Introduction
Breast cancer, the most common malignancy and the
second leading cause of cancer in women, is a heterogeneous disease
with a variety of pathological entities (1). Approximately 232,340 new cases of
invasive breast cancer were diagnosed in the USA in 2013, and
almost 39,620 women will succumbed to this disease (2). The mechanism underlying tumor
initiation and progression of this disease remain unclear, but
partly due to deregulation of microenvironment homeostasis. The
‘seed and soil’ hypothesis indicates that an appropriate
microenvironment (the soil) is crucial for the optimal growth of
tumor cells (the seeds) (3).
Protein tyrosine phosphatase receptor type O
(PTPRO), a type III member of the receptor-type PTP family, was
initially reported to be expressed in human renal glomerulus
(4). PTPRO is a transmembrane
protein, and the PTP domain located in its intracellular region
catalyzes the dephosphorylation of tyrosine peptides. Tyrosine
phosphorylation plays a critical role in cellular processes such as
cell proliferation, differentiation and apoptosis (5). PTPRO has attracted attention as an
important tumor suppressor in multiple cancers. Overexpression of
PTPRO was reported to induce the apoptosis of the terminally
differentiated leukemic cell line U937 (6). It was also demonstrated that PTPRO
acts as a tumor suppressor in human lung cancer cell line A549
(7). Furthermore, the promoter
region of the PTPRO encoding gene is frequently methylated in human
breast cancer as well as in other cancers, and this was found to be
correlated with downregulation of PTPRO expression (7,8).
However, to the best of our knowledge, most previous
studies have focused on PTPRO expression in cancer cells while
little attention has been given to the role of PTPRO expression in
the tumor niche. In order to investigate the involvement of PTPRO
in the breast cancer niche, we employed an orthotopic mouse mammary
gland tumor model. As PTPRO was eliminated in the breast cancer
niche, a significant increase in tumor size and the number of
metastases were observed. These tumor-suppressive effects in the
cancer niche were correlated with a reduction in tumor
angiogenesis, circulating tumor cells (CTCs) and the induction of
tumor apoptosis. Therefore, our results describe here for the first
time, that PTPRO expression in the tumor niche represents a
potential therapeutic strategy for breast cancer.
Materials and methods
Cell culture
Py8119 cells were isolated from spontaneously
arising tumors in MMTV-PyMT C57Bl/6 female mice by serial
trypsinization and limiting dilution (9). Cells were cultured in F12K culture
medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 5%
fetal bovine serum (FBS; Wisent, Nanjing, China) and MITO serum
extender (BD Biosciences, Franklin Lakes, NJ, USA) and maintained
in a humidified incubator at 37°C with CO2.
Animal experiments
The ptpro+/− C57Bl/6 mice were kindly
provided by Dr Bixby from the University of Miami. The PTPRO gene
type was determined as described previously (10). Seven-week-old female C57Bl/6 mice
and ptpro−/− C57Bl/6 mice (10 mice in each group) were
used for the in vivo animal experiments. The animals were
maintained under specific pathogen-free conditions in the animal
housing facility of Nanjing Medical University. All animal
protocols were approved and monitored by the Institutional Animal
Care and Use Committee of Nanjing Medical University.
Orthotopic mammary tumor model
Py8119 cells tagged with luciferase-GFP
(luciferase-GFP plasmid; kindly provided by Dr Brian Rabinovich, MD
Anderson Cancer Center, Houston, TX, USA) were implanted
orthotopically into the inguinal mammary fat-pad area
(106 cells/side/mouse). The tumor size was measured with
a caliper in 2 dimensions. Tumor volume was calculated with the
equation: V = (L × W2) × 0.5, where L is the length and
W is the width of the tumor. Six weeks after inoculation of the
breast cancer cells, all mice were sacrificed and observed grossly
by the Whole Body Imaging System (Illumatool 9900; Lightools
Research, Encinitas, CA, USA). Lungs were removed during autopsy
and GFP-expressing metastatic colonies were observed under a
fluorescence microscope (Nikon, Melville, NY, USA).
Histology
Tumors and associated mammary glands were removed
from the wild-type and ptpro+/− C57Bl/6 mice, fixed in
10% neutral buffered formalin and embedded in paraffin.
Paraffin-embedded sections were stained with hematoxylin and eosin.
For immunohistochemistry (IHC) staining, the specimens were
deparaffinized, and rehydrated through xylene and graded alcohols.
