Introduction
Reduced expression in immortalized cells
(REIC)/Dickkopf (Dkk)-3 gene is a member of the Dkk family, which
consists of four members (Dkk-1 to -4). Dkk proteins regulate the
canonical Wnt/β-catenin signaling pathway, which plays a critical
role in cell proliferation and differentiation (1,2).
Dkk-1, -2 and -4 interact with the low-density lipoprotein-related
receptor 5 or 6 (LRP5/6) and affect Wnt/β-catenin signaling
(3–5). REIC/Dkk-3 does not associate with
LRP5/6, and its involvement in Wnt/β-catenin signaling remains
controversial (6–8).
Unlike other Dkk family members, REIC/Dkk-3 is a
tumor-suppressor gene whose expression is markedly reduced in
various types of cancer cells and tissues (9–16).
Overexpression of REIC/Dkk-3 with an adenovirus vector carrying the
human REIC/Dkk-3 gene (Ad-REIC) induces endoplasmic reticulum (ER)
stress-mediated apoptosis in cancer cells (17,18).
We previously demonstrated that the N-terminal region of REIC/Dkk-3
is responsible for its cancer cell-specific induction of apoptotic
activity (19). In addition,
infection of normal cells with Ad-REIC resulted in the production
of interleukin (IL)-7, which contributes to systemic anticancer
immunoreactivity (17). Based on
these findings, a phase I-IIa study of Ad-REIC gene therapy in
prostate cancer patients is ongoing (20).
Recently, we found that secreted REIC/Dkk-3 protein
induces differentiation from monocytes to dendritic cell (DC)-like
cells (21). DCs control immune
homeostasis by regulating both innate and adaptive immunity. Since
DCs play a critical role in initiating cancer immunity, they have
become an attractive target for cancer immune therapy. The
mechanisms by which cytokines regulate DC development from
hematopoietic stem cells have been extensively analyzed in
vitro (22,23). For example, the addition of
granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-4
to the culture medium is a standard procedure to induce DC
differentiation from monocytes, and it has been applied to the
preparation of DC vaccines for cancer therapy (24,25).
Differentiation of DC-like cells was observed when monocytes were
treated with exogenous REIC/Dkk-3 protein at doses higher than 1
μg/ml (21); however, the
naturally circulating REIC/Dkk-3 protein is found at 40–60 ng/ml in
serum (26). To our knowledge, this
activation is unique to the REIC/Dkk-3 protein among the Dkk family
members, which has a relatively low (35–40%) sequence similarity in
the Dkk family (1). In the present
study, we analyzed the REIC/Dkk-3 protein to identify the region
responsible for the induction of DC differentiation. In addition,
the role of the REIC/Dkk-3 protein in immune activation was
confirmed by examining its anticancer effects in response to
intraperitoneal administration, and its effect on the activation of
immunocompetent cells in blood.
Materials and methods
Construction of the expression
plasmids
Recombinant REIC-Dkk-3 proteins were expressed using
a previously developed supergene expression (SGE) system (27,28).
The expression plasmid DNA [pIDT-SMART (C-TSC)-REIC] for expression
of the full-length REIC/Dkk-3 (FL-REIC) protein was described
previously (27). The cDNA fragment
encoding an N-terminal truncated form of C-REIC [Arg142-Ile350] was
amplified with PCR primers containing the EcoRI and
BamHI restriction sites. The PCR products were first cloned
into the p3xFLAG-CMV-9 expression vector (Sigma-Aldrich, St. Louis,
MO, USA) to express FLAG-tag fused C-REIC protein. To obtain
efficient recombinant protein expression with the SGE system, the
open reading frame was cloned into the pIDT-SMART (C-TSC)
vector.
Preparation of the human REIC/Dkk-3
protein
Both FL-REIC and C-REIC were transiently expressed
in FreeStyle™ 293-F cells (Life Technologies, Carlsbad, CA, USA)
using Freestyle 293 Expression Medium and the 293 Fectin
transfection reagent (Life Technologies), according to the
manufacturer’s instructions. Briefly, exponentially growing cells
(1×106 cells/ml) with 180 ml media were prepared in a
500-ml flask. After transfection with 180 μg each of
expression plasmid DNAs and 293 Fectin complex, the cells were
cultivated using an orbital shaker (125 rpm) at 37°C in the
presence of 8% CO2 for 4 days. Secreted proteins in the
culture media were concentrated by Amicon Ultra centrifugal filter
units (Millipore, Billerica, MA, USA), and the buffer was then
replaced with 20 mM HEPES buffer (pH 7.2) using Sephadex G25M
column chromatography (GE Healthcare, Piscataway, NJ, USA).
Subsequently, the proteins were purified by anion exchange column
chromatography (DEAE-Toyopearl 650M; Tosoh, Tokyo, Japan) and
eluted with a linear NaCl gradient (0 to 0.7 M). The solution of
the recombinant proteins was changed to PBS using a Sephadex G25M
column, and then sterilized with 0.22 μm Millex-GV syringe
filters (Millipore) and stored at −70°C until use for the
biological experiments.
