Introduction
Glycogen synthase kinase-3β (GSK-3β) is a
multifunctional serine/threonine protein kinase that functions as a
transducer in the Wnt/β-catenin, Notch, Hedgehog and other
signaling pathways to regulate multiple physiological processes,
including glycogen metabolism, cell proliferation, differentiation
and apoptosis (1,2). GSK-3β is one of the essential cell
signal transducers and is recognized for its diverse functions in
cell survival (3).
Although its functions in normal cell physiology
have been thoroughly investigated, the role of GSK-3β in cancer is
still unclear and is the subject of much debate (4,5). In
solid tumors, Thiel et al (6) found that treating gastric cancer cells
with RNAi targeting GSK-3β or GSK-3β inhibitors induced an increase
in cox-2 and promoted the progression of gastric cancer. However,
Tsuchiya et al (7) showed
that GSK-3β functions as a tumor suppressor in colon cancer by
promoting the degradation of β-catenin. In a previous study of
leukemia by our group (8), we
demonstrated that GSK-3β is unusually highly expressed in the
cytoplasm and nuclei of leukemic cells in children with acute
lymphoblastic leukemia (ALL), suggesting that GSK-3β may play a
role as an oncogene in ALL. There is a great deal of interest in
finding effective drugs directed against GSK-3β for the treatment
of various types of cancers (9).
Tetramethylpyrazine (TMP)
(2,3,5,6-tetramethylpyrazine;
C8H12N2) is one of the bioactive
ingredients extracted from the rhizome of the Chinese herb
Ligusticum. Studies have demonstrated that TMP has potent
inhibitory effects on a variety of tumors through affecting the
proliferation and migration of tumor cells (10–12).
In our previous study, TMP was found to inhibit the growth of HL-60
cells by inducing their differentiation (13). There have been no studies concerning
the effect of TMP on ALL cells.
In the present study, we treated Jurkat and SUP-B15
ALL cell lines with TMP and evaluated the effects of TMP on
proliferation, apoptosis and the cell cycle. We also analyzed the
expression of GSK-3β and its downstream transcription factors and
apoptosis-related molecules in ALL cells in hopes of providing new
insight into the anti-leukemia mechanism of TMP.
Materials and methods
Chemicals and reagents
Both the human acute T lymphoblastic leukemia Jurkat
and Ph+ ALL SUP-B15 cells were obtained from the
American Type Culture Collection (ATCC; USA), and preserved in our
laboratory. RPMI-1640 and fetal bovine serum (FBS) were obtained
from Gibco and HyClone, USA, respectively. A nuclear and total
protein extraction kit and Annexin V-FITC/PI apoptosis detection
kit were purchased from KeyGen Biotechnology, China. Western
blotting detection reagents and Cell Counting Kit-8 (CCK-8) were
obtained from Beyotime Institute of Biotechnology (Jiangsu, China).
TMP was purchased in 4 mg/2 ml injection ampules from Rongsheng
Pharmaceutical Co., Ltd., China. TRIzol reagent was supplied by
Life Technologies, USA. Real-time polymerase chain reaction (PCR)
primers were synthesized by Huada Biotechnology Co. Ltd., Shanghai,
China, and its mixture was obtained from Takara, Japan. Antibodies
against GSK-3β, NF-κB, c-myc, bcl-2, p27KIP1, β-actin
and lamin B1 were purchased from Abcam Co., USA. Antibody against
survivin was purchased from GeneTex Co., USA.
Cell culture
Both Jurkat and SUP-B15 cells were cultured in
RPMI-1640 supplemented with 10% heat-inactivated FBS, 100
μg/ml streptomycin, 100 U/ml penicillin and 1 mM
L-glutamine. They were maintained at 37°C in a humidified
atmosphere of 5% CO2.