Sections mounted on slides were subjected to antigen retrieval by a
pressure cooker, and then incubated with the antibodies specific
for mouse CD31 and CD34 (both from Abcam, Cambridge, MA, USA).
Immunocomplexes were visualized by the DAB method. All histologic
analyses were examined by one pathologist. Image-Pro software
(Media Cybernetics Inc., Rockville, MD, USA) was used to calculate
the vessel density (vessels/mm2). TUNEL staining was
performed as described previously (11). Briefly, DNA fragmentation associated
with apoptosis in the tumor cells was detected in situ by
the addition of digoxigenin-labeled nucleotides to label the free
3′-end of DNA fragments using the Apoptosis Detection kit according
to the manufacturer’s instructions (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China).
Circulating tumor cell detection
Six weeks after inoculation of the breast cancer
cells in the mammary flat pads, blood was collected by cardiac
puncture from the mice. All the procedures were performed under
deep anesthesia with isofluorane. Eight hundred microliters of the
blood sample was added to 25 ml 1X red blood cell lysis buffer
(BioLegend, San Diego, CA, USA). After gently vortexing, the
samples were incubated at room temperature for 15 min. Samples were
washed with PBS, resuspended in 500 μl PBS for flow cytometric
analysis. Flow cytometric analysis was performed by using a BD
FACSCalibur analyzer, and results were analyzed with CellQuest Pro
software (both from BD Biosciences, San Jose, CA, USA).
Experimental in vivo metastasis
study
An intracardiac injection model was used for the
present study. Briefly, luciferase tagged py8119 cells were
harvested and injected into the left cardiac ventricle of
anesthetized female mice. Each mouse was injected with
2×105 cells in 0.1 ml of phosphate-buffered saline.
Development of metastasis induced by py8119 cells was monitored at
regular intervals by whole mouse fluorescence and bioluminescence