Analysis of REIC/Dkk-3 degraded
products
During the optimization of purification procedures
for REIC/Dkk-3 proteins, degraded products were often detected on
SDS-PAGE. This degradation converged to a 17-kDa band on SDS-PAGE,
which was no longer degraded with long incubation times. This
limited degradation product (C17-REIC) was analyzed for its
amino-terminal sequence with a protein sequencer (Applied
Biosystems 491), and carboxyl terminal amino acids were determined
by amino acid analyzer (L-8500; Hitachi, Japan) after
hydrazinolysis of the protein.
Preparation of the human monocytes
Human peripheral blood monocytes (PBMCs) were
prepared from the blood of healthy donors by a standard method
involving Ficoll-Paque centrifugation. The cell collection rate was
determined by the trypan blue exclusion method. The survival rate
was confirmed to be 99% or greater. For preparation of the
monocytes, PBMCs were resuspended in LGM-3 (serum-free lymphocyte
growth medium-3; Lonza, Walkersville, MD, USA). The cells adhering
to a plastic dish (subjected to incubation in a 10-cm dish at 37°C
for 2 h) were used as monocytes. In some experiments,
CD14+ monocytes were separated using CD14+
magnetic-activated cell sorting microbeads (MACS; Miltenyi Biotec,
Bergisch Gladbach, Germany). Purified CD14+ monocytes
were resuspended in LGM-3 medium.
Treatment of the human monocytes
CD14+ monocytes were cultured in LGM-3
medium with or without DC differentiation factors. As a positive
control, 2 ng/ml each of GM-CSF and IL-4 (both from R&D
Systems, Minneapolis, MN, USA) were added to the medium. As for
REIC/Dkk-3 proteins, 10 μg/ml of purified recombinant
proteins was added. After cultivation for 7 days, the solution was
stirred manually, and after 3 min, the number of DC-like cells per
randomly selected visual field was counted with magnification of
the slightly expanded photographs. The data were converted into a
graph (n=5 visual fields). The cells were observed with a phase
contrast microscope.
Western blotting
Purified CD14+ monocytes were incubated
for 6 h in LGM-3 medium with 2 ng/ml GM-CSF or 10 μg/ml REIC
protein. Total cellular proteins were prepared from the treated
cells, and western blot analysis was performed as previously
described (21). Proteins were
identified using the following antibodies: anti-phospho-Akt
(Ser473), anti-phospho-glycogen synthase kinase 3β (GSK-3β) (Ser9),
anti-GSK-3β, anti-phosphorylated signal transducers and activators
of transcriptions (STAT)3 (Tyr705) and anti-phospho-STAT5 (Tyr694)
(Cell Signaling Technology, Beverly, MA, USA).
Tumor-suppressive effects of FL-REIC and
C17-REIC proteins in vivo
Murine renal carcinoma (RENCa) cells
(1×106) were subcutaneously injected into mice (BALB/c,
female, n=5). On days 3, 5, 7, 10, 12 and 14 after injection
(provided that day 3 after injection was designated as the day of
the start of administration of REIC proteins), 100 μg each
of FL-REIC or C17-REIC, both proteins dissolved in 100 μl of
PBS, or PBS as a control was intraperitoneally injected into mice.
On day 17, the therapeutic effects were evaluated as tumor volume,
and anticancer immune activity was measured before mice were
euthanized. All experiments were conducted in accordance with the
guidelines for animal experiments of our institution.
Flow cytometry
EDTA (0.2% solution, 30 μl) was added to 750
μl of mouse blood collected from the inferior vena cava as
an anticoagulant. Antibodies (1 μl each) with different
fluorescent labels (purchased from eBioscience) were added to 30
μl of blood, stirred and incubated at 4°C for 60 min to
stain immunocompetent cells as follows: DCs (anti-CD11c antibody
and anti-CD80 antibody) or cytotoxic T cells (anti-CD8 antibody and
anti-CD69 antibody).
Subsequently, erythrocytes were lysed in a red blood
cell lysis buffer. Cells were washed twice with PBS and resuspended
in 200 μl of PBS to generate a solution for analysis. A
total of 3×104 cells were collected using a FACSCalibur
flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and
analyzed using CellQuest software (Becton Dickinson). An
appropriate gate was set on the basis of the forward scatter
pattern characteristic of these cells, and only cells within the
gate were analyzed.
Statistical analysis
Data are expressed as the means ± standard error.
Differences between two groups were analyzed using the unpaired
Student’s t-test, and p<0.05 was considered statistically
significant.