Cell proliferation assay
Cell proliferation was detected with the CCK-8 assay
following the manufacturer’s instructions. Both Jurkat and SUP-B15
cells were seeded at a density of 1×104 cells/well in
96-well microtiter plates and cultured for 24, 48 and 72 h with
different concentrations of TMP (20, 40, 80, 160 and 320
μg/ml). Control cells were not treated with TMP. At each
indicated time point, the cells were combined with 10 μl
CCK-8 kit solution and incubated for an additional 3 h. Absorbance
was measured at 450 nm (A value). Three replicate wells were used
for each analysis. The cell growth inhibition rate (IR) (IR = 1 - A
of treated wells/A of control wells) and the half maximal
inhibitory concentration (IC50) value were
calculated.
Cell apoptosis by Annexin V/PI assay
The cell apoptosis rate was measured by flow
cytometry (FACSCalibur; BD Biosciences, USA) with Annexin V/PI
staining asssay. After being synchronized, Jurkat cells were
incubated with TMP (0, 60, 120 and 180 μg/ml) for 48 h,
while SUP-B15 cells were incubated with TMP (0, 100, 200 and 300
μg/ml) for 48 h. Cells (>1×106) were collected
and washed twice with cold 0.01 M phosphate-buffered saline (PBS).
According to the manufacturer’s instructions, cells were
resuspended in binding buffer and stained with Annexin V-FITC (5
μl) and PI (5 μl), respectively, and then analyzed by
flow cytometry. Early apoptosis was defined as being Annexin
V-positive and PI-negative, while late apoptosis and necrosis were
defined as being both Annexin V-positive and PI-positive.
Cell cycle assay
The flow cytometric analysis was employed to
determine the cell cycle distribution of the TMP treated Jurkat and
SUP-B15 cells. Briefly, ~1×106 cells were collected.
After fixation with 70% cold ethanol at −20°C overnight, the cells
were washed twice with PBS, and labeled with 50 mg/ml PI containing
100 μg/ml DNase-free RNase A. The cells were cultured for 30
min at 37°C in the dark and analyzed for DNA content by flow
cytometry.
Preparation of RNA extraction and
quantitative real-time PCR analysis
The expression of GSK-3β, c-myc, cox-2, survivin,
bcl-2 and p27 mRNA in the TMP-treated Jurkat and SUP-B15 cells was
analyzed by quantitative real-time PCR analysis. After the
co-culture of cells with TMP at different concentrations for 48 h,
total RNAs were isolated using TRIzol reagent according to the
manufacturer’s protocol. Reverse transcription was used to
synthesize complementary DNAs. The primer sequences and annealing
temperature used for PCR are listed in Table I. cDNA (0.8 μl) was added to
10 μl of the PCR mixture containing 3.4 μl of
H2O, 0.4 μl each of 5′ and 3′ primers (10
μmol•l−1), and 5 μl of 2X SYBR Premix Ex
Taq II.
 | Table IPrimers and annealing
temperatures. |
Table I
Primers and annealing
temperatures.
| Primer | Sequences | Size (bp) | Annealing
temperature (°C) |
|---|
| p27 |
5′-CGTGTCCTCAGAGTTAGCCG-3′
5′-GGCAAGTACGAGTGGCAAGA-3′ | 171 | 58 |
| Cox-2 |
5′-CCTGCCCTTCTGGTAGAAA-3′
5′-GGACAGCCCTTCACGTTATT-3′ | 215 | 58 |
| Survivin |
5′-AGGACCACCGCATCTCTACAT-3′
5′-AAGTCTGGCTCGTTCTCAGTG-3′ | 118 | 64 |
| GSK-3β |
5′-GGCTACCATCCTTATTCCTCCT-3′
5′-GTCCACGGTCTCCAGTATTAGC-3′ | 101 | 64 |
| Bcl-2 |
5′-CTGCACCTGACGCCCTTCACC-3′
5′-CACATGACCCCACCGAACTCAAAGA-3′ | 119 | 61 |
| β-actin |
5′-AAGATGACCCAGATCATGTTTGAGACC-3′
5′-GCCAGGTCCAGACGCAGGAT-3′ | 191 | 56 |
The reaction conditions were as follows: 39 cycles
for 30 sec at 95°C, 5 sec at 95°C, 30 sec at annealing temperature,
and 1 min at 65°C. The results were normalized against β-actin and
appeared as a target mRNA:β-actin ratio.