imaging using the Xenogen IVIS-Spectrum System (Perkin-Elmer,
Waltham, MA, USA).
Statistical analysis
All statistical analysis was carried out using
GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA) and
SPSS 16.0 software (IBM, Armonk, NY, USA). P-values were calculated
by the Student’s t-test and the Fisher’s exact test. Data sets with
P≤0.05 were considered statistically significant.
Results
PTPRO expression in the tumor niche is
essential for the inhibition of tumor growth and metastasis
We first determined the effect of PTPRO expression
in the tumor niche on the regulation of tumorigenesis.
GFP-expressing Py8119 mouse breast cancer cells were implanted
orthotopically into female wild-type or ptpro−/− C57Bl/6
mice. After 2 weeks of inoculation, the incidence of tumor
formation in both groups was 100%. No significant differences in
the frequency of tumor formation were observed.
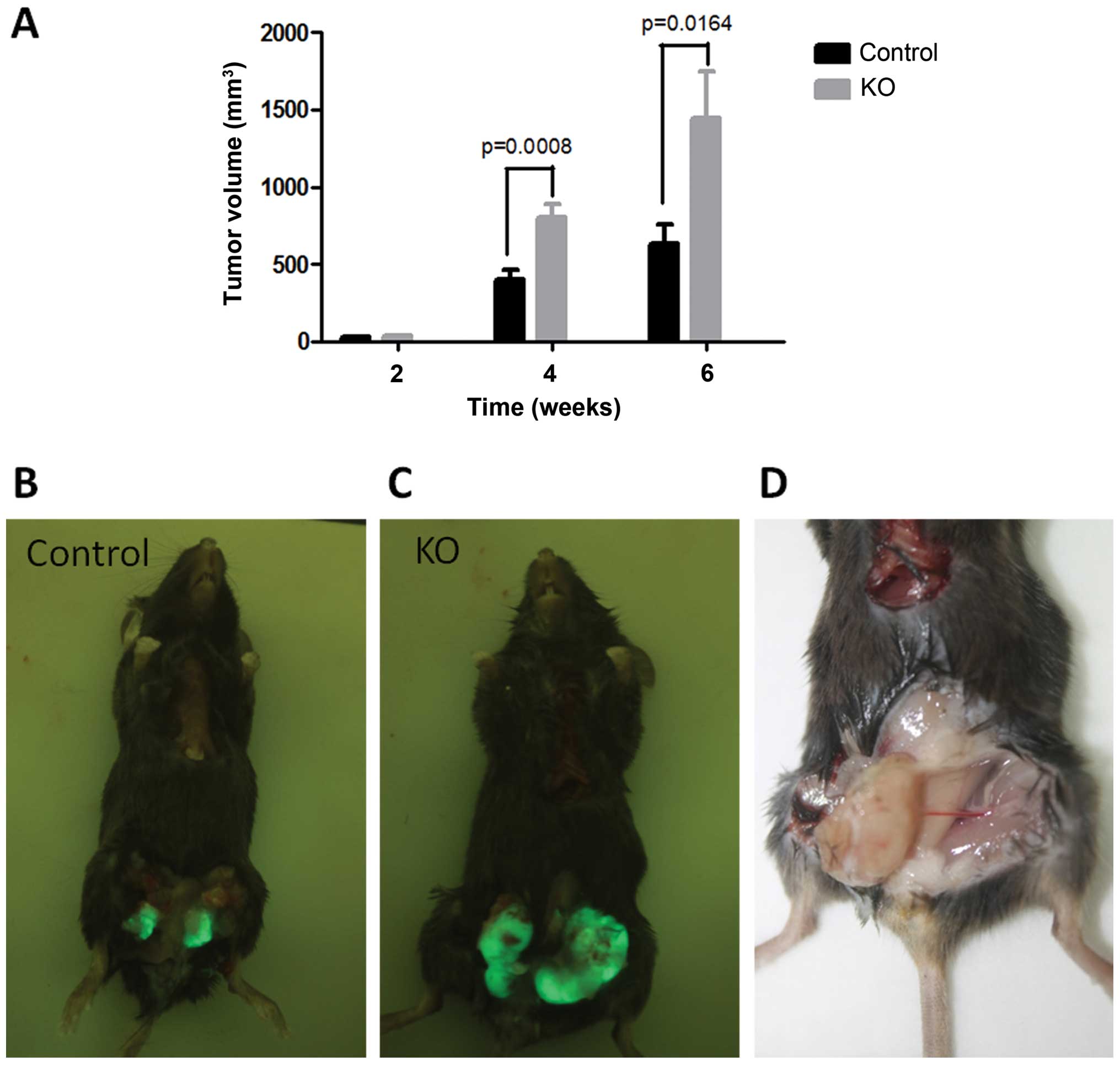
We next investigated whether PTPRO expression in the
niche could influence tumor growth and metastasis. Tumor size at
the orthotopic site was measured by a vernier caliper at week 2, 4,
and 6 after inoculation. The tumor growth rate in the 2 groups was
initially similar, and the mean tumor volume of the 2 groups became
significantly different after 4 weeks of inoculation (Fig. 1). Significantly larger tumors were
observed in the ptpro−/− mice when compared with the
wild-type mice, which indicate that PTPRO expression in the niche
may act as a tumor suppressor. After the termination of the
experiment, the numbers of peritoneum metastases (Fig. 2A) and bone metastases (Fig. 2B) were counted using the Whole Body
Imaging System, and the metastatic tumors on the surface of the
lung were observed under a fluorescence microscope (Fig. 2B). In the ptpro−/− group,
metastasis formed in most of the mice (6/10), while in the
wild-type group only one mouse was detected with metastasis (1/11)
(Table I).
 | Table IMetastases observed in mice. |
Table I
Metastases observed in mice.