Results
Production and purification of the
REIC/Dkk-3 protein
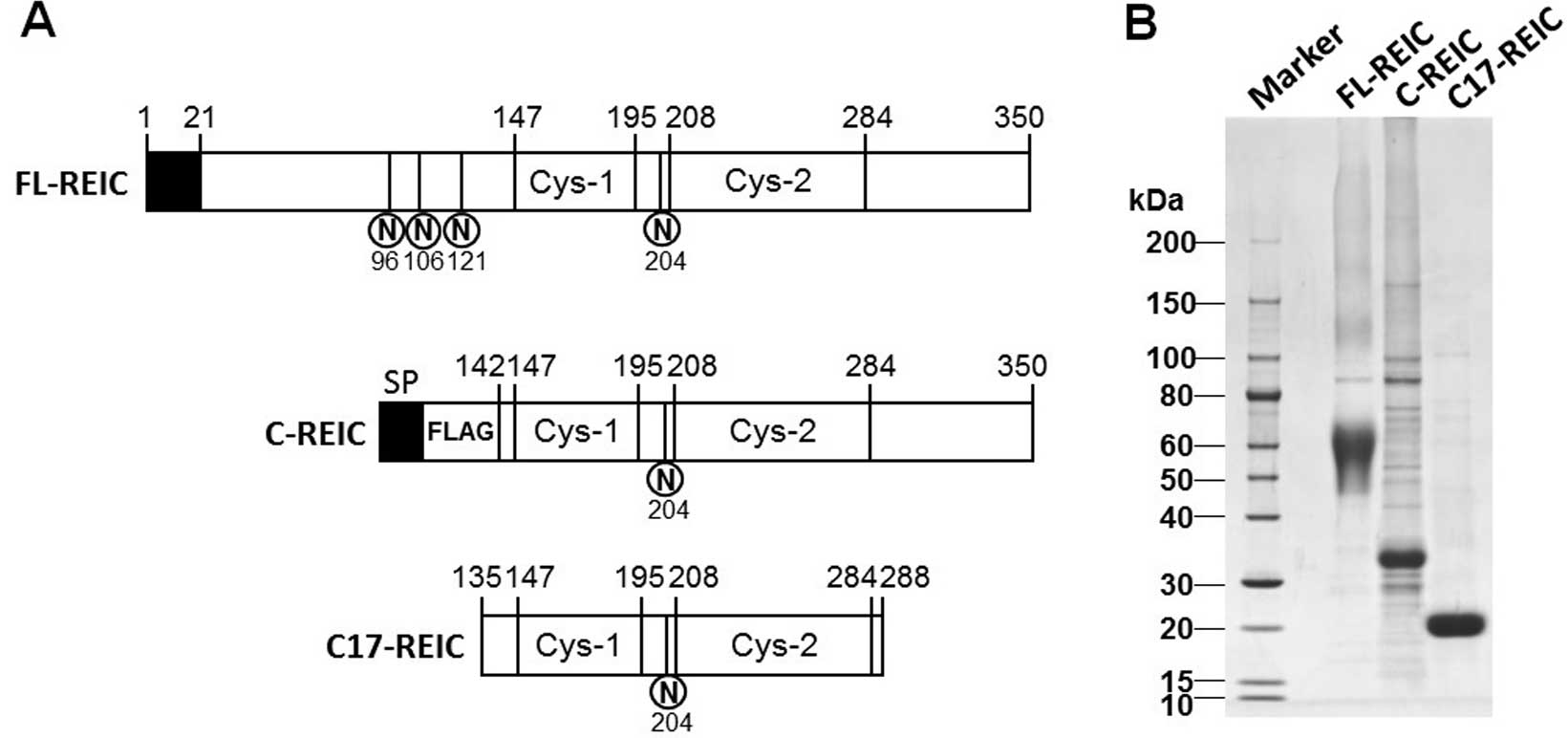
To elucidate the molecular mechanism underlying the
induction of anticancer immune responses by REIC/Dkk-3, the
FL-REIC/Dkk-3 protein and the C terminal domain of REIC/Dkk-3
(C-REIC) containing two cysteine (Cys)-rich domains were produced
in Freestyle 293-F cells (Life Technologies) (Fig. 1A). Secreted REIC/Dkk-3 protein was
recovered from the culture medium of transfected 293-F cells on day
4. Approximately 100 mg of purified FL-REIC was obtained from a
1-liter culture using this system. Since the expression of C-REIC
protein with the original signal peptide showed a low yield, the
signal peptide was replaced by the Met-preprotrypsin leader
sequence (PPT LS) preceding the FLAG coding sequence of the
p3xFLAG-CMV-9 vector.
The stability of the FL-REIC protein was tested by
incubation at 37°C, which resulted in the detection of degraded
products on SDS-PAGE. Although the degradation mechanism was
unclear, this proteolytic degradation was enhanced with unpurified
FL-REIC protein in a low-salt buffer. However, the degraded protein
products converged in a band of ~17 kDa on SDS-PAGE, and longer
incubation periods did not result in additional degradation
products. Amino acid sequencing of this product resulted in the
identification of Ser135 as the amino terminal residue and Phe288
as the carboxyl terminal residue (Fig.
1A). The purity of the REIC/Dkk-3 protein was determined as
greater than 90% by SDS-PAGE (Fig.
1B).
