Introduction
Leukemia is a common cancer that affects a
significant segment of the population, particularly children
(1). A recent study by the American
Cancer Society (ACS) showed that 47,150 new cases of leukemia were
diagnosed in the United States in 2012, whereas ~23,540 adults and
children died of leukemia during 2012 (2). In clinical therapy, the major strategy
for patients with leukemia include bone marrow transplantation,
radiotherapy and chemotherapy (3,4).
However, the cure rate is low and the side-effects are
debilitating; thus, the search for new agents for leukemia patients
is urgent.
Apoptosis is the major method by which anticancer
agents eliminate cancer cells (5).
Apoptosis is controlled by extrinsic and intrinsic pathways
(6). The extrinsic pathway involves
the death receptor, in which the death domains target caspase-8
when combined with their corresponding ligands. The activation of
caspase-8 then activates caspase-3 to ultimately induce apoptosis.
The intrinsic pathway of apoptosis is associated with DNA damage.
Oligomerization of Bax or Bak promotes the release of mitochondrial
cytochrome c into the cytoplasm. Cytochrome c
combines with the caspase-9 precursor to form an apoptosis complex.
This activation of caspase-9 then activates caspase-3 and
poly(ADP-ribose) polymerase (PARP) to induce apoptosis (7). The mitochondrial depolarization and
activation of caspase family proteases are the central steps in the
process of apoptosis (8), and their
associated signaling pathways include intrinsic
(mitochondrial-dependent) and endoplasmic reticulum (ER) stress
signals (9,10). ER stress occurs when ER homeostasis
is lost due to an overload of protein folding in the ER (11). ER stress triggers an evolutionarily
conserved response termed the unfolded protein response (UPR)
(12). The UPR alters
transcriptional and translational programs to cope with the
accumulation of unfolded or misfolded proteins. Failure to resolve
a protein-folding defect and restore ER homeostasis induces the UPR
to initiate apoptosis. Several mechanisms have been proposed that
link the distressed ER to apoptosis, including Bcl-2 family
proteins (13,14).
Numerous phytochemicals are present in many
herbal-based dietary supplements or herbal medicines, which may be
effective in clinical application as cancer suppressors (15,16).
Wogonin is one of the major flavonoids found in the root of the
Chinese herb Scutellaria baicalensis Georgi (also called
Huang-Qin), which is widely used in the treatment of a number of
diseases due to its antiviral, antibacterial, anti-inflammatory,
antioxidant and anticancer effects (17,18).
Previous studies both in vitro and in vivo have shown
that wogonin has anticancer effects in various types of cancer,
such as human colorectal (19) and
lung (20) cancer, gallbladder
carcinoma (21), breast cancer
(22), hepatocellular carcinoma
(23), osteosarcoma (24) and glioma (25,26).
Recent studies have also shown that wogonin exhibits anticancer
effects in hematologic malignancies. For example, wogonin induced
apoptosis in a human myeloma cell line by downregulating p-AKT and
overexpressing Bax (27). Wogonin
also induced cell cycle arrest and erythroid differentiation in the
imatinib-resistant K562 leukemia cell line and primary chronic
myelogenous leukemia cells (28).
In addition, wogonin reversed the multidrug resistance of human
K562/A02 myelogenous leukemia cells via inhibition of the Nrf2/ARE
signaling pathway (29). Moreover,
wogonin attenuated etoposide-induced oxidative DNA damage and
apoptosis in the bone marrow cells of mice via suppression of
oxidative DNA stress and modulation of OGG1 expression (30). These findings indicated that wogonin
is a promising chemo-protective agent and may be valuable for the
treatment of leukemia. However, the precise mechanisms involved in
the induced apoptosis of leukemia cells by wogonin remain to be
further elucidated.
The aim of the present study was to investigate the
effects of wogonin on the apoptosis of HL-60 cells. The apoptotic
mechanisms and pathways induced by wogonin were also investigated,
with particular focus on caspase-, mitochondrial- and ER
stress-dependent apoptosis. We further examined whether wogonin
induces HL-60 cell apoptosis through the PI3K-AKT signaling
pathway. Our results support the potential of wogonin as a
chemotherapeutic agent for the treatment of leukemia.
Materials and methods
Reagents and antibodies
Wogonin was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich)
as a stock solution of 100 mM. Wogonin was further diluted in
RPMI-1640 (Invitrogen, Big Cabin, OK, USA) plus 10% fetal bovine
serum (FBS; Invitrogen, Grand Island, NY, USA) to the appropriate
final concentrations. The primary polyclonal rabbit anti-human
antibodies: PI3K, phosphorylated (p)-PI3K (Tyr458), AKT, p-AKT
(Ser473), PARP-1, pancreatic ER stress kinase (PERK), eukaryotic
initiation factor 2α (eIF2α), activating transcription factor 6
(ATF6), inositol-requiring enzyme 1α (IRE1α) and β-actin were
obtained from Cell Signaling Technology (Beverly, MA, USA). The
secondary horseradish peroxidase (HRP)-labeled mouse anti-rabbit
IgG polyclonal antibodies for western blot analysis were provided
by Merck Millipore (Beijing, China). Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) were purchased from
BD Biosciences (Palo Alto, CA, USA), SH-6 was provided by Santa
Cruz Biotechnology (Dallas, TX, USA).
