cells. Another study (7) found that niclosamide inhibits the
growth and induces apoptosis of acute myeloid leukemia cells in
vitro and in nude mice, while cells from normal bone marrow
were spared.
Niclosamide has been confirmed as a potential
anticancer drug. Previous studies found that niclosamide exerted
inhibitory effects on tumors of epithelial origin. However, its
role in mesenchymal-derived tumors (e.g., osteosarcoma) remains to
be determined. The aim of this study was to investigate the role of
niclosamide in human osteosarcoma cells.
Materials and methods
Materials and chemicals
Niclosamide was purchased from Wuhan Shengtianyu
Biotech (Wuhan, China). It was dissolved in dimethyl sulfoxide
(DMSO) and stored at 4°C at the concentration of 2 mM. The cell
counting kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies (Kumamoto, Japan). Hoechst 33342 was obtained from
Bioyear Biotech (Wuhan, China). The Annexin V-FITC apoptotic
detection kit was purchased from BestBio Biotech (Shanghai, China).
Propidium iodide (PI)/RNase was purchased from Tianjin Sungene
Biotech Co., Ltd. (Tianjin, China). The primary antibodies against
bcl-2, bax, and pro-caspase-3 and the secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). All the reagents were stored at 4°C prior to use.
Cell culture
The human MG-63 and U2OS osteosarcoma cell lines
were obtained from the China Center for Type Culture Collection
(Wuhan, China). The cells were cultured in DMEM medium with high
glucose (HyClone, Logan, UT, USA) containing 10% fetal bovine serum
(Gibco, Carlsbad, CA, USA). The cells were incubated in a constant
environment at 37°C with 95% air and 5% CO2. Medium
renewal was performed every 2–3 days. The cells were subcultured
when 90% confluence was reached.
CCK-8 assay
Cells (5×103) were seeded in 96-well
plates and cultured overnight. Niclosamide (0, 0.1, 0.5, 1, 2, 3,
4, 5 and 10 μM) was added and incubated for 24 and 48 h,
respectively. The medium was replaced with 100 μl fresh medium and
10 μl CCK-8 solution. The cells were incubated for an additional 4
h and the absorbance was measured at 450 nm by an Enspire
microplate reader (PerkinElmer, Inc., Waltham, MA, USA). The cell
viability and IC50 values were calculated.
Hoechst 33342 staining
Cells (5×105) were transferred in 6-well
plates and cultured overnight. Niclosamide (0, 1, 2 and 5 μM) was
added and incubated for 24 h. The medium was replaced with 0.5 ml
Hoechst 33342. The plate was agitated for 10 min in the dark and
analyzed using a fluorescence microscope (Olympus BX51, Olympus,
Tokyo, Japan).
Cell cycle analysis
After various treatments, the cells were collected
and fixed with cold 70% ethanol at room temperature (RT) for 1 h.
The cells were then washed twice with PBS and stained with 0.5 ml
PI/RNase at RT for 30 min. The samples were analyzed by flow
cytometry (FC 500, Beckman Coulter, Brea, CA, USA; BD FACSAria III,
Becton Dickinson, Franklin Lakes, NJ, USA).
Annexin V-FITC/PI double staining
Following treatment with various concentrations of
niclosamide, the cells were collected and washed twice with PBS.
Annexin V binding buffer (400 μl) and Annexin V-FITC (5 μl) were
added. The cells were homogenized and incubated in the dark at 4°C
for 15 min. PI (10 μl) was added and incubated for 5 min. The
samples were analyzed by flow cytometry (FC 500, Beckman
Coulter).
After various treatments, the medium was removed.
Then, 400 μl Annexin V binding buffer, 5 μl Annexin V-FITC and 10
μl PI solution, respectively, were added. The cells were incubated
at RT in the dark for 30 min and analyzed using a fluorescence
microscope (Olympus BX51, Olympus).
Western blotting
Cells were similarly treated with various
concentrations of niclosamide for 24 h, lysed, collected and
centrifuged at 12,000 × g at 4°C for 30 min. The supernatants were
obtained and protein concentration was determined by BCA assay.
An equivalent amount of proteins was then separated
by SDS-polyacrylamide gel and transferred onto PVDF membranes. The
membranes were incubated with 5% skimmed milk, followed by
incubation with primary antibodies overnight at 4°C and secondary
antibody at RT for 2 h. The immunoblots were developed and
visualized with a Supersignal West Pico Assay kit (Thermo Fisher
Scientific, Inc., Rockford, IL, USA) and autoradiography film.
Statistical analysis
Data were presented as mean (M) ± standard deviation
(SD). The Student’s t-test was applied to analyze the differences
between each niclosamide concentration and the control. P<0.05
was considered significantly different.