The cysteine-rich domain of REIC/Dkk-3 is
responsible for the induction of DC-like cell differentiation
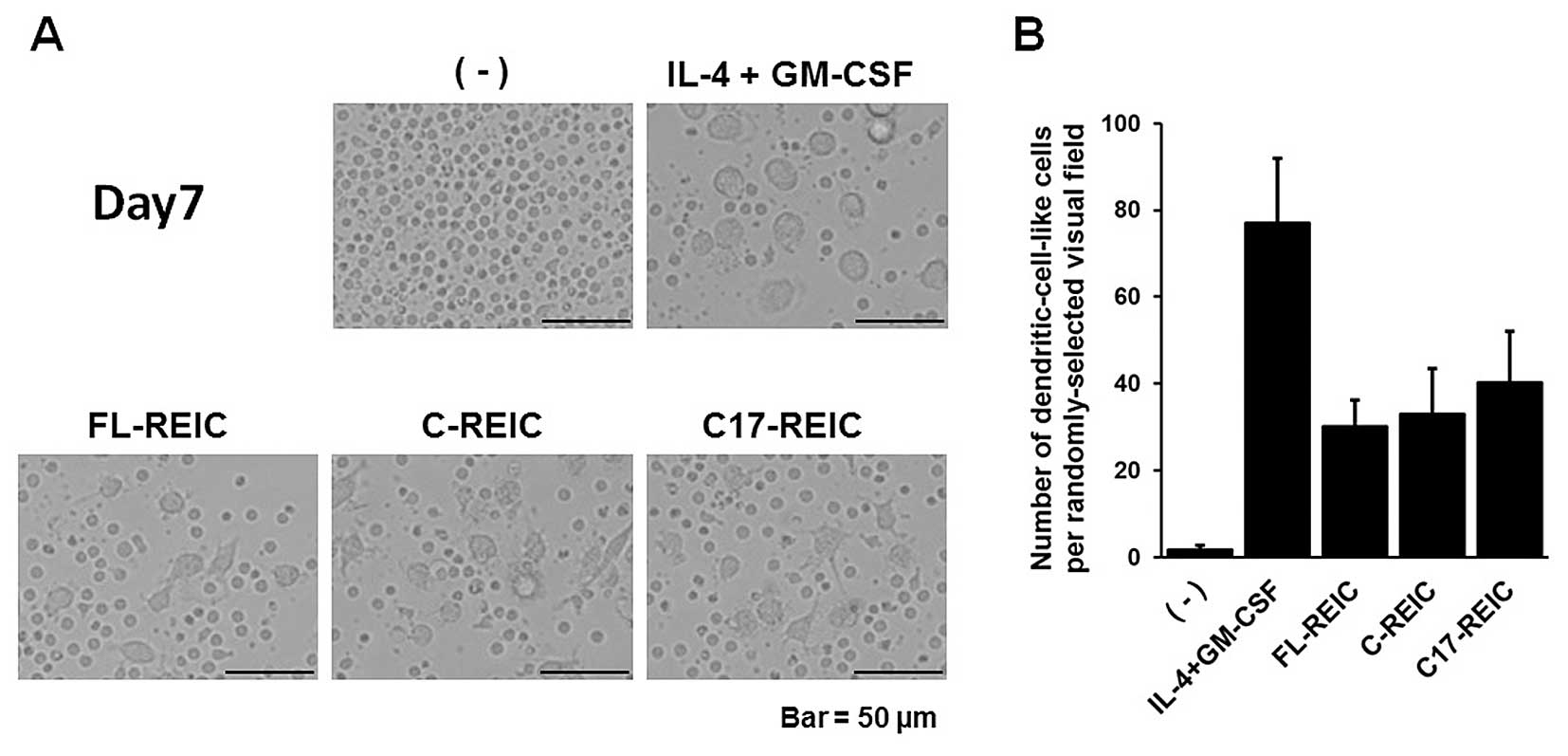
Previous studies from our group showed that FL-REIC
is a DC-like cell differentiation factor for monocytes when used at
a range of 1–10 μg/ml (21).
To investigate whether the C-REIC and C17-REIC proteins induce DC
differentiation, CD14+ monocytes were incubated with 10
μg/ml of purified REIC proteins. After a 7-day culture,
imaging with a phase-contrast microscope showed DC-like
differentiation of cells treated with GM-CSF/IL-4 and each of the
three REIC proteins (FL-REIC, C-REIC and C17-REIC) (Fig. 2A). The number of DC-like cells per
randomly-selected visual field (n=5) was counted, and the three
types of REIC proteins had a comparable effect (Fig. 2B). These results indicated that
C17-REIC, composed of two Cys-rich domains, is essential for the
induction of DC-like cell differentiation.