| TM | PM | LM | BM |
|---|
| WT | 1/11 | 1/11 | 0/11 | 0/11 |
| KO | 6/10a | 2/10 | 3/10 | 1/10 |
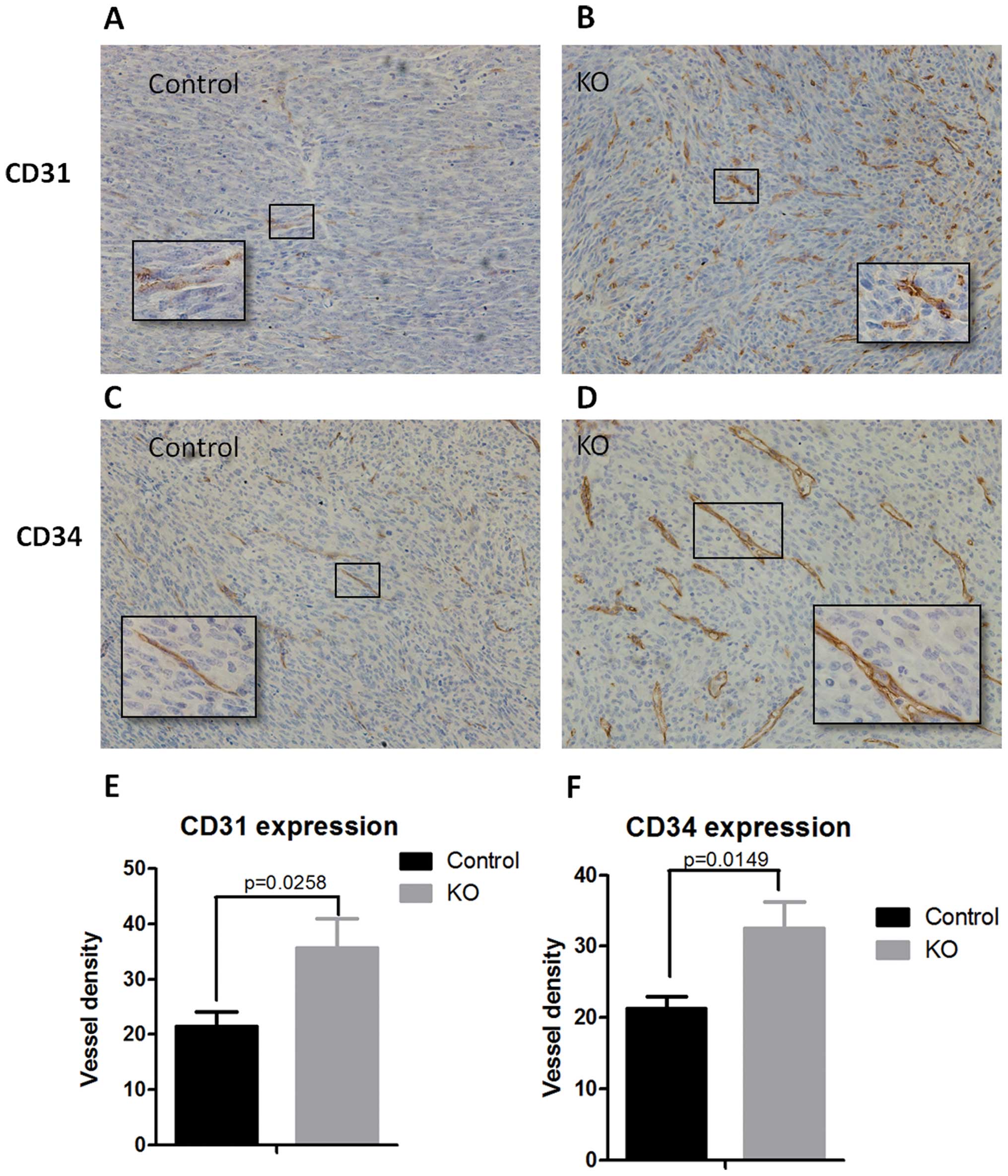
Increased vessel density in the
ptpro−/− niche
Angiogenesis, regarded as a hallmark of cancer
progression, is a multi-step process which consists of the
development of new blood vessels from pre-existing ones and is
essential for tumor growth and metastatic spread of tumor cells
(12). Our results showed that
PTPRO expression in the niche was essential for the inhibition of
tumor growth and metastasis. Therefore, we aimed to determine the
microvessel density in the tumors of wild-type and
ptpro−/− mice. Immunohistochemical staining was
performed with the CD31 (Fig. 3A and
B) and CD34 (Fig. 3C and D)
antibodies. Notably, the quantification of IHC data indicated that
the microvessel density was significantly higher in the tumor
tissues of the ptpro−/− group when compared with that in
the wild-type group (Fig. 3E and
F).