Western blot analysis
Western blot analysis was conducted for GSK-3β,
c-myc, cox-2, survivin, bcl-2, NF-κB and p27 expression. Jurkat and
SUP-B15 cells of the treated and control group were lysed in lysis
buffer containing protease inhibitor. According to the nuclear and
total protein extraction kit manufacturer’s protocol, the following
procedures were followed. Nuclear and total protein samples (100
μg) were separated on 10% polyacrylamide resolving gels and
5% stacking gels and then transferred at 100 V and 250 mA for 90
min. The membranes were incubated with primary antibodies overnight
at 4°C. Loading controls of total and nuclear protein samples were
confirmed with β-actin and lamin B1 antibodies, respectively.
Statistical analysis
The results are presented as the mean ± SEM of three
independent experiments. Treated groups were compared with the
control group by one-way analysis of variance (ANOVA). p<0.05
was considered to indicate a statistically significant result.
Results
Effect of TMP on the proliferation of
Jurkat and SUP-B15 cells
To investigate the antiproliferative effect of TMP
on ALL cells, Jurkat and SUP-B15 cells were exposed to different
concentrations of TMP for 24, 48 and 72 h. As shown in Fig. 1, TMP notably inhibited the growth of
both cell lines in a dose- and time-dependent manner, although
Jurkat cells were more sensitive to TMP. The IC50 values
of TMP in the Jurkat and SUP-B15 cells at 48 h were 120 and 200
μg/ml, respectively. Concentrations of 60, 120 and 180
μg/ml of TMP were used to treat the Jurkat cells, and
