Introduction
In addition to surgery and chemotherapy,
radiotherapy is commonly used to treat a wide variety of cancers,
although the therapeutic response is known to be varied in each
cancer (1,2). Precise evaluation of the efficacy of
therapy including radiotherapy requires non-invasive diagnostic
methods to evaluate whole tumor regions (2). Positron emission tomography (PET) can
non-invasively provide functional information of interest,
including data on proliferation and metabolism of tumors, thereby
providing information on temporal pathophysiological changes in
tumors after therapy (2). PET with
2-deoxy-2-[18F] fluoro-D-glucose ([18F]FDG)
can evaluate therapeutic efficacy before morphologic change as
determined by computed tomography (CT) and magnetic resonance
imaging (MRI) and it has been increasingly recognized to be useful
for therapeutic response evaluation (3,4). Since
increased glucose metabolism is not specific to cancer cells,
[18F]FDG highly accumulates in inflammatory and
granulomatous lesions (5,6). When patients have accompanying
unexpected/subclinical inflammation, [18F]FDG PET
sometimes gives false-positive results. Since radiotherapy often
causes inflammatory reactions in irradiated areas (1,7), high
[18F]FDG uptake has been reported to be observed in
irradiated areas (2,7). It could be difficult to distinguish
inflammation from a metabolically active residual tumor, and it is
generally necessary to wait several months after the end of
radiotherapy to minimize the influence of radiation-induced
inflammation before examination of radiotherapy efficacy.
Therefore, the introduction of tracers that are less affected by
inflammation than [18F]FDG is required to complement the
limitation of [18F]FDG PET.
Amino acids are generally required nutrients for
proliferating tumor cells and their pooling increases by
upregulation of their transporters in many tumor types, while
inflammatory cells have a low protein metabolism compared with
glucose metabolism (2,8). Amino acid PET tracers are known to
accumulate less in inflammatory lesions than [18F]FDG
(8). However, one of the most
widely used amino acid PET tracers,
[methyl-11C]methionine, is metabolized in cells,
resulting in numerous radiolabeled metabolites, which could
decrease tumor specificity and complicate the interpretation of PET
data (8). Therefore, several
radiolabeled non-natural amino acids that are resistant to in
vivo metabolism are expected to be more useful for the
above-mentioned purpose (8). We
recently demonstrated that 2-amino-[3-11C]isobutyric
acid ([3-11C] AIB), a PET tracer based on a non-natural
amino acid, is highly accumulated in tumors, but less in
inflammatory lesions compared with [18F]FDG in a mouse
model (9). [3-11C]AIB
has the potential to complement the limitation of
[18F]FDG and more precisely evaluate the efficacy of
radiotherapy in patients with unexpected/subclinical inflammation.
To date, there is no study evaluating the tumor uptake change of
[3-11C]AIB after irradiation in either patients or
animal models. In the present study, the early change of
[3-11C] AIB uptake in tumors by effective radiotherapy
was evaluated. To clarify the contributing factor in the change in
[3-11C]AIB uptake, serial quantitative PET with
[3-11C]AIB was conducted to quantify tumor uptake; then
tumor uptake was compared with the changes in tumor volume,
histological features and the expression of amino acid transporters
in a mouse model bearing a subcutaneous tumor before and early
after X-ray irradiation.
Materials and methods
Tumor model, X-ray irradiation and
PET
A human small cell lung cancer line SY
(Immuno-Biological Laboratories, Takasaki, Japan) was maintained in
RPMI-1640 (Sigma, St. Louis, MO, USA) containing 5% fetal bovine
serum (Sigma) in a humidified incubator maintained at 37°C with 5%
CO2. The animal experimental protocol was approved by
the Animal Care and Use Committee of the National Institute of
Radiological Sciences, and all the animal experiments were
conducted in accordance with the institutional guidelines regarding
animal care and handling. Male nude mice (BALB/c-nu/nu, 6-weeks
old; Clea Japan, Tokyo, Japan) were maintained under specific
pathogen-free conditions. Two million SY cells were subcutaneously
injected into a hindlimb under isoflurane anesthesia. When
subcutaneous tumors reached a diameter of ~10 mm, the tumors were
irradiated with a single dose 15 Gy of 200 kVp X-rays at a rate of
0.98 Gy/min using a Pantak HF-320 X-ray generator (Shimadzu, Kyoto,
Japan). Other parts of the mouse body were protected by a brass
shield to limit unnecessary radiation exposure.