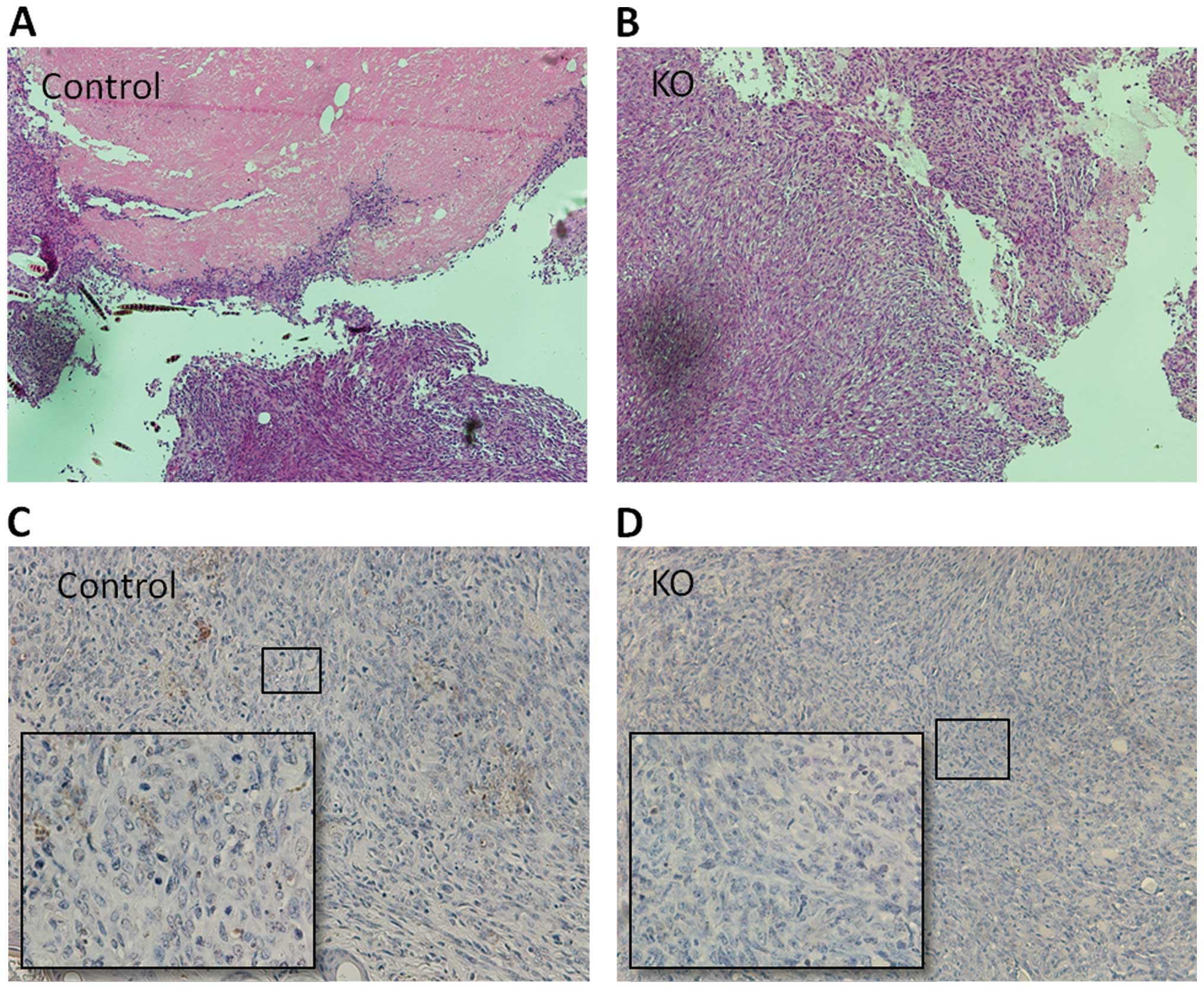
PTPRO expression in the tumor niche is
necessary for induction of apoptosis and necrosis
To further investigate the role of PTPRO as a tumor
inhibitor in the tumor niche, we also performed TUNEL assay to
determine the apoptosis induced by PTPRO expression in the tumor
niche. In wild-type mice, moderate numbers of apoptotic cells were
clearly observed in the tumor tissues (Fig. 4C). In contrast, few apoptotic cells
were noted in the tumor tissues of the ptpro−/− mice
(Fig. 4D). Importantly, the
incidence of necrotic nodules was also higher in the tumor tissues
of the wild-type mice (3/10) than that in the tissues
ptpro−/− mice (0/10) (Fig.
4A and B).