REIC/Dkk-3-mediated phosphorylation of
STAT3, STAT5 and GSK-3β plays a role in DC-like cell
differentiation
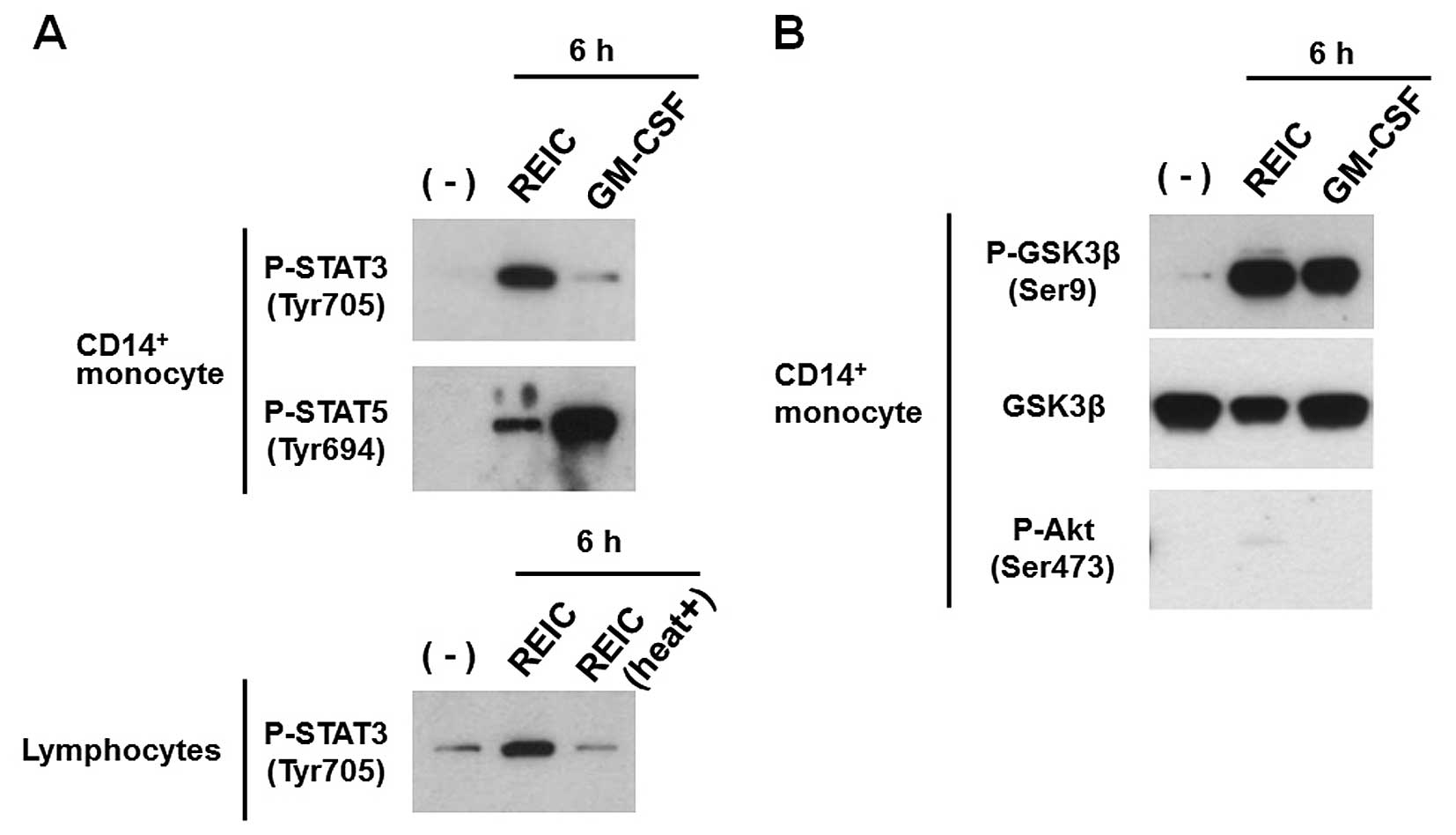
Recently, intracellular activation of both STAT3 and
STAT5 was found to play a role in the development of DCs (29–31).
In our previous study, we showed that REIC/Dkk-3 phosphorylates
STAT1 and STAT3 in monocytes (21).
Furthermore, phosphorylation of GSK-3β on Ser9 by GM-CSF is thought
to be involved in DC maturation (32). To evaluate the phosphorylation of
STAT3, STAT5 and GSK-3β induced by REIC/Dkk-3, monocytes were
treated for 6 h with REIC/Dkk-3 or GM-CSF. Both REIC/Dkk-3 and
GM-CSF induced the phosphorylation of STAT3, STAT5 and GSK-3β in
the monocytes, although the effective dose for REIC/Dkk-3 was much
higher than that of GM-CSF (Fig. 3A and
B). By contrast, heat treatment of REIC/Dkk-3 protein abrogated
the effect on the phosphorylation of STAT3 in lymphocytes (Fig. 3A). Consequently, activation of STAT
signaling and GSK-3β inactivation depending on Ser9 phosphorylation
is a biological activity unique to the REIC/Dkk-3 protein, and it
is not caused by possible contaminants, such as
lipopolysaccharides. Since phosphorylated Akt also induces the
phosphorylation of GSK-3β (33,34),
we analyzed the activation of the PI3K/Akt pathway. Our results
showed that Akt was not activated in response to REIC/Dkk-3
treatment (Fig. 3B). Taken
together, these results revealed that REIC/Dkk-3 induces the
phosphorylation of GSK-3β in monocytes independently from the
PI3K/Akt pathway.