concentrations of 100, 200 and 300 μg/ml were used to treat
the SUP-B15 cells in subsequent experiments. The results suggested
that TMP is an antitumor agent for human ALL cells.
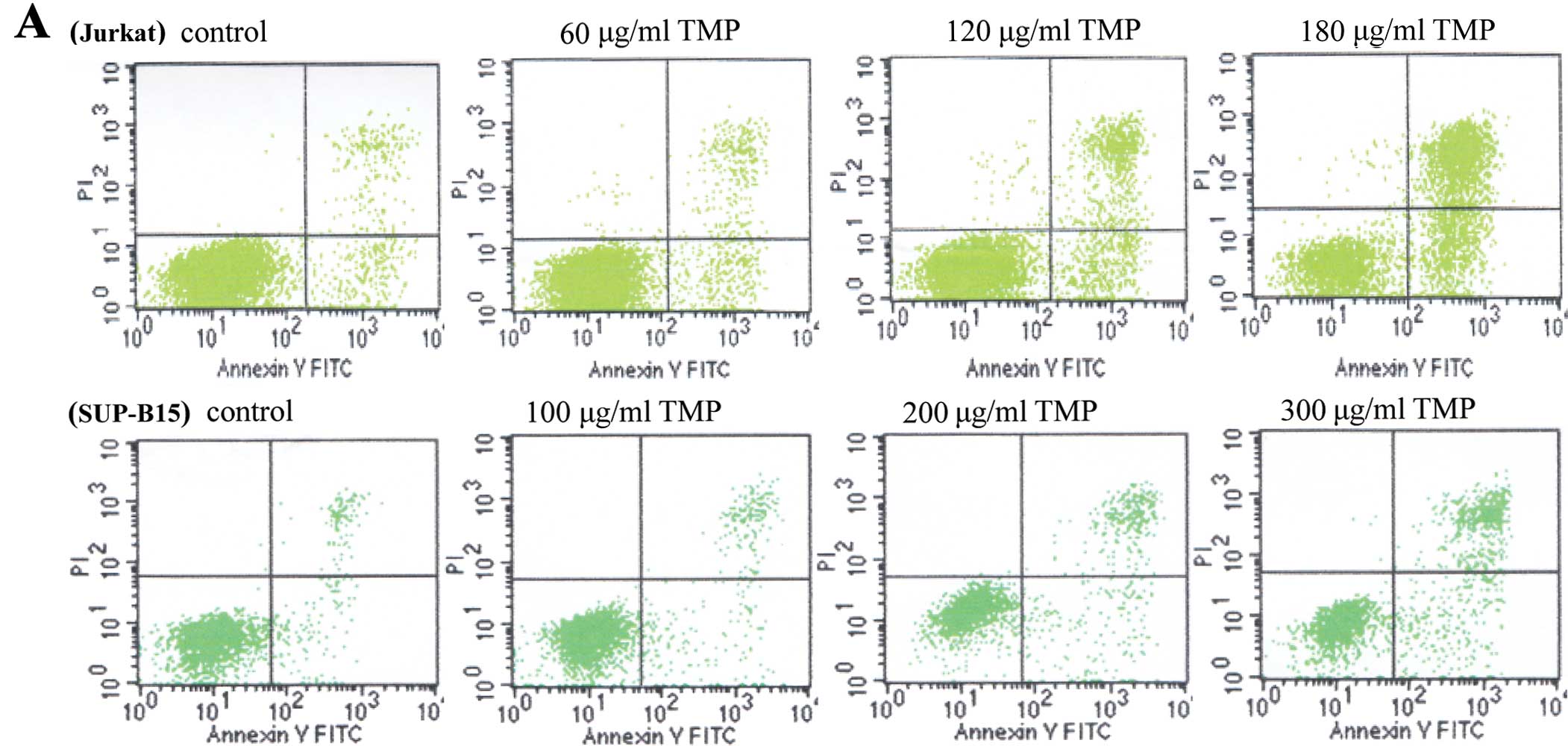
TMP induces apoptosis in Jurkat and
SUP-B15 cells
To confirm whether TMP exerts its inhibitory effect
through inducing apoptosis, Jurkat and SUP-B15 cells were incubated
with concentration gradients of TMP from 20 to 320 μg/ml for
48 h and apoptosis was detected by the Annexin V/PI double staining
assay using flow cytometry. In Fig.
2, the apoptotic rates of Jurkat cells in the control, 60, 120
and 180 μg/ml TMP groups were 3.05±0.05, 4.03±0.2, 6.15±0.3
and 16.64±0.65%, respectively, whereas the rates of SUP-B15 cells
in the control, 100, 200 and 300 μg/ml TMP groups were
2.47±0.15, 4.15±0.3, 8.29±0.29 and 11.2±0.3%, respectively. These
results indicate that TMP induced apoptosis in the Jurkat and
SUP-B15 cells in a dose-dependent manner.
TMP induces G0/G1 cell cycle arrest in
the Jurkat and SUP-B15 cells
Based on the evidence of growth inhibition of Jurkat
and SUP-B15 cells following TMP treatment, we examined cell cycle
distribution using flow cytometry. The results showed that TMP
treatment increased the proportion of cells in the G0/G1 phase,
whereas it decreased the proportion of cells in the S and G2/M
phase (Table II).
 | Table IITMP causes abnormal cell cycle
distribution in the Jurkat and SUP-B15 cells. |
Table II
TMP causes abnormal cell cycle
distribution in the Jurkat and SUP-B15 cells.