[3-11C]AIB (radiochemical purity >99%) was
synthesized from iodo[11C] methane and methyl
N-(diphenylmethylen)-D,L-alaniate using tetrabutylammonium
fluoride-promoted α-[11C]methylation (10). PET studies were conducted in two
schedules, schedule 1 (n=5) and schedule 2 (n=6). The time-points
of PET scan of schedule 1 were one day before irradiation (day -1)
and one and three days after irradiation (days 1 and 3), and those
of schedule 2 were days -1, 1 and 5 (Fig. 1). The 10-min PET scans were
conducted at 30 min after intravenous injection of ~11.1 MBq of
[3-11C]AIB using the small-animal PET system (Inveon;
Siemens Medical Solutions, Malvern, PA, USA) under isoflurane
anesthesia. Body temperature was maintained around 37°C by a
heating lamp and warm water during scans. Images were reconstructed
using a 3D maximum a posteriori (18 iterations with 16
subsets, β=0.2) without attenuation correction. The region of
interest (ROI) was manually drawn over tumors and tracer uptake was
quantified as the standardized uptake value (SUV)max.
Histological analysis
As a separate experiment, tumors (n=5 for each
time-point) were fixed in 10% (v/v) neutral buffered formalin and
embedded in paraffin for sectioning. Sections (0.1 μm
thickness) were stained with hematoxylin and eosin (H&E).
Apoptotic cells were detected by terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end labeling
(TUNEL) staining using an (Merck Millipore, Billerica, MA, USA).
Ki-67 staining was conducted using an anti-Ki-67 antibody (MIB-1;
Dako, Glostrup, Denmark) as described previously (11). TUNEL- and Ki-67-positive cells were
quantified in at least five randomly selected fields of each
section at ×400 magnification.
Real-time quantitative RT-PCR
As a separate experiment, first-strand cDNAs from
three tumors were synthesized using a FastLane Cell cDNA kit
(Qiagen, Hilden, Germany). Real-time RT-PCR was conducted in
triplicate with predesigned and preoptimized TaqMan probes to
detect three system A transporters (SLC38A1, SLC38A2, and SLC38A4)
and 18S rRNA (Applied Biosystems, Foster City, CA, USA) using
Mx3000P qPCR systems (Agilent Technologies, Santa Clara, CA, USA).
Gene expression levels were normalized to 18S rRNA expression in
each sample.
Statistical analysis
Tumor uptake, positive cells of TUNEL or Ki-67, and
mRNA expression data were analyzed by one-way ANOVA or two-way
repeated-measures ANOVA, followed by the Student-Newman-Keuls
multiple comparison test. The correlation between tumor uptake and
other factors was examined by simple regression analysis. A value
of P<0.05 was considered statistically significant.
Results
Serial PET before and after X-ray
irradiation
To determine the schedule of PET studies after
X-irradiation, we measured the tumor sizes after 15 Gy radiation.
Tumor sizes increased up to 2 days after irradiation and then
decreased thereafter (Fig. 1 upper
panel). All tumors had almost disappeared around 10 days after
irradiation (Fig. 1 upper panel).
[18F]FDG tumor uptake is reported to increase
temporarily at early time-points (4 h to several days) after
radiotherapy (12–14). As it is important to determine the
change in [3-11C]AIB tumor uptake at early time-points
after irradiation, we selected day 1 for the first post-radiation
PET study. We additionally selected two time-points, days 3 and 5,
to evaluate change in tumor uptake on the way to tumor shrinkage.
Due to the availability of the PET machine (5 days/week), we set
two schedules of PET study, schedules 1 and 2, as shown in Fig. 1 (lower panel).