Construction of the AKT constructs
The adenoviral constructs were generated using a
protocol from Qbiogene’s AdEasy vector system (Carlsbad, CA, USA)
with the following modifications. The constitutively active AKT
constructs were amplified by PCR and cloned into the pShuttle-CMV
adenovirus transfer vector (BD Clontech Laboratories, Palo Alto,
CA, USA). HA-AKT was cloned into the HindIII and
EcoRV sites and was transformed into BJ5183 bacteria,
resulting in Ad-HA-AKT. All constructs were purified using Qiagen
Plasmid Maxiprep kits according to the manufacturer’s instructions.
The constructs were confirmed by restriction enzyme digestion and
sequencing analysis.
The adenoviruses were amplified by infecting human
acute promyelocytic leukemia HL-60 (ATCC® CCL-240™)
cells. Infected cells were harvested and centrifuged at 1,250 × g
at 4°C for 10 min, and the resulting cellular lysate pellet was
resuspended in Dulbecco’s modified Eagle’s medium (DMEM) without
supplements. The cells were disrupted using three freeze/thaw
cycles and the suspension was subsequently spun at 1,250 × g at 4°C
for 15 min to release the virus particles. The supernatant
containing the viral particles was spun at 100,000 × g (Beckman
SW28 rotor) for 16 h at 4°C through an isopycnic CsCl gradient. The
viral band was isolated and dialyzed against 10% glycerol in
phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 1.47 mM
KH2PO4 and 8.1 mM
Na2HPO4, pH 7.6) for 16–24 h at 4°C. The
viral particle concentration (titer) was determined by absorbance
at 260 nm. viruses were aliquoted and stored at −80°C until
used.
Cell culture and treatments
The HL-60 suspension cells were purchased from the
Peking Union Medical College Cell Library (Beijing, China). The
cells were cultured in RPMI-1640 supplemented with 100 U/ml of
penicillin, 100 μg streptomycin and 10% FBS at 37°C in a
humidified atmosphere containing 5% CO2. The media were
changed every 2–3 days and subcultured when the cell population
density reached 70–80% confluency. Cells were seeded at an
appropriate density according to each experimental design.
Assays of caspase-3, 8 and 9
activities
Cell lysates (30 μg) from the HL-60 cells
obtained after treatment with wogonin at the desired doses and time
periods, were analyzed for caspase acivity spectrophotometrically
at 405 nm using a microtiter plate reader. The assays were
performed by incubating the cell lysates with 0.2 mM of the
caspase-specific colorimetric tetrapeptide substrates,
Ac-LEHD-p-nitroaniline (pNA) (for caspase-9), Ac-IETD-pNA (for
caspase-8) or Ac-DEVD-pNA (for caspase-3) for 1 h at 37°C as
described in a previous study (31). The increase in the absorbance at 405
nm which corresponds to the amount of pNA liberated from the
peptide substrates was converted into units of enzyme activity
using a standard curve generated with free pNA. One unit of
caspase-3, 8 or 9 activity corresponded to the amount of enzyme
that will release 1 pmol of pNA from 0.2 mM DEVD-pNA, IETD-pNA or
Ac-LEHD-pNA/min, respectively. Lysates from the HL-60 cells treated
with DMSO were also used in these assays as the control group.
Cell viability assay
Cell viability was assessed using the MTT assay.
HL-60 cells were plated into 96-well clusters at a density of
5×104 cells/well. After a 24-h incubation under
group-specified experimental conditions, the clustered HL-60 cells
were processed for detection of cell viability by MTT assays. Spent
medium was removed and 10 μl MTT solution (5 mg/ml) was
added to 100 μl of respective growth medium without phenol
red, and the plates were incubated at 37°C for 4 h in a humidified
5% CO2 atmosphere. Then, the formazan crystals formed by
mitochondrial reduction of MTT were solubilized in DMSO (100
μl/well), and the absorbance was read at 540 nm using a
microplate reader (Bio-Rad, Hercules, CA, USA). Percent inhibition
of cytotoxicity was calculated as a fraction of the control group
and expressed as a percentage of cell viability.
Determination of apoptosis in the HL-60
cells
Double-staining for Annexin V-FITC and PI was
performed to estimate the apoptotic rate of the HL-60 cells.