| A, Jurkat
cells |
|---|
|
|---|
| TMP
(μg/ml) | Cell cycle
distribution (%)
|
|---|
| G0/G1 | S | G2/M |
|---|
| 0 | 47.07±3.35 | 38.88±1.58 | 14.05±1.65 |
| 60 | 54.72±0.92a | 32.11±1.9 | 13.17±2.20 |
| 120 | 60.05±4.02a | 30.58±5.13a | 9.37±3.45 |
| 180 | 70.83±1.09a | 26.55±0.92a | 2.62±0.45a |
|
| B, SUP-B15
cells |
|
| TMP
(μg/ml) | Cell cycle
distribution (%)
|
| G0/G1 | S | G2/M |
|
| 0 | 25.87±1.09 | 67.03±0.92 | 7.1±1.65 |
| 100 | 34.05±4.02a | 59.12±5.13a | 6.83±2.20 |
| 200 | 46.84±2.22a | 47.72±1.9a | 5.44±3.45 |
| 300 | 52.47±1.03a | 43.4±1.58a | 413±0.45 |
GSK-3β expression in the TMP-treated
Jurkat and SUP-B15 cells
To determine whether GSK-3β participates in the
apoptotic induction by TMP, we evaluated the expression of GSK-3β
with quantitative real-time PCR and western blot analysis. As shown
in Fig. 3, GSK-3β expression
decreased significantly both at the protein and RNA levels compared
with the control group in the Jurkat and SUP-B15 cells treated with
TMP. These results imply a potential inhibitory effect of TMP on
GSK-3β expression in ALL.
TMP decreases the expression of GSK-3β
downstream of NF-κB and c-myc in the Jurkat and SUP-B15 cells
GSK-3β has been identified to play a vital role in
the NFκB- and c-myc-mediated survival of cancer cells. Since NF-κB
and c-myc are downstream transcription factors of GSK-3β, we
examined the effect of TMP on the levels of NF-κB and c-myc in the
Jurkat and SUP-B15 cells. The results showed that the expression of
NF-κB and c-myc in the Jurkat and SUP-B15 cells was decreased in
both the whole-cell and nuclear lysates (Fig. 4). These findings suggest that TMP
may exert its effect on ALL cell apoptosis by downregulating the
transcriptional activity of NF-κB and c-myc through GSK-3β
regulation.
Effect of TMP on the expression of
survivin, bcl-2, cox-2 and p27 in the Jurkat and SUP-B15 cells
Bcl-2, cox-2 and survivin are downstream molecules
of NF-κB or c-myc that are relevant for apoptosis, and we evaluated
the effect of TMP on their expression levels in the Jurkat and
SUP-B15 cells (Fig. 5). The results
revealed that bcl-2 and survivin protein expression was decreased
significantly in both cell lines after exposure to TMP compared
with the control groups, whereas changes in the mRNA levels were
not significant. Consistent with the PCR results, the cox-2 protein
level was decreased significantly in both the Jurkat and SUP-B15
cells. Considering the fact that TMP resulted in the arrest of
Jurkat and SUP-B15 cells in the G0/G1 phase of the cell cycle and
p27/Kip1 regulates and blocks cell cycle progression
through the G1-S transition, real-time RT-PCR and western blotting
were conducted to examine the p27 expression in the ALL cells
(Fig. 5). The results showed that
TMP upregulated the expression of p27 in Jurkat and SUP-B15 cells,
suggesting a potential mechanism through which TMP affects the cell
cycle.
Discussion
ALL is a heterogeneous group of malignant clonal
diseases that originate from pluripotent hematopoietic stem cells.
Although improved therapeutic strategies have achieved long-term
survival rates of more than 80% in children, the survival rate is
less than 40% in adults (14).
Patients for whom induction chemotherapy fails require intensive
chemotherapy and may suffer from severe side-effects of the
treatments. Therefore, novel agents are urgently required. The
pathogenesis of ALL is the result of multiple factors and genes
working in concert. In particular, aberrant cell signal
transduction plays a vital role in its occurrence and development.