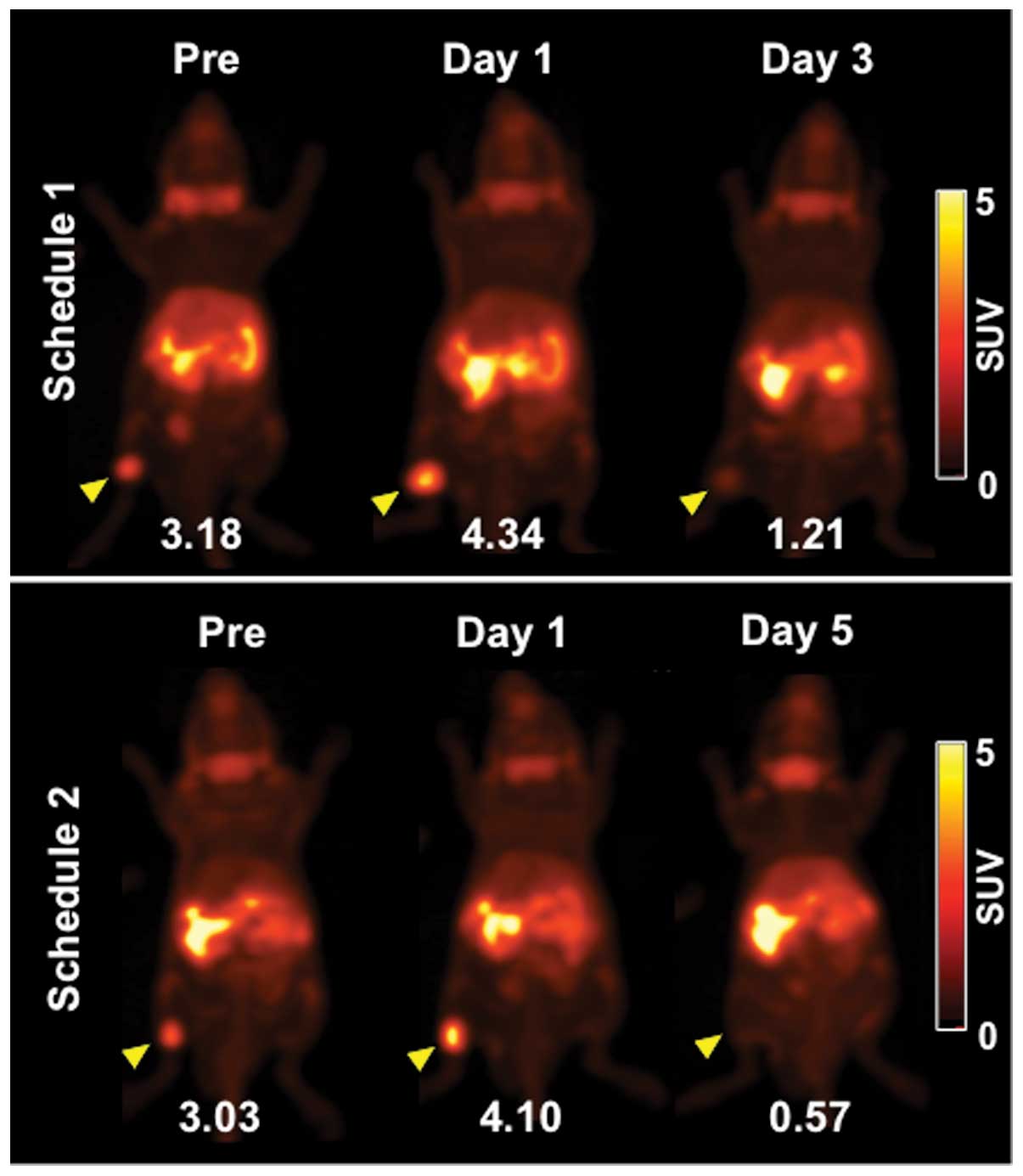
Representative PET images (maximum intensity
projection) of [3-11C]AIB before (day -1) and after
irradiation are shown in Fig. 2.
[3-11C]AIB PET clearly visualized tumors on day -1 and
more clearly on day 1 (Fig. 2).
Tumor uptake of [3-11C]AIB on day 3 was markedly
decreased and that on day 5 was barely detected (Fig. 2). Serial changes in tumor volume of
the mice used for the PET studies are shown in Fig. 3 (upper panels). The volume of all
tumors was increased on day 1 compared with that on day 0, although
the change was slight in three tumors (Fig. 3, upper panels). The volume of two
tumors on day 3 in schedule 1 increased, while that of the
remaining tumors decreased (Fig. 3,
upper panels). On day 5, a great reduction in volume was observed
in all tumors (Fig. 3, upper
panels). Temporal changes in tumor uptake are shown in Fig. 3 (middle panels). Although tumor
uptake was increased on day 1, with the exception of two mice,
there was no significant difference in mean SUVmax compared with
that on day -1 (Fig. 3, middle
panels). On days 3 and 5, tumor uptake was significantly decreased
compared with that on day -1 (P<0.05 for day 3 and P<0.01 for
day 5) (Fig. 3, middle panels).