Results
Niclosamide inhibits the proliferation of
MG-63 and U2OS
To determine the inhibitory effect of niclosamide on
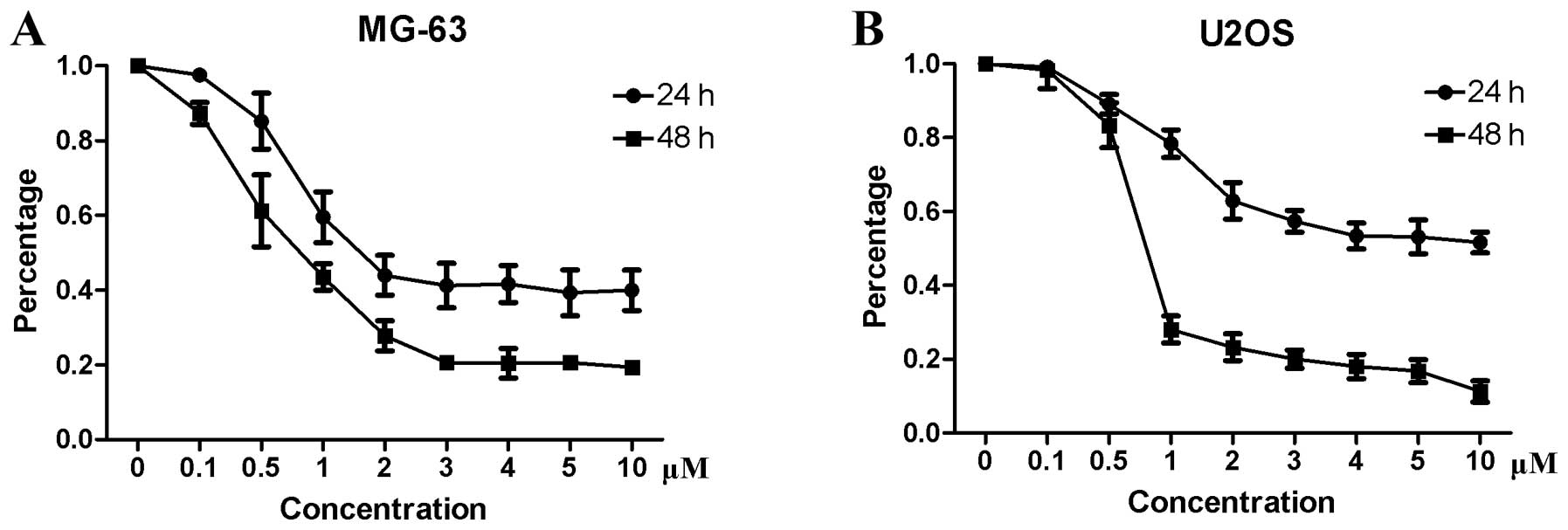
osteosarcoma cells, a CCK-8 assay was performed. The results showed
that niclosamide inhibited the proliferation of MG-63 (Fig. 1A) and U2OS (Fig. 1B) in a time- and dose-dependent
manner. The IC50 values for MG-63 and U2OS were 1.5 and
5 μM, respectively.
Niclosamide induces cell cycle arrest of
MG-63 and U2OS
Cell cycle analysis was performed with PI staining
by flow cytometry. After MG-63 cells were treated by niclosamide
for 24 h, the number of cells in the G1 phase decreased gradually,
while the number of cells increased in G2 and S phase. For U2OS
cells, the cell cycle was mainly arrested in G2 phase. The
dose-dependent effects were obvious, especially in U2OS cells
(Fig. 2).
Niclosamide induces apoptosis of MG-63
and U2OS
To determine whether niclosamide induces apoptosis
of MG-63 and U2OS cells, Hoechst staining, Annexin V FITC/PI
staining and western blotting were performed.
As shown in Fig. 3,
the cell nuclei were dyed blue, while the nuclei of apoptotic cells
were dyed bright blue or even white. The results showed that the
number of apoptotic cells increased gradually in a dose-dependent
manner. Niclosamide therefore induced the apoptosis of MG-63 and
U2OS cells.
Fig. 4 shows the
flow cytometry results of Annexin V-FITC/PI double staining.
Niclosamide induced both early and late apoptosis of MG63 and U2OS
cells, which was more evident in U2OS cells.
The two cell lines were observed with a fluorescence
microscope after double staining (Fig.
5). Under the light field, the appearance of cells was more
sparse in the treatment groups. When the cells were observed with
blue/green spectral filters, the number of apoptotic cells
significantly increased in a dose-dependent manner.
The expression of bcl-2, bax and pro-caspase-3 was
examined by western blotting (Fig.
6). The results showed that pro-caspase-3 decreased
significantly, which indicated the activation of apoptosis. The
expression of bcl-2 decreased and an increased expression of bax
was observed. The bax/bcl-2 ratio increased following treatment
with niclosamide, which further confirmed that niclosamide
inhibited cell growth by inducing apoptosis.