As noted above, GSK-3β acts as an oncogene in ALL and plays an
important role in ALL tumorigenesis and progression. In the present
study, we adopted GSK-3β as a promising drug target for new,
effective and low-toxicity anticancer drugs (15). The small-molecule inhibitors of
GSK-3β are mainly divided into two categories: ATP-competitive and
non-ATP competitive agents. The former category includes bis-indole
and pyrazine, for example, and lithium is a representative of the
latter group (16,17). TMP, which is a pyrazine, has been
widely used in the clinic for the treatment of cardiovascular and
neurovascular diseases (18,19),
and it has an excellent safety profile. Recently, beyond its
traditional function, TMP has been found to have antitumor effects
(20). In the present study, we
showed a novel effect of TMP on human Jurkat and SUP-B15 ALL cell
lines, i.e., targeting the GSK-3β pathway. Our research found that
TMP inhibited the proliferation of Jurkat and SUP-B15 cells in a
dose- and time-dependent manner by inducing apoptosis and arresting
the cell cycle at the G0/G1 phase. Thus, we report that TMP may be
an effective anti-ALL agent. Studies have demonstrated that therapy
targeting GSK-3β inhibits the proliferation of several cancer cell
lines (21,22). Our experiments showed that GSK-3β
expression was significantly decreased in the Jurkat and SUP-B15
cells following treatment with TMP. Therefore, we showed for the
first time that TMP exerts antitumor effects on ALL cells by
inhibiting GSK-3β signaling.
The substrates of GSK-3β are mainly glycogen
synthase and transcription factors such as elf2, HSF-1, c-jun,
c-myc and c-myb, among others (23). Here, we found that TMP decreased the
total and nuclear expression of transcription factors NF-κB and
c-myc in the Jurkat and SUP-B15 cells. NF-κB is a pleiotropic
transcription factor that regulates the transcription of its
downstream target genes that are critical for the regulation of
tumorigenesis, apoptosis and a wide variety of cellular functions
(24). In normal cells, NF-κB is
sequestered in the cytoplasm in its inactive form through binding
with the inhibitor proteins of the IκB family (25). However, NF-κB was found to be
persistently activated in leukemic cells and regulated the
anti-apoptotic mechanism (26).
Kotliarova et al (27) used
GSK-3β inhibitors and RNAi technology to reduce the activity of
GSK-3β, leading to a decrease in NF-κB activity, which caused the
apoptosis of glioma cells. Ougolkov et al (28) found that the inhibition of GSK-3β
activity can silence the expression of the downstream
anti-apoptotic target genes of NF-κB in chronic lymphocytic
leukemia (CLL) B cells, thereby promoting the apoptosis of CLL B
cells. All previous studies suggest that NF-κB is affected by
GSK-3β inactivity.
c-myc, which belongs to the myc family of
transcription factors, is a regulator gene that codes for a
nucleoprotein that binds to nuclear DNA. By promoting or inhibiting
transcription of target genes, c-myc participates in the start of
intracellular signal transduction and in the expression of a wide
variety of genes (29). C-myc
regulates target gene expression by forming a dimer with MAX
proteins. Studies have shown that c-myc is constitutively highly
expressed in acute leukemia (30).
Dysfunction of c-myc led to the unregulated expression of many
genes, some of which are involved in cell proliferation and
apoptosis.
Further experiments in the present study showed that
the expression levels of cox-2, bcl-2 and survivin, the downstream
target genes of NF-κB and c-myc, were decreased.
Our previous results showing that the expression of
GSK-3β was suppressed by TMP as well as other related literature
suggest that GSK-3β may be a potential target for TMP in ALL cells.
In our experiments, TMP caused both Jurkat and SUP-B15 cells to be
arrested in the G0/G1 phase, which may be associated with an
increase in the expression of p27KIP1.
p27KIP1 protein plays a pivotal role in the regulation
of the cell cycle as an inhibitor of cyclin-dependent kinases
(CDKs) through inhibiting the checkpoint kinase CDK2/cyclin E1
complex and blocking cell cycle transition from the G0/G1 to the S
phase (31,32). The inhibition of GSK-3β has been
reported to induce cell cycle arrest at the G1 phase via the
activation of p27KIP1 (8), which is consistent with our
results.