There was no correlation between tumor volume and uptake in
schedule 1 (Fig. 3, lower left
panel). Although there was a correlation in schedule 2 (Fig. 3, lower right panel), the correlation
was lost when the data of day 5 were excluded (data not shown).
Histological analysis
Many mitotic cells were observed in the
H&E-stained sections of the untreated tumors (Fig. 4A, left column). The number of
mitotic cells was markedly decreased on day 1 (Fig. 4A, left column). On day 3, although
the mitotic cells increased, necrotic cells and fibrosis were
observed (Fig. 4A, left column). On
day 5, few living cells were observed and fibrotic areas were
widely spread (Fig. 4A, left
column). Tumor sections were also stained for TUNEL and Ki-67
(Fig. 4A, middle and right
columns), and the percentages of positive cells were quantified. We
were not able to quantify the results of day 5 since only a few
living cells were observed at this time-point, as described above.
In the untreated sections stained with TUNEL, few TUNEL-positive
(apoptotic) cells (<0.5%) were observed (Fig. 4A, middle column and Fig. 4B, left panel). X-irradiation-induced
apoptotic cells were ~1.4% on day 1 and increased to 8% on day 3
(Fig. 4B, left panel). Although
there was no significant difference between 0 Gy and day 1, there
was a significant difference between 0 Gy and day 3 (P<0.01). In
the Ki-67-stained sections, ~20% of untreated cells were positively
stained (Fig. 4A, right column and
4B, right panel). Although Ki-67-positive cells were significantly
decreased to ~7% on day 1 (P<0.01 vs. 0 Gy), they were increased
to ~20% on day 3, which was as high as that in the untreated tumors
(Fig. 4B, right panel). There were
no significant correlations between [3-11C]AIB tumor
uptake, and percentages of TUNEL- or Ki-67-positive cells (data not
shown).
Amino acid transporter expression
analysis
The amino acid transporter system A consists of
three transporters SLC38A1, SLC38A2, and SLC38A4 (8,15,16).
According to mRNA expression analysis of these three transporters
by real-time RT-PCR, expression levels of both SLC38A1 and SLC38A2
were detected in the tumors (Fig.
5, upper panels), but not that of SLC38A4 (data not shown). The
expression of SLC38A1 on day 1 tended to be increased compared with
that with 0 Gy, although there was no significant difference, and
it decreased on days 3 and 5 with significant differences
(P<0.05 vs. 0 Gy; Fig. 5, upper
left panel). That of SLC38A2 on day 1 was nearly equal to that with
0 Gy and decreased on days 3 and 5 with significant differences
(P<0.05 vs. 0 Gy; Fig. 5, upper
right panel). There was a significant correlation between
[3-11C]AIB tumor uptake and SLC38A1 expression, but not
SLC38A2 (Fig. 5, middle and lower
panels).
Discussion
In the present study, to determine whether
[3-11C]AIB is useful to monitor early metabolic change
of tumors after radiotherapy, we conducted PET studies evaluating
the tumor uptake change after irradiation in a tumor mouse model on
two different schedules. One day after irradiation (day 1), the
tumor uptake of [3-11C]AIB tended to increase, although
there were no statistically significant differences compared with
that one day before irradiation (day -1). This increased uptake was
transient and decreased thereafter. Temporarily increased tumor
uptake at early time-points (4 h to several days) after irradiation
has also been observed in [18F]FDG PET of patients
(12–14). Although this mechanism has not been
clarified yet, there are two possible reasons; the increased uptake
may be caused by an elevated inflammatory reaction or by
transiently enhanced glucose metabolism (12–14).
[3-11C]AIB was reported to accumulate less in
inflammatory lesions compared with [18F]FDG in a mouse
model with acute inflammation (9).
Furthermore, few inflammatory infiltrates were observed in the
tumor sections in the present study. Hence, we can exclude the
possibility that the increased tumor uptake of
[3-11C]AIB on day 1 was caused by induced inflammatory
reaction, and the increased uptake is thought to reflect other
pathophysiological change(s). Although the clinical implication of
temporarily increased uptake of [18F]FDG early after
radiotherapy is still unclear, it could be a predictor of favorable
outcome of radiotherapy in brain tumors (13). In xenograft tumor models, increased
[18F]FDG uptake and apoptotic cells were observed in a
tumor with high radiosensitivity, but not in tumors with lower
radiosensitivity (17). Since the
present study was conducted using a single effective radiation dose
for a single tumor model, further studies using tumor models with
various radiosensitivities are needed to determine whether
temporarily increased [3-11C]AIB uptake after
radiotherapy is related to the efficacy of radiotherapy.