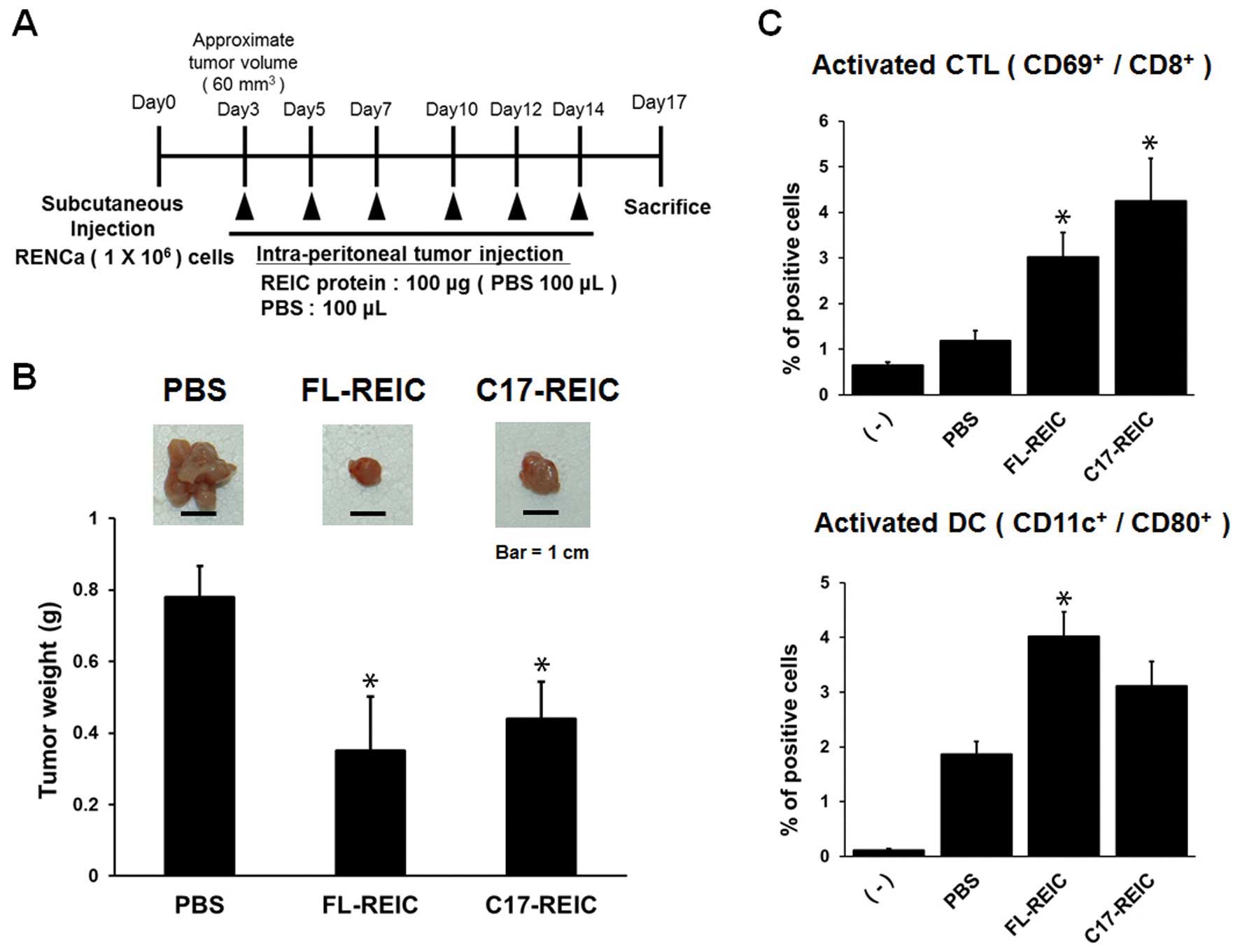
Intraperitoneal injection of REIC/Dkk-3
suppresses tumor growth via induction of cancer immunity
We previously demonstrated that intratumoral
administration of FL-REIC inhibited tumor growth in vivo
through the induction of cancer immunity (21). To investigate the antitumor
potential of REIC/Dkk-3, FL-REIC and C17-REIC proteins were
intraperitoneally injected into tumor-bearing mice (Fig. 4A). Significant tumor growth
suppression was observed 17 days after the injection of the
REIC/Dkk-3 proteins. Tumor volumes were statistically significantly
smaller in the group treated with both FL-REIC and C17-REIC than in
the group treated with PBS (Fig.
4B). These antitumor effects of the REIC/Dkk-3 proteins were
accompanied by in vivo induction of CTL
(CD69+/CD8+) and DCs
(CD11c+/CD80+) in the peripheral blood
(Fig. 4C). Taken together, these
results revealed that intraperitoneally injected REIC/Dkk-3
proteins exhibited antitumor effects mediated by the activation of
systemic immunity, and the cysteine-rich core domain was essential
for these biological responses.
Discussion
In the present study, we demonstrated the
feasibility of anticancer protein therapy by using recombinant REIC
proteins. High level production of recombinant REIC proteins was
achieved by using Freestyle 293 cell suspension cultures and SGE
high-level expression vector systems (27) with transient gene expression. During
the process of FL-REIC protein purification, we identified a stable
region designated as C17-REIC, composed of two Cys-rich domains.
In vitro and in vivo assays using truncated forms of
the REIC protein revealed that the Cys-rich core domain (C17-REIC)
is critical for inducing cancer immunity, acting as a DC-like cell
differentiation factor from monocytes. The N-terminal sequence of
C17-REIC, SVGDEEGRRS, is the same sequence previously reported as
the binding sequence for dynein light chain, Tctex1 (35). Although the detailed mechanism
underlying the interaction between the secretory REIC/Dkk-3 protein
and the intracellular Tctex1 protein remains unclear, the
proteolytic processing of C17-REIC observed in vitro may
reflect its intracellular biological action. Furthermore, we
demonstrated that the C17-REIC protein acts as a tumor suppressor
similar to the FL-REIC/Dkk-3 protein. Since the therapeutic effects
of the protein depend on its structural integrity, it is important
to minimize the risk of degradation, denaturation, aggregation, and
precipitation, and storage conditions are important. Therefore, the
fact that the robust C17-REIC domain is the domain responsible for
protein function suggests that REIC possesses favorable features
for protein-based therapy.