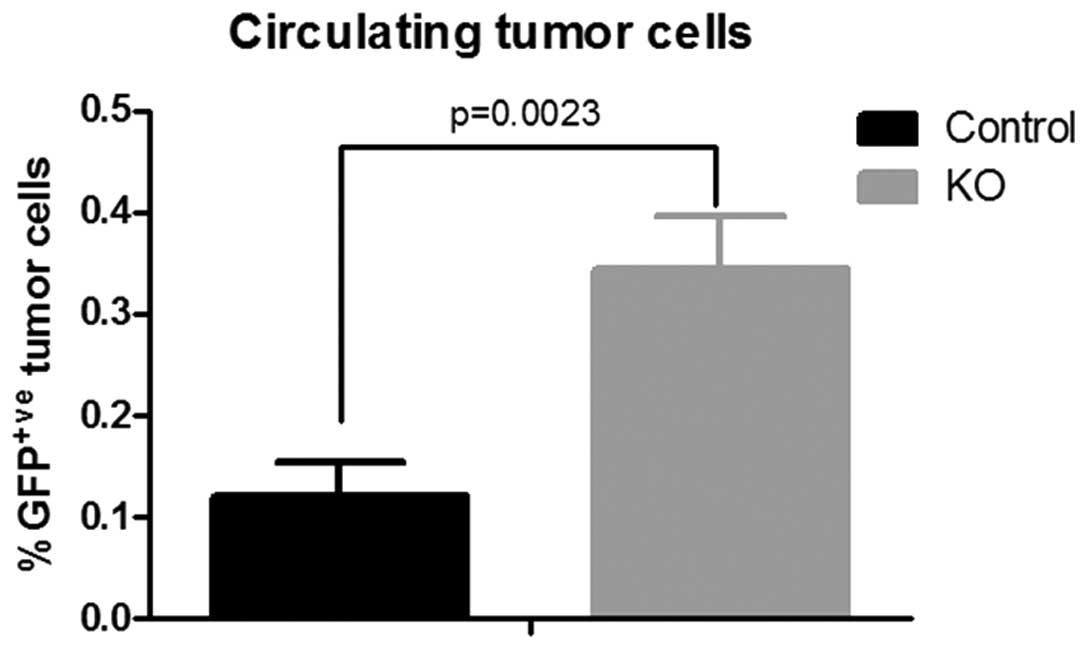
Escape of tumor cells from the primary
tumor is facilitated by the ptpro−/− niche
Emerging evidence indicates that tumors are composed
of tumor parenchyma and stroma, two discrete but interactive parts
that cross-talk to regulate tumor growth and metastasis (13). Paracrine signaling between tumor
cells and the tumor niche may play a critical role in the formation
of metastasis. The fact that less metastasis was observed in the
wild-type mice encouraged us to investigate the number of CTCs in
the orthotopic tumor model. GFP-labeled Py8119 cells were
inoculated into the mammary fat pad of the wild-type and
ptpro−/− mice, and peripheral blood was then collected
for calculation of CTCs by flow cytometry after 6 weeks. A
significantly higher percentage of GFP+ cells (CTCs) was
observed in the blood of the ptpro−/− mice (Fig. 5). The results indicate that PTPRO
expression in the tumor niche may prevent the escape of tumor cells
from the primary tumor.
PTPRO expression in the premetastatic
niche prevents the formation of metastasis
The ‘seed and soil’ hypothesis postulates that an
appropriate host microenvironment is essential for the formation of
metastasis (3). Therefore, we
recruited the intracardiac injection model to investigate the
direct effect of PTPRO expression in the pre-metastatic niche on
metastasis formation. The luciferase-tagged Py8119 cells were
inoculated intracardiacally into female wild-type or
ptpro−/− C57Bl/6 mice. The metastatic tumor growth was
monitored at regular intervals by fluorescence and bioluminescence
imaging. Notably, 4 weeks after the intracardiac injection, the
signals of total flux in the ptpro−/− group were
significantly higher than that in the control group (Fig. 6), which indicated that more
metastases formed in the mice of the ptpro−/− group. Our
results showed that PTPRO expression may play an important role in
the prevention of metastasis formation in the pre-metastatic
niche.
Discussion
PTPRO, also known as glomerular epithelial protein 1
(GLEPP1), was initially identified in research for
podocyte-specific proteins that may regulate glomerular structure
and function (4). Subsequent
studies indicated that PTPRO may act as a tumor suppressor in
leukemia (8), hepatocellular
carcinoma (14), lung cancer
(7) as well as breast cancer
(15,16). In a recent study, we also
demonstrated that PTPRO in cancer cells could inhibit cell
proliferation and enhance apoptosis by downregulation of signal
transducers and activators of transcription 3 (STAT3) (10). In the past two decades, most
investigators have focused primarily on the PTPRO expression in
tumor cells. However, emerging evidence indicates that the tumor
niche and microenvironment are important components which could
cross-talk with tumors and modulate tumor growth (17,18).