Briefly, after incubation with various doses of wogonin for 48 h,
the HL-60 cells were trypsinized and washed twice with PBS, and
centrifuged at 800 rpm for 5 min. Then, 1×106 cells were
suspended in binding buffer and double-stained with Annexin V-FITC
and PI for 30 min at room temperature. Subsequently, the
fluorescence of each sample was quantitatively analyzed by a
FACSCalibur flow cytometer and CellQuest software. The results were
interpreted as follows: PI-positive and Annexin V-FITC-positive
stained cells were considered as apoptotic.
Quantitative reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total messenger RNA from the HL-60 cells was
isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA)
according to the manufacturer’s instructions. The amount and
quality of the RNA were determined using a NanoDrop 2000
spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
First-strand cDNA was synthesized from 300 ng of RNA with
SuperScript® III First-Strand Synthesis system (Applied
Biosystems, Foster City, CA, USA) and used as the template for PCR
with the FastStart HiFi PCR system (Roche Applied Science,
Indianapolis, IN, USA). The fold-change in gene expression was
obtained by dividing the treated group signal by that of the base
expression level signal of the corresponding gene in the untreated
cells. Results were normalized using qRT-PCR signal from β-actin of
the respective samples.
The primers of the target genes were:
glucose-regulated protein 78 (GRP78) forward, 5′-TCT GCT TGA TGT
GTG TCC TCT T-3′ and reverse, 5′-GTC GTT CAC CTT CGT AGA CCT-3′;
C/EBP-homologous protein (CHOP) forward, 5′-GGA GAA GGA GCA GGA GAA
TGA-3′ and reverse, 5′-AGA CAG ACA GGA GGT GAT GC-3′; GRP94
forward, 5′-TCG GGA AGC AAC AGA GAA GG-3′ and reverse, 5′-TCA TCT
TCC TTA ACC CTC CGC-3′; Bcl-2 forward, 5′-GCT GAG GCA GAA GGG TTA
TG-3′ and reverse, 5′-GCC CCC TTG AAA AAG TTC AT-3′; Bax forward,
5′-AGG GTT TCA TCC AGG ATC GAG CAG-3′ and reverse, 5′-ATC TTC TTC
CAG ATG GTG AGC GAG-3′; β-actin forward, 5′-GGC GGA CTA TGA CTT AGT
TG-3′ and reverse, 5′-AAA CAA CAA TGT GCA ATC AA-3′.
Western blot assay
For the western blot analysis, HL-60 cells were
harvested, washed once in ice-cold PBS, gently lysed in ice-cold
lysis buffer (250 mM sucrose, 1 mM EDTA, 0.05% digitonin, 25 mM
Tris, pH 6.8, 1 mM dithiothreitol, 1 μg/ml leupeptin, 1
μg/ml pepstatin, 1 μg/ml aprotinin, 1 mM benzamidine
and 0.1 mM phenylmethylsulphonyl fluoride) for 30 min, and
centrifuged at 12,000 rpm at 4°C. The protein concentration was
measured using the BioRad Bradford protein assay reagent, and
subjected to SDS-PAGE. Proteins were transferred to polyvinylidene
fluoride membranes and incubated successively in 5% bovine serum
albumin in Tris-buffered saline Tween-20 buffer (25 mmol/l Tris, pH
7.5, 150 mmol/l NaCl and 0.1% Tween-20) for 1 h, then incubated
overnight at 4°C with PI3K, p-PI3K (Tyr458), AKT, p-AKT (Ser473),
PARP-1, PERK, eIF2α, ATF6, IRE1α or β-actin, followed by reaction
with HRP-labeled mouse anti-rabbit IgG polyclonal antibodies for 1
h. After each incubation, the membranes were washed extensively in
TTBS and the immunoreactive band was detected using ECL-detecting
reagents.
Data analysis
Results are expressed as the mean ± SEM. Statistical
comparisons were performed using a Student’s t-test, one-way
analysis of variance (ANOVA), or two-way repeated measures ANOVA
with the Student’s t-test for post hoc analyses. In all cases,
values of P<0.05 were considered to indicate statistically
significant results.
Results
Cytotoxic effects of wogonin on the HL-60
cells
In the pre-experiment, we applied wogonin at doses
of 10, 25, 50, 75, 100 and 150 μM. The results showed that
wogonin inhibited the viability of HL-60 cells in a dose-dependent
manner (Fig. 1a) and the effect was
predominant at 75–150 μM. In addition, HL-60 cells were
treated with 75 μM wogonin for 6, 12, 24, 48, 72 and 96 h.
We found that 75 μM wogonin inhibited the viability of the
HL-60 cells in a time-dependent manner (Fig. 1b) and the effect was predominant at
48–96 h. Therefore, HL-60 cells were treated with wogonin for 48 h
in the subsequent experiments.
Wogonin induces the apoptosis of HL-60
cells
To further investigate whether wogonin induces the
apoptosis of HL-60 cells, the cells were treated with 75 μM
wogonin for 48 h and the apoptotic rate of the HL-60 cells was
detected using Annexin V-FITC/PI staining. The results revealed
that 75 μM wogonin induced apoptosis in the HL-60 cells
(Fig. 1c).