The results of the present study shed light on the
molecular mechanisms underlying the induction of DC-like cell
differentiation from monocytes by REIC/Dkk-3. The REIC/Dkk-3
protein induced the phosphorylation of GSK-3β at levels comparable
with the cytokine GM-CSF. Since GSK-3β phosphorylation is induced
by various cytokines (33), the
intracellular signaling pathway elicited in response to REIC/Dkk-3
stimulation may be shared with that of cytokines.
DC vaccine therapy is a promising option for cancer
therapy. DCs exist in various populations characterized by
different surface markers (23). In
our previous study, we showed that the surface markers of DCs
induced by REIC/Dkk-3 protein treatment were similar to those
induced by GM-CSF and IL-4, except that the CD1a antigen was
negative (21). Since REIC/Dkk-3 is
ubiquitously expressed in normal tissues, whereas its expression is
suppressed in many tumor tissues, REIC/Dkk-3 may play an important
role in cancer immunity by regulating the differentiation of DCs.
Indeed, intraperitoneal tumor injection of the REIC/Dkk-3 protein
inhibited tumor growth and induced the activity of immunocompetent
cells in blood in a mouse model of subcutaneous renal
adenocarcinoma. The REIC/Dkk-3 gene is expressed in most human
tissues (9) and the concentration
of the secreted protein in normal human serum is 40–60 ng/ml
(26), indicating that the risk of
immunogenicity is low.
The findings of the present study support the
hypothesis that the REIC/Dkk-3 protein is suitable for anticancer
immunity medical treatment. REIC/Dkk-3 protein therapy holds
promise as a method of immunotherapy. Ad-REIC gene therapy is a
highly effective approach in various cancers, and has been shown to
exert antitumor effects locally and systemically. In the future,
REIC/Dkk-3 protein therapy may contribute to enhance the systemic
antitumor effects of Ad-REIC therapy.
Acknowledgments
The present study was supported by METI’s FY2010
Supplementary Budget for Regional Innovation Creation R&D
Programs ‘Development of an intelligent injection system for
nanobiotargeted therapy’ and a Grant for Promotion of Science and
Technology in Okayama Prefecture (by MEXT) ‘Creation of
nanobiotargeted therapy using REIC as a therapeutic gene for
cancer’. We thank Ms. Tomoko Honjo (Okayama University) and Ms.
Remi Sunami (Momotaro-Gene Inc.) for valuable assistance. Okayama
University and Momotaro-Gene Inc. are applying for patents on the
SGE systems. M.W., M.S., and H.K. are inventors of the patents.
Okayama University and Momotaro-Gene Inc. are applying for patents
on the Partial region polypeptide of the REIC/Dkk-3 protein. J.F.,
M.W. and H.K. are inventors of the patents. Momotaro-Gene Inc.
holds the patents of Ad-REIC and REIC protein agents and develops
the agents as a cancer therapeutic medicine. H.K., M.S. and M.W.
own stock in Momotaro-Gene Inc. Okayama University and
Momotaro-Gene Inc. are working together for the development of the
Ad-REIC agent. Okayama University also received research funds for
the joint research. H.K. is the chief science officer for
Momotaro-Gene Inc.
References
|
1
|
Krupnik VE, Sharp JD, Jiang C, et al:
Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mao B, Wu W, Li Y, et al:
LDL-receptor-related protein 6 is a receptor for Dickkopf proteins.
Nature. 411:321–325. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li L, Mao J, Sun L, Liu W and Wu D: Second
cysteine-rich domain of Dickkopf-2 activates canonical Wnt
signaling pathway via LRP-6 independently of dishevelled. J Biol
Chem. 277:5977–5981. 2002. View Article : Google Scholar
|
|
5
|
Cheng Z, Biechele T, Wei Z, et al: Crystal
structures of the extracellular domain of LRP6 and its complex with
DKK1. Nat Struct Mol Biol. 18:1204–1210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura RE and Hackam AS: Analysis of
Dickkopf3 interactions with Wnt signaling receptors. Growth
Factors. 28:232–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujii Y, Hoshino T and Kumon H: Molecular
simulation analysis of the structure complex of C2 domains of DKK
family members and β-propeller domains of LRP5/6: explaining why
DKK3 does not bind to LRP5/6. Acta Med Okayama. 68:63–78. 2014.
|
|
8
|
Fujita K and Janz S: Attenuation of WNT
signaling by DKK-1 and -2 regulates BMP2-induced osteoblast
differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer.