In summary, our experimental results showed that TMP
can induce apoptosis in ALL cells by downregulating GSK-3β and TMP
plays a role as an antitumor agent. TMP, which is an approved
treatment option for vascular diseases, exhibits broad potential
for the treatment of leukemia, and our results provide a
theoretical basis for its clinical application.
Abbreviations:
|
GSK-3β
|
glycogen synthase kinase-3β
|
|
TMP
|
tetramethylpyrazine
|
|
ALL
|
acute lymphoblastic leukemia
|
References
|
1
|
Doble BW and Woodgett JR: GSK-3: Tricks of
the trade for a multi-tasking kinase. J Cell Sci. 116:1175–1186.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jope RS and Johnson GV: The glamour and
gloom of glycogen synthase kinase-3. Trends Biochem Sci. 29:95–102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grimes CA and Jope RS: The multifaceted
roles of glycogen synthase kinase 3β in cellular signaling. Prog
Neurobiol. 65:391–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beurel E and Jope RS: The paradoxical pro-
and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic
apoptosis signaling pathways. Prog Neurobiol. 79:173–189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel S and Woodgett J: Glycogen synthase
kinase-3 and cancer: Good cop, bad cop? Cancer Cell. 14:351–353.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiel A, Heinonen M, Rintahaka J,
Hallikainen T, Hemmes A, Dixon DA, Haglund C and Ristimäki A:
Expression of cyclo-oxygenase-2 is regulated by glycogen synthase
kinase-3beta in gastric cancer cells. J Biol Chem. 281:4564–4569.
2006. View Article : Google Scholar
|
|
7
|
Tsuchiya K, Nakamura T, Okamoto R, Kanai T
and Watanabe M: Reciprocal targeting of Hath1 and beta-catenin by
Wnt glycogen synthase kinase 3beta in human colon cancer.
Gastroenterology. 132:208–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Y, Gu X, Li R, Luo Q and Xu Y: Glycogen
synthase kinase-3β inhibition induces nuclear factor-κB-mediated
apoptosis in pediatric acute lymphocyte leukemia cells. J Exp Clin
Cancer Res. 29:1542010. View Article : Google Scholar
|
|
9
|
Wang Z, Smith KS, Murphy M, Piloto O,
Somervaille TC and Cleary ML: Glycogen synthase kinase 3 in MLL
leukaemia maintenance and targeted therapy. Nature. 455:1205–1209.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH
and Zhou SY: Inhibition of cyclooxygenase-2 by tetramethylpyrazine
and its effects on A549 cell invasion and metastasis. Int J Oncol.
40:2029–2037. 2012.PubMed/NCBI
|
|
11
|
Chen L, Lu Y, Wu JM, et al: Ligustrazine
inhibits B16F10 melanoma metastasis and suppresses angiogenesis
induced by vascular endothelial growth factor. Biochem Biophys Res
Commun. 386:374–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi B, Liu D, He M, Li Q, Liu T and Shao J:
Role of the ROS/AMPK signaling pathway in
tetramethylpyrazine-induced apoptosis in gastric cancer cells.
Oncol Lett. 6:583–589. 2013.PubMed/NCBI
|
|
13
|
Wu Y, Xu Y, Shen Y, Wang C, Guo G and Hu
T: Tetramethylpyrazine potentiates arsenic trioxide activity
against HL-60 cell lines. Braz J Med Biol Res. 45:187–196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Apostolidou E, Swords R, Alvarado Y and
Giles FJ: Treatment of acute lymphoblastic leukaemia: A new era.
Drugs. 67:2153–2171. 2007. View Article : Google Scholar
|
|
15
|
Naito S, Bilim V, Yuuki K, Ugolkov A,
Motoyama T, Nagaoka A, Kato T and Tomita Y: Glycogen synthase
kinase-3beta: A prognostic marker and a potential therapeutic
target in human bladder cancer. Clin Cancer Res. 16:5124–5132.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen P and Goedert M: GSK3 inhibitors:
Development and therapeutic potential. Nat Rev Drug Discov.