Metastases are believed to be the major cause of
death in breast cancer patients. It was reported that between 25
and 50% of patients diagnosed with breast cancer eventually develop
deadly metastases (19). The
formation of metastases requires the following key steps: tumor
cell growth, angiogenesis, escape from the primary tumor, entrance
into blood vessels, survival in vessels, and adaption to the
pre-metastatic niche.
In the present study, we investigated the effect of
PTPRO expression in the tumor niche on tumor growth, angiogenesis,
CTCs and metastasis of breast cancer. Our results indicated that
PTPRO expression in the tumor niche is essential for the inhibition
of tumor growth. Significantly larger tumors and a higher
metastasis frequency were observed in the ptpro−/− mice
when compared with the wild-type mice. We also found that the
tumors harvested from the control mice showed more apoptosis and
necrosis than that of the ptpro−/− group.
Angiogenesis promotes tumor growth and metastasis,
and various PTPs have been implicated to regulate angiogenesis
(20,21). Thus, we investigated the vascularity
in the tumors of the ptpro−/− and control groups. CD31
(PECAM-1) and CD34 expression levels were recruited as markers for
angiogenesis. Consistent with the tumor growth rate and the
frequency of metastasis, we demonstrated that the microvessel
density was significantly higher in the tumor tissues of the
ptpro−/− group when compared with that in the wild-type
group. Therefore, we conclude that the increased tumor volume and
frequency of metastasis in the ptpro−/− group may be
partly due to the loss of PTPRO signaling which could inhibit
angiogenesis in the tumor microenvironment.
CTCs, rare malignant cells found in the peripheral
blood, are useful as indicators of prognosis both initially and
after therapy (22). Furthermore,
the enumeration of CTCs could be a surrogate for the status of
tumor cells in the circulating system. Our observation of increased
numbers of CTCs in the ptpro−/− mice provides further
evidence that PTPRO expression in the tumor niche inhibits tumor
metastasis by preventing tumor cells from entering blood vessels.
On the other hand, the formation of metastases is a highly
inefficient process (23). From
model systems, it has been estimated that ~1×106 tumor
cells per gram of tumor tissue can be introduced daily into the
blood stream (24). The fate of
CTCs is mainly determined by the acquirement of resistant to
anoikis and the adaption to the new microenvironment of distant
organs. To further explore the role of PTPRO expression in the
pre-metastatic niche, we inoculated the same number of Py8119 cells
intracardially into female wild-type or ptpro−/− C57Bl/6
mice. Notably, more metastases were observed in the mice of the
ptpro−/− group. These observations appear to indicate
that PTPRO expression may play an important role in the prevention
of metastasis formation in the pre-metastatic niche.
Our study had limitations. The tumor niche is
composed of vascular endothelial cells, adipocytes, fibroblasts,
platelets, mast cells and macrophages. We are not able to rule out
the function of PTPRO in any particular cell type. Further studies
are required to elucidate the role of PTPRO in different types of
cells in the tumor niche.
In summary, our studies showed that PTPRO expression
in the tumor niche acts as a tumor suppressor and inhibits the
growth and metastasis of breast cancer cells. The mechanisms
underlying the suppressive effect of PTPRO may be due to the
induction of apoptosis and the suppression of angiogenesis.
Acknowledgements
We are grateful to Dr Lesley Ellies (University of
California, San Diego) for providing us with the Py8119 cell line.
The present study was supported in part by The National Natural
Science Foundation of China (nos. 81270952, 81070684, 81071753,
81172502, 81202077 and 81272916), The Natural Science Foundation of
Jiangsu Province (BK2010581, BK2011853, BK2011855 and BK20141023),
the Program for Development of Innovative Research Team in The
First Affiliated Hospital of NJMU (IRT-008), a project funded by
the Priority Academic Program Development of Jiangsu Higher
Education Institutions (PAPD) and Jiangsu Province Innovation
Project for Graduate Student (CXZZ13-0585).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
4
|
Thomas PE, Wharram BL, Goyal M, Wiggins
JE, Holzman LB and Wiggins RC: GLEPP1, a renal glomerular
epithelial cell (podocyte) membrane protein-tyrosine phosphatase.
Identification, molecular cloning, and characterization in rabbit.