Discussion
Niclosamide, an antitapeworm drug, has been FDA
approved for almost 50 years (3,4). In
recent years, it has been suggested that niclosamide inhibited
cancer growth and metastasis in epithelium-derived tumors,
including prostate (8), lung
(9,10), head and neck (11), and ovarian cancer (12,13).
However, its effects on mesenchymal tumors (e.g., osteosarcoma) are
unclear. Thus, we performed this study to determine this issue. The
results of the present study demonstrate that niclosamide inhibits
proliferation, induces cell cycle arrest and apoptosis in human
osteosarcoma cells in a time- and dose-dependent manner.
In the development of new chemotherapeutic agents,
issues that need to be addressed concern evaluation of the efficacy
and safety of such drugs. Niclosamide, which has been approved by
FDA for 50 years (3,4), is safe for oral ingestion.
Furthermore, niclosamide induces apoptosis in acute myelocytic
leukemia cells but spares cells from normal bone marrow (7). Thus, niclosamide is generally regarded
as safe and efficient in many tumors, including colon cancer
(5,14), breast cancer (3,4), and
prostate cancer (8). In the present
study, we evaluated the efficacy of niclosamide on human cancer
cells in vitro. The results show that it is a potential
anticancer drug for osteosarcoma.
Previous studies revealed that niclosamide induces
growth inhibition and cell apoptosis by cell cycle arrest (15,16).
However, the cell cycle arrest of various cancer cells by
niclosamide is not phase-specific. Niclosamide induces different
cell cycle distribution in different cancer cells. For example, the
flow cytometric analysis showed that niclosamide induced S-phase
arrest in human erythroleukemia K562 cells (17). However, the cell population in G1
phase transiently increased following treatment with niclosamide in
primary human glioblastoma cells (6). In Du145 prostate cancer cells,
G0/G1-phase arrest has been observed (18). In this study, cell cycle analysis
was performed in two different osteosarcoma cell lines, MG-63 and
U2OS. The cell cycle distribution was found to be different from
the results of previous studies. Niclosamide induces G2/S arrest in
MG-63 cells, but G2 phase arrest in U2OS cells. Thus, niclosamide
is a cancer cell-specific cell-cycle inhibitor.
Bcl-2, an antiapoptotic protein, inhibits a number
of programs in cell apoptosis (19). Bax is identified as a
bcl-2-interacting protein that inhibits the activity of bcl-2 and
promotes apoptotic cell death (20). An increased bcl-2/bax ratio is
usually detected when apoptotic death occurs (21). In the present study, we found that
niclosamide induces apoptosis of MG-63 and U2OS cells by decreasing
the bcl-2/bax ratio, a finding that was consistent with a previous
study (4). Caspase-3 plays a key
role in the process of apoptosis (22,23).
It is associated with morphological changes of apoptosis and
apoptotic body formation (22).
When apoptosis occurs, caspase-3 is activated and cleaved into two
subunits (23). The decreased
expression of procaspase-3 in our study further confirmed that
niclosamide induces apoptosis in human osteosarcoma cells.
To the best of our knowledge, this is a pilot study
and some issues deserve mentioned. First, apoptosis is induced by
niclosamide but the major mechanism is not clear. It is well known
that niclosamide inhibits oxidative phosphorylation in the
mitochondria of cestodes. Niclosamide affects cancer cells in
several different pathways, including Wnt/β-catenin (24,25),
mTORC1 signaling (26,27), STAT 3 (10), and ROS (28). Further studies are required to
determine the exact mechanism in osteosarcoma cells. Second, it has
been found that niclosamide induces apoptosis in cancer cells but
spares normal cells (7). The reason
for this difference in apoptosis induction between the normal cells
and cancer cells remains to be identified through a more
comprehensive investigation of the apoptotic mechanism.
Furthermore, the present study was performed in vitro but
not in vivo. A limitation of this is that certain chemicals
show excellent anticancer effects in vitro but not in
vivo. Animal studies and clinical studies are therefore needed
before niclosamide is proven as an effective anticancer drug.
In conclusion, the present study has demonstrated
that niclosamide inhibits proliferation, induces cell cycle arrest
and apoptosis of human osteosarcoma cells. Niclosamide may be
developed into a novel and potential chemotherapeutic drug for
osteosarcoma.
Acknowledgements
This study was supported by Hubei Province’s
Outstanding Medical Academic Leader Program.