For schedule 1, there was no correlation between
tumor uptake and volume. For schedule 2, although there was a
positive correlation between tumor uptake and volume, the tumor
volume on day 5 was greatly decreased. This greatly decreased tumor
uptake could be caused by the drastic reduction in viable cells as
shown in the H&E-stained sections on day 5. When the data of
day 5 were eliminated, there was no correlation between tumor
uptake and volume for schedule 2. These findings raise the
possibility that a temporal change in tumor uptake by irradiation
is independent of tumor volume at the earlier time-points. Although
decreased tumor uptake of [18F]FDG after effective
therapy provides useful information for the evaluation of efficacy,
in general it is necessary to wait several months after
radiotherapy to minimize the influence of radiation-induced
inflammation. Our previous studies showed that the accumulation of
[3-11C]AIB was less in an inflammatory lesion compared
with [18F]FDG in a mouse model with acute inflammation
(9) as mentioned above. Therefore,
[3-11C]AIB PET has the potential for evaluating the
response to radiotherapy at earlier time-points than
[18F]FDG PET. Although [3-11C]AIB tumor
uptake was higher than [18F]FDG in tumor-bearing mice
(9,10), [18F]FDG tumor uptake is
generally higher than the amino acid-based PET probes, such as
[methyl-11C] methionine, in patients. Considering the
high sensitivity of [18F]FDG in detecting tumors in
patients, combined PET of both [18F]FDG and
[3-11C]AIB may be clinically superior to
[3-11C]AIB PET alone for increased sensitivity and to
precisely evaluate the therapeutic efficacy. Further clinical
studies are needed to assess the role of [3-11C]AIB PET
in the evaluation of therapeutic efficacy.
To explore the factor(s) responsible for
[3-11C]AIB tumor uptake change after irradiation, we
conducted histological and expression analyses of tumors before and
after irradiation. Based on the findings of the H&E-stained
sections, tumor cells transiently stopped proliferation early after
irradiation and then started to proliferate again, and then cell
death was induced. These findings were consistent with the analysis
of the TUNEL- and Ki-67-stained sections in the present study.
Although radiation-induced cell death occurred following cell
regrowth after temporary cell cycle arrest in the irradiated SY
cells in the present study as well as in other cells (1), the tumor uptake was not correlated
with either apoptotic or proliferative cells. These findings
suggest that [3-11C]AIB PET could lead to fewer
false-positive results due to transient cell regrowth activity as
well as inflammation reaction. AIB is incorporated into cells
mainly through amino acid transporter system A that consists of
three transporters SLC38A1, SLC38A2 and SLC38A4 (8,15,16).
In the expression analysis, SLC38A1 and SLC38A2 were detected in
the tumors, but SLC38A4 was not detected. Some proteins, including
amino acid transporters, often show ectopic expression in malignant
transformed cells (8,15,16).
Although SLC38A2 is expressed in normal lung, SLC38A1 and SLC38A4
are not expressed (15,16). The SLC38A1 expression in SY tumors
could be caused by oncogenic transformation. The SLC38A1 expression
showed a tendency to increase temporarily on day 1, but not
SLC38A2, and the expression of both decreased thereafter. The
temporal change in tumor uptake of [3-11C]AIB was
correlated with that of SLC38A1 expression, but not with SLC38A2.
The ectopic expression of SLC38A1 may play a major role in the high
uptake of [3-11C]AIB in SY tumors. This may be the
reason why the temporal change of [3-11C]AIB uptake by
irradiation was correlated with that of SLC38A1 expression. Since
each type of cancer has a distinct expression pattern of amino acid
transporters (15,16), it would be important to investigate
the relationship between [3-11C]AIB uptake and amino
acid transporter expression after irradiation in several tumor
models to elucidate the implication of temporal change of
[3-11C]AIB after radiotherapy.
The present study showed that the temporal change in
[3-11C]AIB tumor uptake at early time-points after
irradiation was correlated with the amino acid transporter
expression, but independent of tumor volume and cell proliferation
activity, suggesting that [3-11C]AIB PET has the
potential for evaluating an early metabolic change after
radiotherapy not affected by transient proliferation activity. Our
findings support further preclinical and clinical studies to
investigate the role of metabolic change detected by
[3-11C]AIB PET in the evaluation of radiotherapy
efficacy before morphological change of tumors.