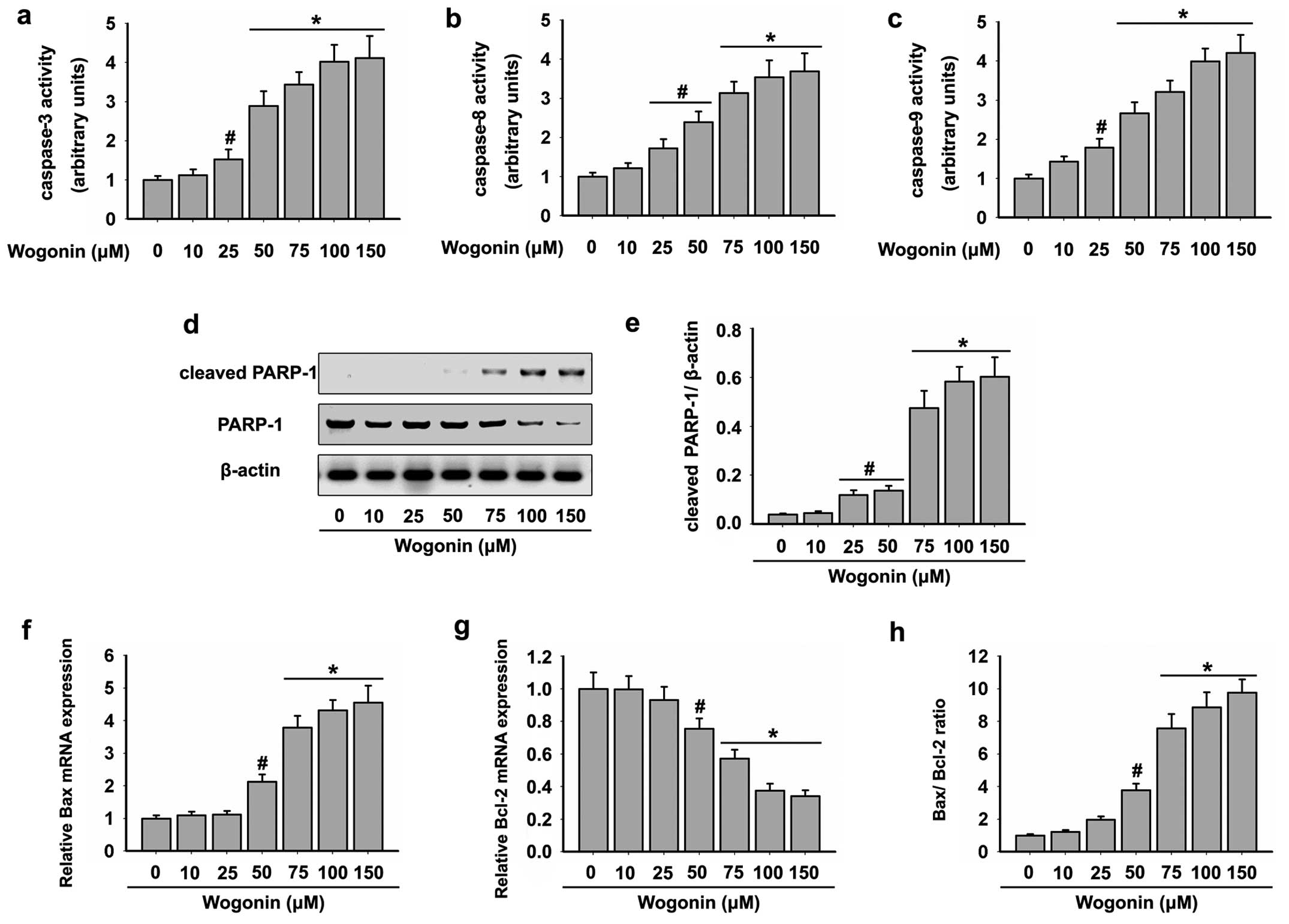
Effects of wogonin on the caspase
activity of HL-60 cells
Apoptosis induced by various cytotoxic agents is
highly dependent on the activation of caspases, which play pivotal
roles in cleaving specific target proteins (32). As shown in Fig. 1c, wogonin caused strong apoptotic
death of the HL-60 cells. Therefore, we assessed whether wogonin
activates caspase pathways in the HL-60 cells. Cells were exposed
to increasing doses of wogonin for 48 h, and then analyzed for
caspase activation by colorimetric assays. As shown in Fig. 2a-c, wogonin triggered a
dose-dependent increase in the specific activities of caspase-3, -8
and -9. A mean specific activity peak was noted after incubation of
the HL-60 cells with doses of 75–150 μM wogonin for 48
h.
Effects of wogonin on the PARP-1 activity
of HL-60 cells
We next performed western blot analysis to determine
the level of cleaved PARP-1 that induces the enhanced apoptosis.
