Introduction
The multiple myeloma cell is a neoplastic
plasma-cell disorder that is characterized by clonal proliferation
of malignant plasma cells in the bone marrow microenvironment. This
disorder causes monoclonal protein proliferation in the blood or
urine and it is associated with organ dysfunction (1). It accounts for ~1% of neoplastic
diseases and ~10% of hematologic cancers. The median age of
diagnosis is ~70 years (2). The
recent introduction of autologous stem-cell transplantation and the
availability of agents such as thalidomide, lenalidomide and
bortezomib have changed the management of myeloma (3,4).
Although overall survival has increased, the disease is not
curable.
Chemokines are low molecular weight cytokines that
are specialized for recruiting leukocyte to inflammatory sites and
for correctly positioning lymphocytes in secondary lymphoid organs
(5). Chemokines are also involved
in different pathological processes including the growth and
dissemination of solid tumors and hematological malignancies
(6,7). For example, several
chemokines/chemokine receptors are associated with multiple myeloma
activity. The migration of myeloma cells to the bone marrows is
mediated by CXCR4, which is highly expressed in myeloma cells, and
by its ligand CXCL12 which is produced by stromal cells (8). CCR1, the receptor for CCL3/MIP-1α, is
involved in osteolytic bone diseases and is highly expressed in
myeloma patients (9).
CX3CL1 (also known as fractalkine) is a chemokine
constitutively expressed in many hematopoietic and
non-hematopoietic tissues. It is synthesized as a membrane-bound
protein, but can also be released by proteolytic cleavage (10,11).
Membrane-bound CX3CL1 functions as an adhesion molecule, whereas
the secreted form triggers chemotaxis of lymphocytes and monocytes
to inflammatory sites (10,12). The receptor for CX3CL1, CX3C
chemokine receptor 1 (CX3CR1), is expressed on human NK cells,
monocytes, T lymphocytes and mast cells (10,13).
Previous studies have shown that CX3CR1 expression
is upregulated in solid tumors such as in breasts or prostate
(14,15), while pancreatic adenocarcinoma
models (16) have shown that CX3CR1
is involved in the metastatic spread of tumor cells to specific
tissues expressing CX3CL1. Several studies of CX3CR1 have been
performed with different types of B cell lymphoma (17) and chronic lymphocytic leukemia (CLL)
(18) in hematological
malignancies. However, the expression of CX3CR1 has not been
investigated in multiple myeloma, since CX3CR1 expression has not
been confirmed in B cell lineages from pro B cells to plasma cells
(19). Therefore, the functional
role of CX3CR1 in multiple myeloma remains unclear. The present
study, which was an investigation of the role of the CX3CL1/CX3CR1
axis in multiple myeloma, indicates that this axis is involved in
the interaction between the tumor cells and their bone
microenvironment.
Materials and methods
Reagents and antibodies
Recombinant human CX3CL1 and anti-human CX3CL1 were
purchased from R&D Systems (Minneapolis, MN, USA). The
following mAbs were used: anti-AKT1 (C-20), anti-ERK1/2 (C-16),
anti-PCNA (PC-10) and anti-NFkbp65 (C-20) from Santa Cruz
biotechnology (Santa Cruz, CA, USA), and anti-phospho AKT
(Ser-473), anti-phospho p44/42 ERK (Thr-202/Tyr-204), anti-STAT3
and anti-phospho STAT3 (Ser727) from Cell Signaling Technology
(Beverly, MA, USA).