Acknowledgments
We thank Takamitsu Morioka for the expertise in
assessing the histological findings; Yuriko Ogawa for technical
assistance; Yuichiro Yoshida, Masanao Ogawa, and Nobuki Nengaki in
the Radiochemistry section for technical support of
[3-11C]AIB production; staff in the Cyclotron operation
section for cyclotron operation; and staff in the Laboratory Animal
Sciences section for animal management. The present study was
supported by grants from the ministry of Education, Culture,
Sports, Science and Technology of Japan (KAKENHI 23510289 and
24591803).
References
|
1
|
Halperin EC, Perez CA and Brady LW: Perez
and Brady’s Principles and Practice of Radiation oncology. 5th.
Lippincott Williams & Wilkins; Philadelphia, PA: 2008
|
|
2
|
Van de Wiele C, Lahorte C, Oyen W, Boerman
O, Goethals I, Slegers G and Dierckx RA: Nuclear medicine imaging
to predict response to radiotherapy: a review. Int J Radiat oncol
Biol Phys. 55:5–15. 2003. View Article : Google Scholar
|
|
3
|
Juweid ME and Cheson BD: Positron-emission
tomography and assessment of cancer therapy. N Engl J Med.
354:496–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bading JR and Shields AF: Imaging of cell
proliferation: status and prospects. J Nucl Med. 49:64S–80S. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shreve PD, Anzai Y and Wahl RL: Pitfalls
in oncologic diagnosis with FDG PET imaging: physiologic and benign
variants. Radiographics. 19:61–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Love C, Tomas MB, Tronco GG and Palestro
CJ: FDG PET of infection and inflammation. Radiographics.
25:1357–1368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haberkorn U, Strauss LG, Dimitrakopoulou
A, et al: PET studies of fluorodeoxyglucose metabolism in patients
with recurrent colorectal tumors receiving radiotherapy. J Nucl
Med. 32:1485–1490. 1991.PubMed/NCBI
|
|
8
|
McConathy J and Goodman MM: Non-natural
amino acids for tumor imaging using positron emission tomography
and single photon emission computed tomography. Cancer Metastasis
Rev. 27:555–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuji AB, Kato K, Sugyo A, et al:
Comparison of 2-amino-[3-¹¹C]isobutyric acid and
2-deoxy-2-[18F]fluoro-D-glucose in nude mice with
xenografted tumors and acute inflammation. Nucl Med Commun.
33:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato K, Tsuji AB, Saga T and Zhang M-R: An
efficient and expedient method for the synthesis of
11C-labeled α-aminoisobutyric acid: a tumor imaging
agent potentially useful for cancer diagnosis. Bioorg Med Chem
Lett. 21:2437–2440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida C, Tsuji AB, Sudo H, et al:
Therapeutic efficacy of c-kit-targeted radioimmunotherapy using
90Y-labeled anti-c-kit antibodies in a mouse model of small cell
lung cancer. PLoS one. 8:e592482013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hautzel H and Müller-Gärtner HW: Early
changes in fluorine-18-FDG uptake during radiotherapy. J Nucl Med.
38:1384–1386. 1997.PubMed/NCBI
|
|
13
|
Maruyama I, Sadato N, Waki A, et al:
Hyperacute changes in glucose metabolism of brain tumors after
stereotactic radiosurgery: a PET study. J Nucl Med. 40:1085–1090.
1999.PubMed/NCBI
|
|
14
|
Rozental JM, Levine RL, Mehta MP, et al:
Early changes in tumor metabolism after treatment: the effects of
stereotactic radiotherapy. Int J Radiat Oncol Biol Phys.
20:1053–1060. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mackenzie B and Erickson JD:
Sodium-coupled neutral amino acid (System N/A) transporters of the
SLC38 gene family. Pflugers Arch. 447:784–795. 2004. View Article : Google Scholar
|
|
16
|
Nakanishi T and Tamai I: Solute carrier
transporters as targets for drug delivery and pharmacological
intervention for chemotherapy. J Pharm Sci. 100:3731–3750. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furuta M, Hasegawa M, Hayakawa K, et al:
Rapid rise in FDG uptake in an irradiated human tumour xenograft.
Eur J Nucl Med. 24:435–438. 1997.PubMed/NCBI
|