The cleavage of PARP-1 was significantly higher in the HL-60 cells
following wogonin treatment in a dose-dependent manner. The maximum
activation of cleaved PARP-1 was detected following treatment of
wogonin at doses of 75–150 μM for 48 h (Fig. 2d and e). This result was in
accordance with the activities of caspase-3, 8 and 9. In addition,
wogonin treatment also resulted in significant reduction in total
PARP-1 levels (Fig. 2d and e).
Effects of wogonin on the mitochondrial
death pathway in HL-60 cells
The activation of caspase-9 by wogonin suggested
that the mitochondrial apoptotic pathway was triggered in the HL-60
cells. Since Bcl-2 family proteins are known to control the
mitochondrial-mediated apoptosis pathway by maintaining a balance
between pro- and anti-apoptotic members (33), we examined the effects of wogonin on
the expression levels of Bcl-2 family proteins in the HL-60 cells.
Our results showed that wogonin increased the mRNA level of
pro-apoptotic Bax (Fig. 2f), but
decreased the mRNA level of Bcl-2 (Fig.
2g) in HL-60 cells. In addition, the Bax/Bcl-2 ratio was
increased following treatment with wogonin in the HL-60 cells
(Fig. 2h). These data demonstrated
that wogonin induced HL-60 cell apoptosis through
mitochondrial-mediated mechanisms.
Effects of wogonin on the ER stress in
HL-60 cells
Treatment with wogonin has been shown to induce
accumulation of misfolded nascent glycoproteins in the ER lumen in
various types of cancer cells, leading to ER stress and UPR
activation (23,24,26,34).
The expression of ER stress markers was analyzed in the HL-60 cells
following treatment with wogonin. In the HL-60 cells, wogonin
increased the mRNA expression of GRP94 (Fig. 3a), GRP78 (Fig. 3b) and CHOP (Fig. 3c) confirming induction of ER stress,
and their increased expression was correlated with cleaved PARP-1
and activity of caspase-3, -8 and -9, markers of apoptosis.
When ER stress occurs, GRP78 is released from three
key branches: PERK, IRE1 and ATF6 and binds misfolded proteins,
thereby activating the UPR (35,36).
Next, we analyzed the expression levels of the following key UPR
signal transduction molecules: PERK, eIF2α, ATF6 and IRE1α.
Exposure of the HL-60 cells to wogonin resulted in the upregulated
expression of p-PERK, p-eIF2α and cleaved ATF6 and activation of
IRE1α (Fig. 3d-f). These data
suggested that wogonin induced HL-60 cell apoptosis through ER
stress-mediated mechanisms.
Effects of wogonin on the PI3K-AKT
activation in HL-60 cells
To further understand the molecular mechanisms by
which wogonin induces HL-60 cell apoptosis, we examined the
expression of PI3K-AKT, critical signaling proteins associated with
cell apoptosis. The levels of p-PI3K and p-AKT were detected, and
the results demonstrated that wogonin significantly blocked the
constitutive phosphorylation of PI3K at Tyr458 and AKT at Ser473 in
a dose-dependent manner (Fig.
4a).
To determine the role of PI3K-AKT in wogonin-induced
apoptosis and ER stress in HL-60 cells, constitutively active
HA-tagged AKT constructs were made in AKT at Ser473, which were
mutated to an aspartic acid residue to mimic phosphorylated AKT.
The expression of HA-AKT significantly increased the phosphorylated
AKT activity (Fig. 4b) after
wogonin treatment. Active AKT significantly increased the cell
viability (Fig. 5a) and reduced the
percentage of HL-60 apoptotic cells (Fig. 5b) when compared with the cells in
the wogonin only treatment group. Moreover, active AKT also reduced
wogonin-induced caspase-3, -8, -9 (Fig.
5c) and PARP-1 (Fig. 5d)
activities and the Bax/Bcl-2 ratio (Fig. 5e-g) when compared with cells in the
wogonin only treatment group. In addition, upregulated expression
of p-PERK, p-eIF2α and cleaved ATF6 and activation of IRE1α induced
by wogonin were reduced in the HL-60 cells expressing active AKT
(Fig. 6a-c). These results
indicated that the pro-apoptotic effects of wogonin in HL-60 cells
were associated with inhibition of the activation of the PI3K/AKT
signaling cascade.
Discussion
Wogonin, one of the active components extracted from
Scutellariae radix, exhibits antioxidant, anti-inflammatory
and antitumor activities. Wogonin has been shown to induce
anti-proliferation, cell cycle arrest and differentiation in
hematologic malignancies. Our study presented data showing that
wogonin induced a cytotoxic effect and apoptosis in HL-60 cells.
Activation of caspase-8 and -9 are key events in the extrinsic and
intrinsic pathways of apoptosis, respectively, while the activation
of caspase-3 and PARP-1 is a critical event in both apoptotic
pathways. Accordingly, caspases-3, -8, -9 and PARP-1 are the main
markers for the activation of apoptosis (6,7).