Cells
Human multiple myeloma cell lines included
RPMI-8226, KMS-12BM, KMS-12PE, L-363, OPM-2, KARPAS-620 and AMO-1
cells. All the cell lines were maintained in RPMI-1640 medium (Wako
Pure Chemical Industries, Inc., Osaka, Japan) supplemented with 20%
fetal bovine serum, 50 μM 2-mercaptoethanol (both from
Invitrogen, Carlsbad, CA, USA), 100 u/ml penicillin and 100
μg/ml streptomycin (both from Meiji Seika Pharma, Tokyo,
Japan). The cells were cultured at 37°C in an incubator with a
humidified 5% CO2 atmosphere.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RNA was extracted using an RNeasy Plus Mini kit
(Qiagen, Hilden, Germany) and converted to cDNA using the
SuperScript III First-Strand Synthesis System for RT-PCR (Life
Technologies Corporation, Carlsbad, CA, USA). The expression of
CX3CR1 was determined by RT-PCR. The PCR was performed for 30
cycles (denaturation, 98°C 5 sec; annealing, 60°C 5 sec; extension,
72°C 10 sec) using Sapphire Amp Fast PCR Master Mix (Takara Bio,
Shiga, Japan) The PCR products were subjected to electrophoresis on
a 1.5% agarose gel with SYBR-Green (Lonza Inc., Rockland, ME, USA)
and then photographed under an ultraviolet transilluminator. GAPDH
was used as a normalization control. The primers for GAPDH were as
follows: sense, 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′ and
antisense, 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′. The primers for
CX3CR1 were as follows: sense, 5′-TGG CCT TGT CTG ATC TGC TGT
TTG-3′ and antisense, 5′-ATG GCT TTG GCT TTC TTG TGG TTC-3′. The
RNA from healthy donor plasma cell was purchased from AllCells
(Alameda, CA, USA).
Western blotting
The western blot analysis was performed following
incubation with or without 10 nM rCX3CL1. Antibodies to
phosphorylated (p) or unphosphorylated JAK, Stat, Akt and Erk1/2
were used. Images of immunoblots were scanned and quantified with
an ImageQuant LAS 4000 Lumino Image Analyzer from Fuji Film
Corporation (Tokyo, Japan).
Adhesion assay
Triplicate wells of 96-well plates were coated with
1 μg fibronectin (Asahi Techno Glass, Co., Ltd., Tokyo,
Japan) and vascular cell adhesion molecule-1 (VCAM-1) (R&D
Systems). RPMI-8226 cells were stimulated by recombinant human
CX3CL1 (10 nM) for 5 min. After stimulation, RPMI-8226 cells
(1×104 cells/100 μl in EMEM with 0.1% BSA) were
seeded and incubated for 20 min at 37°C. Cells that adhered to the
well were evaluated as previously described (20).
Osteoclast differentiation
RPMI-8226 cells were cultured in the absence or
presence of recombinant human CX3CL1 (10 nM) for 48 h, and then the
conditioned cell culture medium was collected. Osteoclast
precursors (RAW 264.7) were suspended in a-MEM supplemented with
10% FBS and cultured in a 24-well culture plates at
1x106/well. After 48 h, the culture medium was replaced
with 50% conditioned medium with or without 100 ng/ml of mouse
recombinant soluble RANKL (Wako Pure Chemical Industries, Inc.).
After 4 days, the cells were dehydrated with ethanol-acetone (1:1)
for 1 min, dried and stained at room temperature with
tartrate-resistant acid phosphatase (TRAP) staining solution.
TRAP-positive cells appeared dark red. We counted TRAP-positive
multi-nucleated cells containing three or more nuclei as
osteoclasts.
Statistical analysis
Data were analyzed for statistical significance
using the Student’s t-test. P<0.05 was considered to indicate a
statistically significant result. The mean and SD were calculated
for all variables.
Results
CX3CR1 expression in multiple
myeloma
We first compared the expression of CX3CR1 in seven
human multiple myeloma cell lines and plasma cells (Cd138-positive
cell fraction) by RT-PCR (Fig. 1).
CX3CR1 expression was detected in three (RPMI-8226, OPM-2 and
KARPAS-620) of the seven human multiple myelomas, even though
CX3CR1 was not expressed in the plasma cells that derived from
healthy donors. CX3CR1 expression was apparently induced during the
process of malignant transformation of normal plasma cells to
multiple myeloma.