J Biol Chem. 269:19953–19962. 1994.PubMed/NCBI
|
|
5
|
Jiang R, Xia Y, Li J, et al: High
expression levels of IKKalpha and IKKbeta are necessary for the
malignant properties of liver cancer. Int J Cancer. 126:1263–1274.
2010.
|
|
6
|
Seimiya H and Tsuruo T: Functional
involvement of PTP-U2L in apoptosis subsequent to terminal
differentiation of monoblastoid leukemia cells. J Biol Chem.
273:21187–21193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motiwala T, Kutay H, Ghoshal K, et al:
Protein tyrosine phosphatase receptor-type O (PTPRO) exhibits
characteristics of a candidate tumor suppressor in human lung
cancer. Proc Natl Acad Sci USA. 101:13844–13849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motiwala T, Majumder S, Kutay H, et al:
Methylation and silencing of protein tyrosine phosphatase receptor
type O in chronic lymphocytic leukemia. Clin Cancer Res.
13:3174–3181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibby K, You WK, Kadoya K, et al: Early
vascular deficits are correlated with delayed mammary tumorigenesis
in the MMTV-PyMT transgenic mouse following genetic ablation of the
NG2 proteoglycan. Breast Cancer Res. 14:R672012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou J, Xu J, Jiang R, et al:
Estrogen-sensitive PTPRO expression represses hepatocellular
carcinoma progression by control of STAT3. Hepatology. 57:678–688.
2013. View Article : Google Scholar
|
|
11
|
Liu Z, Bandyopadhyay A, Nichols RW, et al:
Blockade of autocrine TGF-β signaling inhibits stem cell phenotype,
survival, and metastasis of murine breast cancer cells. J Stem Cell
Res Ther. 2:1–8. 2012. View Article : Google Scholar
|
|
12
|
Carvalho MI, Guimaraes MJ, Pires I, et al:
EGFR and microvessel density in canine malignant mammary tumours.
Res Vet Sci. 95:1094–1099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao Y, Keller ET, Garfield DH, Shen K and
Wang J: Stromal cells in tumor microenvironment and breast cancer.
Cancer Metastasis Rev. 32:303–315. 2013. View Article : Google Scholar
|
|
14
|
Motiwala T, Ghoshal K, Das A, et al:
Suppression of the protein tyrosine phosphatase receptor type O
gene (PTPRO) by methylation in hepatocellular carcinomas. Oncogene.
22:6319–6331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YT, Li FF, Ke C, et al: PTPRO
promoter methylation is predictive of poorer outcome for
HER2-positive breast cancer: indication for personalized therapy. J
Transl Med. 11:2452013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li SY, Li R, Chen YL, et al: Aberrant
PTPRO methylation in tumor tissues as a potential biomarker that
predicts clinical outcomes in breast cancer patients. BMC Genet.
15:672014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Derycke L, Van MV, Depypere H and Bracke
M: Molecular targets of growth, differentiation, tissue integrity,
and ectopic cell death in cancer cells. Cancer Biother Radiopharm.
20:579–588. 2005. View Article : Google Scholar
|
|
18
|
Kopfstein L and Christofori G: Metastasis:
cell-autonomous mechanisms versus contributions by the tumor
microenvironment. Cell Mol Life Sci. 63:449–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorusso G and Ruegg C: New insights into
the mechanisms of organ-specific breast cancer metastasis. Semin
Cancer Biol. 22:226–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai J, Chen Z, Ruan Q, et al: γ-secretase
and presenilin mediate cleavage and phosphorylation of vascular
endothelial growth factor receptor-1. J Biol Chem. 286:42514–42523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen JX, Tuo Q, Liao DF and Zeng H:
Inhibition of protein tyrosine phosphatase improves angiogenesis
via enhancing Ang-1/Tie-2 signaling in diabetes. Exp Diabetes Res.
2012:8367592012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alemar J and Schuur ER: Progress in using
circulating tumor cell information to improve metastatic breast
cancer therapy. J Oncol. 2013:7027322013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paterlini-Brechot P and Benali NL:
Circulating tumor cell (CTC) detection: clinical impact and future
directions. Cancer Lett. 253:180–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang YS, di Tomaso E, McDonald DM, Jones
R, Jain RK and Munn LL: Mosaic blood vessels in tumors: frequency
of cancer cells in contact with flowing blood. Proc Natl Acad Sci
USA. 97:14608–14613. 2000. View Article : Google Scholar : PubMed/NCBI
|