Furthermore, the effect of wogonin on the activation of caspase-3,
-8, -9 and PARP-1 was investigated to attain the precise mechanism
for the induction of apoptosis by wogonin in HL-60 cells. We found
that the induction in activation of caspase-3, -8, -9 and PARP-1 by
wogonin might be a reason for the pro-apoptotic effects in HL-60
cells.
Previous studies have shown that wogonin triggers
the apoptosis of human osteosarcoma (25), hepatocellular carcinoma (23) and glioma cancer cells (26) through the ER stress-dependent
signaling pathways. Moreover, wogonin elicits a potent antitumor
immune effect in human and mouse gastric carcinoma cells through an
ER stress/AKT dependent manner (34). In addition, the apoptosis of cancer
cells can be induced via ER stress. ER stress occurs when ER
homeostasis is lost due to an overload of protein folding in the ER
(11). ER stress triggers an
evolutionarily conserved response termed the UPR (37). The UPR is mediated by three
ER-resident transmembrane proteins that sense ER stress and signal
downstream pathways. These proximal sensors include IRE1α, PERK and
ATF6 (38). Here, we observed that
wogonin upregulated the expression of p-PERK, p-eIF2α, cleaved ATF6
and activation of IRE1α in a dose-dependent manner in HL-60 cells.
It is well known that GRP78 plays a critical cytoprotective role
against ER stress (39-41). In non-stressed mammalian cells,
GRP78 constitutively binds to ATF6, PERK and IRE1 and maintains
them in an inactive status. Upon ER stress, sequestration of GRP78
by unfolded proteins activates these sensors and initiates the UPR
(39,40). The increased mRNA expression levels
of GRP78 and GRP94 were detected in HL-60 cells after treatment
with wogonin. Following GRP78 and GRP94 induction, ER stress
signals lead to activation of CHOP which has been reported to
sensitize cells to apoptosis (38-40).
In our present study, wogonin treatment was found to elevate
expression of CHOP mRNA, which might be another reason for the
pro-apoptotic effects noted in the HL-60 cells.
Bcl-2 family proteins also localize upon ER stress
where their proposed functions include regulation of apoptosis and
the UPR (13,14). The differential effect of the UPR on
cell survival or death has been attributed to the levels of pro- or
anti-apoptotic Bcl-2 family members upon ER stress (13,14).
Anti-apoptotic Bcl-2 family members possess a hydrophobic groove
that binds and inhibits their pro-apoptotic counterparts, which
forms the basis of resistance to chemotherapy (42). To overcome this resistance and
facilitate cell death, small-molecule inhibitors of the Bcl-2
family, aimed at dislodging the pro-apoptotic members from the
hydrophobic groove, have been developed (43,44).
In the present study, we demonstrated that wogonin-induced
apoptosis in HL-60 cells was associated with increased expression
of the pro-apoptotic protein Bax and decreased expression of the
anti-apoptotic protein Bcl-2. Wogonin was found to induce an
increase in the Bax to Bcl-2 ratio, and increased expression of
cleaved caspase-3 and 9 has been demonstrated in human breast
cancer and myeloma cells (27,45).
In the present study, we obtained similar results. Therefore, it is
reasonable to conclude that wogonin-induced apoptosis of HL-60
cells is mediated by the mitochondrial pathway through modulation
of the Bax to Bcl-2 ratio.
The PI3K/AKT signaling pathway is pivotal in
transmitting signals from membrane receptors to downstream targets
that regulate critical cellular responses, such as proliferation,
apoptosis, differentiation and senescence (46). Previous evidence indicates that the
PI3K/AKT signaling pathway plays critical roles in controlling cell
survival by suppressing ER stress-induced cell death (47). The important functions of the
PI3K/AKT signaling pathway in apoptosis regulation have been
extensively studied. Recent studies found that wogonin induced
apoptosis via the PI3K/AKT pathway in HT-29 human colorectal cancer
cells (48) and in a human myeloma
cell line (27). Therefore, we
hypothesized that the PI3K/AKT signaling pathway plays various
roles in the anti-apoptotic effect of woginin in HL-60 cells. Our
data revealed that wogonin downregulated the PI3K/AKT signaling
pathway, and activation of AKT induced by adenoviral vectors
inhibited the pro-apoptotic effects and ER stress induced by
wogonin in the HL-60 cells.
In conclusion, the present study demonstrated that
wogonin acted as a strong and selective inducer of apoptosis in the
HL-60 cells. Obviously, caspase-, mitochondrial- and ER
stress-dependent events are the determinant factors in
wogonin-induced cell death. In addition, wogonin downregulated and
inactivated the PI3K/AKT signaling pathway, which may have a
critical function in wogonin-induced apoptosis in HL-60 cells.