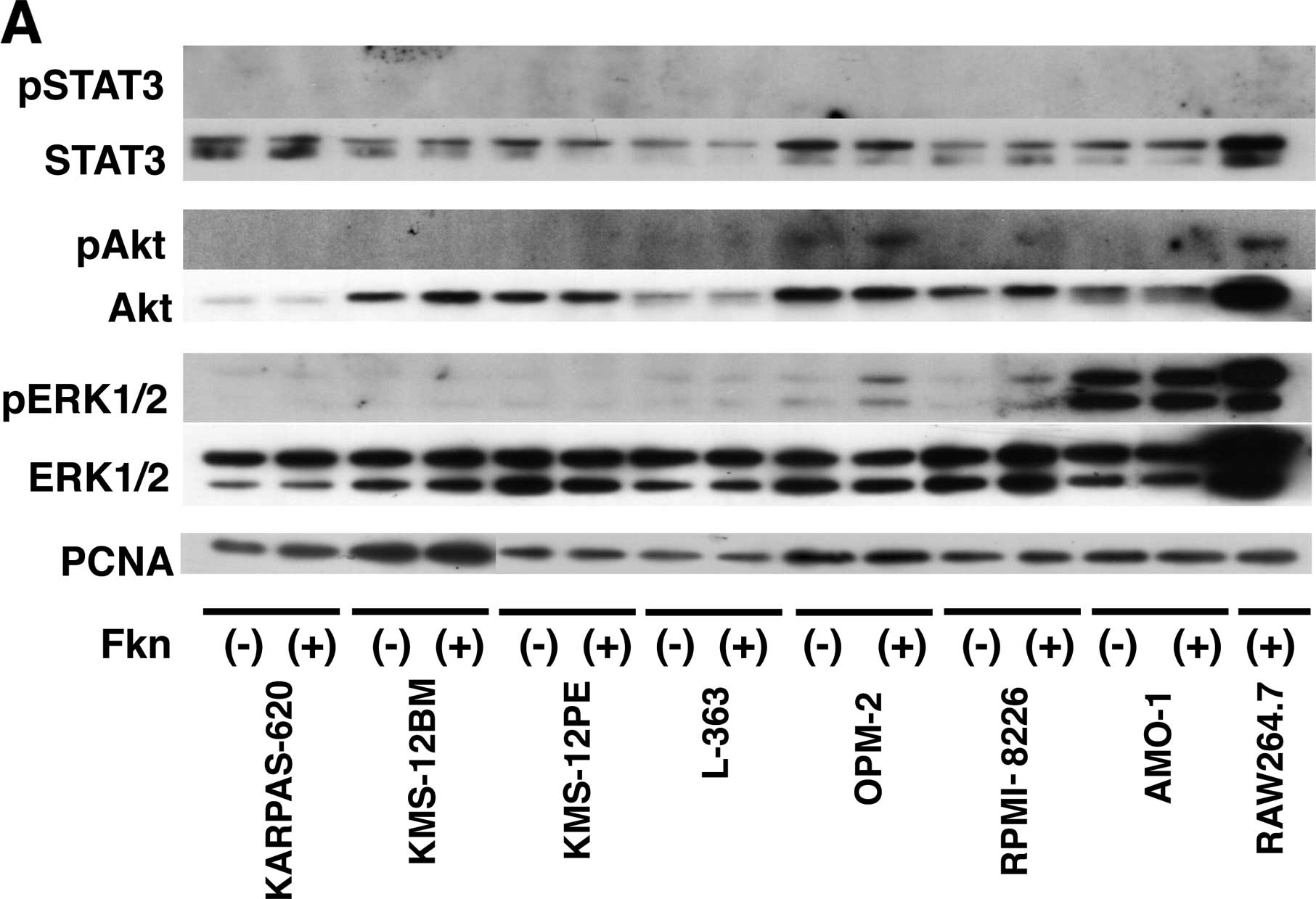
Activation of Akt and ERK signaling by
CX3CL1 in multiple myeloma
The observation of the CX3CR1 expression in human
multiple myeloma cells prompted us to examine the biological
responses of these cells to CX3CL1. The mechanisms underlying the
differences in CX3CL1-induced progression and cell survival were
investigated by analyzing signal transduction. Seven multiple
myeloma cell lines were incubated with or without recombinant
CX3CL1 and were subjected to western blotting using antibodies
against unphosphorylated and phosphorylated Akt, Erk1, Erk2 and
STAT3. Akt, Erk1 and Erk2, but not STAT3, were constitutively
phosphorylated in the two cell lines (Fig. 2A). Incubation of RPMI-8226 cells
with CX3CL1-induced p-Erk1/2 expression after 1 min; this
expression peaked from 2 to 5 min, while p-Akt expression peaked
after 2 min (Fig. 2B). Next, the
RPMI-8226 cells were stimulated with the antibody for 2 min. We
also observed that CX3CL1-induced activation of Akt and ERK in
RPMI-8226 cells was inhibited selectively by an anti-CX3CL1
antibody (Fig. 3). These results
indicate a rapid CX3CL1-driven signaling for progression and cell
survival after stimulation of CX3CL1 in multiple myeloma.