6:712007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows down-regulation in human
immortalized cells and human tumor-derived cell lines. Biochem
Biophys Res Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuji T, Nozaki I, Miyazaki M, et al:
Antiproliferative activity of REIC/Dkk-3 and its significant
down-regulation in non-small-cell lung carcinomas. Biochem Biophys
Res Commun. 289:257–263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abarzua F, Sakaguchi M, Takaishi M, et al:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanimoto R, Abarzua F, Sakaguchi M, et al:
REIC/Dkk-3 as a potential gene therapeutic agent against human
testicular cancer. Int J Mol Med. 19:363–368. 2007.PubMed/NCBI
|
|
13
|
Shien K, Tanaka N, Watanabe M, et al:
Anti-cancer effects of REIC/Dkk-3-encoding adenoviral vector for
the treatment of non-small cell lung cancer. PLoS One.
9:e879002014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirata T, Watanabe M, Kaku H, et al:
REIC/Dkk-3-encoding adenoviral vector as a potentially effective
therapeutic agent for bladder cancer. Int J Oncol. 41:559–564.
2012.PubMed/NCBI
|
|
15
|
Than SS, Kataoka K, Sakaguchi M, et al:
Intraperitoneal administration of an adenovirus vector carrying
REIC/Dkk-3 suppresses peritoneal dissemination of scirrhous gastric
carcinoma. Oncol Rep. 25:989–995. 2011.PubMed/NCBI
|
|
16
|
Uchida D, Shiraha H, Kato H, et al:
Potential of adenovirus-mediated REIC/Dkk-3 gene therapy for use in
the treatment of pancreatic cancer. J Gastroenterol Hepatol.
29:973–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakaguchi M, Kataoka K, Abarzua F, et al:
Overexpression of REIC/Dkk-3 in normal fibroblasts suppresses tumor
growth via induction of interleukin-7. J Biol Chem.
284:14236–14244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanimoto R, Sakaguchi M, Abarzua F, et al:
Down-regulation of BiP/GRP78 sensitizes resistant prostate cancer
cells to gene-therapeutic overexpression of REIC/Dkk-3. Int J
Cancer. 126:1562–1569. 2010.
|
|
19
|
Abarzua F, Kashiwakura Y, Takaoka M, et
al: An N-terminal 78 amino acid truncation of REIC/Dkk-3
effectively induces apoptosis. Biochem Biophys Res Commun.
375:614–618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe M, Nasu Y and Kumon H:
Adenovirus-mediated REIC/Dkk-3 gene therapy: Development of an
autologous cancer vaccination therapy (Review). Oncol Lett.
7:595–601. 2014.PubMed/NCBI
|
|
21
|
Watanabe M, Kashiwakura Y, Huang P, et al:
Immunological aspects of REIC/Dkk-3 in monocyte differentiation and
tumor regression. Int J Oncol. 34:657–663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou GM and Tam YK: Cytokines in the
generation and maturation of dendritic cells: recent advances. Eur
Cytokine Netw. 13:186–199. 2002.PubMed/NCBI
|
|
23
|
Conti L and Gessani S: GM-CSF in the
generation of dendritic cells from human blood monocyte precursors:
Recent advances. Immunobiology. 213:859–870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schuler G, Schuler-Thurner B and Steinman
RM: The use of dendritic cells in cancer immunotherapy. Curr Opin
Immunol. 15:138–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nestle FO: Dendritic cell vaccination for
cancer therapy. Oncogene. 19:6673–6679. 2000. View Article : Google Scholar
|
|
26
|
Zenzmaier C, Sklepos L and Berger P:
Increase of Dkk-3 blood plasma levels in the elderly. Exp Gerontol.
43:867–870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakaguchi M, Watanabe M, Kinoshita R, et
al: Dramatic increase in expression of a transgene by insertion of
promoters downstream of the cargo gene. Mol Biotechnol. 56:621–630.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe M, Sakaguchi M, Kinoshita R, et
al: A novel gene expression system strongly enhances the anticancer
effects of a REIC/Dkk-3-encoding adenoviral vector. Oncol Rep.
31:1089–1095. 2014.PubMed/NCBI
|
|
29
|
van de Laar L, Coffer PJ and Woltman AM:
Regulation of dendritic cell development by GM-CSF: molecular
control and implications for immune homeostasis and therapy. Blood.
119:3383–3393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laouar Y, Welte T, Fu XY and Flavell RA:
STAT3 is required for Flt3L-dependent dendritic cell
differentiation. Immunity. 19:903–912. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tormo AJ and Gauchat JF: A novel role for
STAT5 in DC: Controlling the Th2-response. JAKSTAT.
2:e253522013.
|
|
32
|
Alessandrini A, De Haseth S, Fray M, et
al: Dendritic cell maturation occurs through the inhibition of
GSK-3β. Cell Immunol. 270:114–125. 2011. View Article : Google Scholar
|
|
33
|
Vilimek D and Duronio V:
Cytokine-stimulated phosphorylation of GSK-3 is primarily dependent
upon PKCs, not PKB. Biochem Cell Biol. 84:20–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo J: Glycogen synthase kinase 3beta
(GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett.
273:194–200. 2009. View Article : Google Scholar
|
|
35
|
Ochiai K, Watanabe M, Ueki H, et al: Tumor
suppressor REIC/Dkk-3 interacts with the dynein light chain,
Tctex-1. Biochem Biophys Res Commun. 412:391–395. 2011. View Article : Google Scholar : PubMed/NCBI
|