Therefore, wogonin is a potential chemotherapeutic agent for the
treatment of human leukemia.
References
|
1
|
Canadian Cancer Society/National Cancer
Institute of Canada: Canadian Cancer Statistics. Toronto, ON: ISSN:
0835-29762005
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nau KC and Lewis WD: Multiple myeloma:
diagnosis and treatment. Am Fam Physician. 78:853–859.
2008.PubMed/NCBI
|
|
4
|
Fotoohi AK, Assaraf YG, Moshfegh A,
Hashemi J, Jansen G, Peters GJ, Larsson C and Albertioni F: Gene
expression profiling of leukemia T-cells resistant to methotrexate
and 7-hydroxy-methotrexate reveals alterations that preserve
intracellular levels of folate and nucleotide biosynthesis. Biochem
Pharmacol. 77:1410–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelloff GJ, Crowell JA, Steele VE, Lubet
RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R,
Lawrence JA, et al: Progress in cancer chemoprevention: development
of diet-derived chemopreventive agents. J Nutr. 130(Suppl):
467S–471S. 2000.PubMed/NCBI
|
|
6
|
Chiantore MV, Vannucchi S, Mangino G,
Percario ZA, Affabris E, Fiorucci G and Romeo G: Senescence and
cell death pathways and their role in cancer therapeutic outcome.
Curr Med Chem. 16:287–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meier P and Vousden KH: Lucifer’s
labyrinth-ten years of path finding in cell death. Mol Cell.
28:746–754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lavrik IN, Golks A and Krammer PH:
Caspases: pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadowaki H, Nishitoh H and Ichijo H:
Survival and apoptosis signals in ER stress: The role of protein
kinases. J Chem Neuroanat. 28:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
11
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oakes SA, Lin SS and Bassik MC: The
control of endoplasmic reticulum-initiated apoptosis by the BCL-2
family of proteins. Curr Mol Med. 6:99–109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rong Y and Distelhorst CW: Bcl-2 protein
family members: versatile regulators of calcium signaling in cell
survival and apoptosis. Annu Rev Physiol. 70:73–91. 2008.
View Article : Google Scholar
|
|
15
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phyto-chemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dumontet C and Jordan MA:
Microtubule-binding agents: a dynamic field of cancer therapeutics.
Nat Rev Drug Discov. 9:790–803. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gasiorowski K, Lamer-Zarawska E, Leszek J,
Parvathaneni K, Yendluri BB, Błach-Olszewska Z and Aliev G:
Flavones from root of Scutellaria baicalensis Georgi: drugs of the
future in neurodegeneration? CNS Neurol Disord Drug Targets.
10:184–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li-Weber M: New therapeutic aspects of
flavones: the anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
19
|
He L, Lu N, Dai Q, Zhao Y, Zhao L, Wang H,
Li Z, You Q and Guo Q: Wogonin induced G1 cell cycle arrest by
regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in
human colorectal cancer carcinoma cells. Toxicology. 312:36–47.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Morgan WA, Sanchez-Medina A and
Corcoran O: The ethanol extract of Scutellaria baicalensis and the
active compounds induce cell cycle arrest and apoptosis including
upregulation of p53 and Bax in human lung cancer cells. Toxicol
Appl Pharmacol. 254:221–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong P, Zhang Y, Gu J, Wu W, Li M, Yang J,
Zhang L, Lu J, Mu J, Chen L, et al: Wogonin, an active ingredient
of Chinese herb medicine Scutellaria baicalensis, inhibits the
mobility and invasion of human gallbladder carcinoma GBC-SD cells
by inducing the expression of maspin. J Ethnopharmacol.
137:1373–1380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang KF, Zhang GD, Huang YQ and Diao Y:
Wogonin induces apoptosis and down-regulates survivin in human
breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int
Immunopharmacol. 12:334–341. 2012. View Article : Google Scholar
|
|
23
|
Xu M, Lu N, Zhang H, Dai Q, Wei L, Li Z,
You Q and Guo Q: Wogonin induced cytotoxicity in human
hepatocellular carcinoma cells by activation of unfolded protein
response and inactivation of AKT. Hepatol Res. 43:890–905. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CC, Kuo CL, Lee MH, Lai KC, Lin JP,
Yang JS, Yu CS, Lu CC, Chiang JH, Chueh FS, et al: Wogonin triggers
apoptosis in human osteosarcoma U-2 OS cells through the
endoplasmic reticulum stress, mitochondrial dysfunction and
caspase-3-dependent signaling pathways. Int J Oncol. 39:217–224.
2011.PubMed/NCBI
|
|
25
|
Lee DH, Lee TH, Jung CH and Kim YH:
Wogonin induces apoptosis by activating the AMPK and p53 signaling
pathways in human glioblastoma cells. Cell Signal. 24:2216–2225.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai CF, Yeh WL, Huang SM, Tan TW and Lu
DY: Wogonin induces reactive oxygen species production and cell
apoptosis in human glioma cancer cells. Int J Mol Sci.