3:479–487. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alonso M and Martinez A: GSK-3 inhibitors:
Discoveries and developments. Curr Med Chem. 11:755–763. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li WM, Liu HTHT, Li XY, Wu JY, Xu G, Teng
YZ, Ding ST and Yu C: The effect of tetramethylpyrazine on hydrogen
peroxide-induced oxidative damage in human umbilical vein
endothelial cells. Basic Clin Pharmacol Toxicol. 106:45–52.
2010.
|
|
19
|
Deng L, Guo X, Zhai L, Song Y, Chen H,
Zhan P, Wu J and Liu X: Ligustrazine derivatives. Part 4: Design,
synthesis, and biological evaluation of novel ligustrazine-based
stilbene derivatives as potential cardiovascular agents. Chem Biol
Drug Des. 79:731–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin J, Yu C, Yang Z, He JL, Chen WJ, Liu
HZ, Li WM, Liu HT and Wang YX: Tetramethylpyrazine inhibits
migration of SKOV3 human ovarian carcinoma cells and decreases the
expression of interleukin-8 via the ERK1/2, p38 and AP-1 signaling
pathways. Oncol Rep. 26:671–679. 2011.PubMed/NCBI
|
|
21
|
Kawazoe H, Bilim VN, Ugolkov AV, Yuuki K,
Naito S, Nagaoka A, Kato T and Tomita Y: GSK-3 inhibition in vitro
and in vivo enhances antitumor effect of sorafenib in renal cell
carcinoma (RCC). Biochem Biophys Res Commun. 423:490–495. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thamilselvan V, Menon M and Thamilselvan
S: Anticancer efficacy of deguelin in human prostate cancer cells
targeting glycogen synthase kinase-3 β/β-catenin pathway. Int J
Cancer. 129:2916–2927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McCubrey JA, Steelman LS, Bertrand FE, et
al: GSK-3 as potential target for therapeutic intervention in
cancer. Oncotarget. 5:2881–2911. 2014.PubMed/NCBI
|
|
24
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weston VJ, Austen B, Wei W, Marston E,
Alvi A, Lawson S, Darbyshire PJ, Griffiths M, Hill F, Mann JR, Moss
PA, Taylor AM and Stankovic T: Apoptotic resistance to ionizing
radiation in pediatric B-precursor acute lymphoblastic leukemia
frequently involves increased NF-kappaB survival pathway signaling.
Blood. 104:1465–1473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotliarova S, Pastorino S, Kovell LC, et
al: Glycogen synthase kinase-3 inhibition induces glioma cell death
through c-MYC, nuclear factor-kappaB, and glucose regulation.
Cancer Res. 68:6643–6651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ougolkov AV, Bone ND, Fernandez-Zapico ME,
Kay NE and Billadeau DD: Inhibition of glycogen synthase kinase-3
activity leads to epigenetic silencing of nuclear factor kappaB
target genes and induction of apoptosis in chronic lymphocytic
leukemia B cells. Blood. 110:735–742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hatakeyama S, Watanabe M, Fujii Y and
Nakayama KI: Targeted destruction of c-Myc by an engineered
ubiquitin ligase suppresses cell transformation and tumor
formation. Cancer Res. 65:7874–7879. 2005.PubMed/NCBI
|
|
30
|
Tomita N: BCL2 and MYC dual-hit
lymphoma/leukemia. J Clin Exp Hematop. 51:7–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tapia JC, Bolanos-Garcia VM, Sayed M,
Allende CC and Allende JE: Cell cycle regulatory protein
p27KIP1 is a substrate and interacts with the protein
kinase CK2. J Cell Biochem. 91:865–879. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Porter LA, Kong-Beltran M and Donoghue DJ:
Spy1 interacts with p27Kip1 to allow G1/S
progression. Mol Biol Cell. 14:3664–3674. 2003. View Article : Google Scholar : PubMed/NCBI
|