References
|
1
|
Biermann JS, Adkins DR, Agulnik M, et al:
Bone cancer. J Natl Compr Canc Netw. 11:688–723. 2013.PubMed/NCBI
|
|
2
|
Hogendoorn PC; ESMO/EUROBONET Working
Group. Athanasou N, Bielack S, et al: Bone sarcomas: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21(Suppl 5): v204–v213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Londono-Joshi AI, Arend RC, Aristizabal L,
et al: Effect of niclosamide on basal-like breast cancers. Mol
Cancer Ther. 13:800–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye T, Xiong Y, Yan Y, et al: The
anthelmintic drug niclosamide induces apoptosis, impairs metastasis
and reduces immunosuppressive cells in breast cancer model. PLoS
One. 9:e858872014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sack U, Walther W, Scudiero D, et al:
Novel effect of anti-helminthic Niclosamide on S100A4-mediated
metastatic progression in colon cancer. J Natl Cancer Inst.
103:1018–1036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wieland A, Trageser D, Gogolok S, et al:
Anticancer effects of niclosamide in human glioblastoma. Clin
Cancer Res. 19:4124–4136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin Y, Lu Z, Ding K, et al: Antineoplastic
mechanisms of niclosamide in acute myelogenous leukemia stem cells:
inactivation of the NF-kappaB pathway and generation of reactive
oxygen species. Cancer Res. 70:2516–2527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Lou W, Zhu Y, et al: Niclosamide
inhibits androgen receptor variants expression and overcomes
enzalutamide resistance in castration-resistant prostate cancer.
Clin Cancer Res. 20:3198–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Hu Z, Sun SY, et al: Niclosamide
overcomes acquired resistance to erlotinib through suppression of
STAT3 in non-small cell lung cancer. Mol Cancer Ther. 12:2200–2212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You S, Li R, Park D, et al: Disruption of
STAT3 by niclosamide reverses radioresistance of human lung cancer.
Mol Cancer Ther. 13:606–616. 2014. View Article : Google Scholar :
|
|
11
|
Li R, You S, Hu Z, et al: Inhibition of
STAT3 by niclosamide synergizes with erlotinib against head and
neck cancer. PLoS One. 8:e746702013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yo YT, Lin YW, Wang YC, et al: Growth
inhibition of ovarian tumor-initiating cells by niclosamide. Mol
Cancer Ther. 11:1703–1712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arend RC, Londoño-Joshi AI, Samant RS, et
al: Inhibition of Wnt/β-catenin pathway by niclosamide: A
therapeutic target for ovarian cancer. Gynecol Oncol. 34:112–120.
2014. View Article : Google Scholar
|
|
14
|
Chen W, Chen M and Barak LS: Development
of small molecules targeting the Wnt pathway for the treatment of
colon cancer: a high-throughput screening approach. Am J Physiol
Gastrointest Liver Physiol. 299:G293–G300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Li PK, Roberts MJ, et al:
Multi-targeted therapy of cancer by niclosamide: A new application
for an old drug. Cancer Lett. 349:8–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan JX, Ding K and Wang CY: Niclosamide,
an old antihelminthic agent, demonstrates antitumor activity by
blocking multiple signaling pathways of cancer stem cells. Chin J
Cancer. 31:178–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang AM, Ku HH, Liang YC, et al: The
autonomous notch signal pathway is activated by baicalin and
baicalein but is suppressed by niclosamide in K562 cells. J Cell
Biochem. 106:682–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren X, Duan L, He Q, et al: Identification
of niclosamide as a new small-molecule inhibitor of the STAT3
signaling pathway. ACS Med Chem Lett. 1:454–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Korsmeyer SJ, Shutter JR, Veis DJ, et al:
Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and
cell death. Semin Cancer Biol. 4:327–332. 1993.PubMed/NCBI
|
|
22
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.PubMed/NCBI
|
|
24
|
Lu W, Lin C, Roberts MJ, et al:
Niclosamide suppresses cancer cell growth by inducing Wnt
co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin
pathway. PLoS One. 6:e292902011. View Article : Google Scholar
|
|
25
|
Tomizawa M, Shinozaki F, Motoyoshi Y, et
al: Niclosamide suppresses hepatoma cell proliferation via the Wnt
pathway. Onco Targets Ther. 6:1685–1693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fonseca BD, Diering GH, Bidinosti MA, et
al: Structure-activity analysis of niclosamide reveals potential
role for cytoplasmic pH in control of mammalian target of rapamycin
complex 1 (mTORC1) signaling. J Biol Chem. 287:17530–17545. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balgi AD, Fonseca BD, Donohue E, et al:
Screen for chemical modulators of autophagy reveals novel
therapeutic inhibitors of mTORC1 signaling. PLoS One. 4:e71242009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SL, Son AR, Ahn J, et al: Niclosamide
enhances ROS-mediated cell death through c-Jun activation. Biomed
Pharmacother. 68:619–624. 2014. View Article : Google Scholar : PubMed/NCBI
|