13:9877–9892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang M, Liu LP, Chen Y, Tian XY, Qin J,
Wang D, Li Z and Mo SL: Wogonin induces apoptosis in RPMI 8226, a
human myeloma cell line, by downregulating phospho-Akt and
overexpressing Bax. Life Sci. 92:55–62. 2013. View Article : Google Scholar
|
|
28
|
Yang H, Hui H, Wang Q, Li H, Zhao K, Zhou
Y, Zhu Y, Wang X, You Q, Guo Q, et al: Wogonin induces cell cycle
arrest and erythroid differentiation in imatinib-resistant K562
cells and primary CML cells. Oncotarget. 5:8188–8201.
2014.PubMed/NCBI
|
|
29
|
Xu X, Zhang Y, Li W, Miao H, Zhang H, Zhou
Y, Li Z, You Q, Zhao L and Guo Q: Wogonin reverses multi-drug
resistance of human myelogenous leukemia K562/A02 cells via
downregulation of MRP1 expression by inhibiting Nrf2/ARE signaling
pathway. Biochem Pharmacol. 92:220–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Attia SM, Ahmad SF, Harisa GI, Mansour AM,
El Sayed SM and Bakheet SA: Wogonin attenuates etoposide-induced
oxidative DNA damage and apoptosis via suppression of oxidative DNA
stress and modulation of OGG1 expression. Food Chem Toxicol.
59:724–730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masoud L, Vijayasarathy C,
Fernandez-Cabezudo M, Petroianu G and Saleh AM: Effect of malathion
on apoptosis of murine L929 fibroblasts: a possible mechanism for
toxicity in low dose exposure. Toxicology. 185:89–102. 2003.
View Article : Google Scholar
|
|
32
|
Alnemri ES: Mammalian cell death
proteases: a family of highly conserved aspartate specific cysteine
proteases. J Cell Biochem. 64:33–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Li XJ, Chen Z, Zhu XX, Wang J,
Zhang LB, Qiang L, Ma YJ, Li ZY, Guo QL, et al: Wogonin induced
calreticulin/annexin A1 exposure dictates the immunogenicity of
cancer cells in a PERK/AKT dependent manner. PLoS One.
7:e508112012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lindholm D, Wootz H and Korhonen L: ER
stress and neurode-generative diseases. Cell Death Differ.
13:385–392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Liu CY, Back SH, Clark RL, Peisach
D, Xu Z and Kaufman RJ: The crystal structure of human IRE1 luminal
domain reveals a conserved dimerization interface required for
activation of the unfolded protein response. Proc Natl Acad Sci
USA. 103:14343–14348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Winnay JN and Kahn CR: PI 3-kinase
regulatory subunits as regulators of the unfolded protein response.
Methods Enzymol. 490:147–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schröder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar
|
|
40
|
Pfaffenbach KT and Lee AS: The critical
role of GRP78 in physiologic and pathologic stress. Curr Opin Cell
Biol. 23:150–156. 2011. View Article : Google Scholar :
|
|
41
|
Li J, Ni M, Lee B, Barron E, Hinton DR and
Lee AS: The unfolded protein response regulator GRP78/BiP is
required for endoplasmic reticulum integrity and stress-induced
autophagy in mammalian cells. Cell Death Differ. 15:1460–1471.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
43
|
Lessene G, Czabotar PE and Colman PM:
BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov.
7:989–1000. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vogler M, Dinsdale D, Dyer MJ and Cohen
GM: Bcl-2 inhibitors: small molecules with a big impact on cancer
therapy. Cell Death Differ. 16:360–367. 2009. View Article : Google Scholar
|
|
45
|
Chung H, Jung YM, Shin DH, Lee JY, Oh MY,
Kim HJ, Jang KS, Jeon SJ, Son KH and Kong G: Anticancer effects of
wogonin in both estrogen receptor-positive and -negative human
breast cancer cell lines in vitro and in nude mice xenografts. Int
J Cancer. 122:816–822. 2008. View Article : Google Scholar
|
|
46
|
McCubrey JA, Lee JT, Steelman LS, Blalock
WL, Moye PW, Chang F, Pearce M, Shelton JG, White MK, Franklin RA,
et al: Interactions between the PI3K and Raf signaling pathways can
result in the transformation of hematopoietic cells. Cancer Detect
Prev. 25:375–393. 2001.PubMed/NCBI
|
|
47
|
Hu P, Han Z, Couvillon AD and Exton JH:
Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in
counteracting endoplasmic reticulum stress-induced cell death. J
Biol Chem. 279:49420–49429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD,
Choi CS, Nam JS and Jung JY: Antitumor actions of baicalein and
wogonin in HT-29 human colorectal cancer cells. Mol Med Rep.
6:1443–1449. 2012.PubMed/NCBI
|