Increased adhesion to the extracellular
matrix (ECM) by CX3CL1 in RPMI-8226
We next examined whether CX3CL1 regulates cell
adhesion of human multiple myeloma. The number of RPMI-8226 cells
adhering to fibronectin and VCAM-1 increased by ~17- and 3-fold
respectively, in response to pretreatment with recombinant CX3CL1
(Fig. 4). The adhesion of multiple
myeloma cells to fibronectin and VCAM-1, which are mainly expressed
in bone marrow stromal cells, activates many pathways and results
in the upregulation of the cell cycle regulating proteins and
anti-apoptotic proteins (9,21). These results suggest that
CX3CL1-induced the progression of multiple myeloma in bone
microenvironments.
Induction of osteoclast differentiation
by multiple myeloma via CX3CL1
We previously reported that CX3CL1 expressed by
osteoblasts plays an important role in osteoclast differentiation,
possibly acting through its dual functions as a chemotactic factor
and adhesion molecule for osteoclast precursors expressing CX3CR1
(22,23).
Given the apparent expression of CX3CR1 by multiple
myeloma cells, along with the inducible effect of its ligand CX3CL1
on multiple myeloma cell adhesion to bone microenvironment ECM. We
also investigated the possible synergy between multiple myeloma and
osteoclast precursors in osteoclast differentiation via CX3CL1.
Osteoclast precursors, RAW 264.7 were differentiated by RANKL in
conditioned medium collected from multiple myeloma cells stimulated
by CX3CL1. After 4 days, TRAP-positive multinuclear osteoclasts
were counted (Fig. 5). The
conditioned medium increased the number of TRAP-positive
multinuclear osteoclasts, while treatment with rat anti-CX3CL1 mAb
suppressed the induction of TRAP-positive multinuclear osteoclasts.
These results suggest that CX3CL1 indirectly induces osteoclast
differentiation by promoting the secretion of a factor from
multiple myeloma in bone microenvironments.
Discussion
In the development of multiple myeloma, several
cytokines, such as IL-6, IGF-1, VEGF and TNF-α directly promote
cell survival and angiogenesis. JAK-STAT and IL-6 in particular,
are believed to play a central role in cell survival and disease
progression in multiple myeloma (24). Each chemokine and its receptor forms
an axis that promotes cancer progression via effects on cell
survival and angiogenesis (25).
The role of the CX3CL1/CX3CR1 axis in the interaction between tumor
cells and their microenvironment has been examined in non-Hodgkin
lymphoma (17) and CLL (18), but not in multiple myeloma. In the
present study, we confirmed expression of the chemokine receptor
CX3CR1 in the multiple myeloma cell lines. No CX3CR1 was expressed
in the plasma cells that derived from healthy donors (19).
We therefore investigated whether the chemokine
CX3CL1 and its ligand CX3CR1 may be associated with cell survival
and disease progression. As shown in Figs. 1Figure 23, rapid phosphorylation of Akt and ERK1/2,
which is related to signaling for survival and progression, as well
as JAK-STAT, was observed following chemokine CX3CL1 treatment.
These results may indicate that CX3CR1-positive myeloma cells have
advantages regarding survival and progression.
However, the reason why multiple myeloma upregulates
CX3CR1, but not in plasma cells from healthy donors, remains
unclear. Therefore, further studies are needed to clarify the
regulation of CX3CR1 expression by analysis of transcriptional
factors (26) and chromosomal
translocation related to multiple myeloma progression (27). The CX3CL1/CX3CR1 axis has a known
association with several diseases caused by abnormal inflammation,
such as rheumatoid arthritis (28).
In fact, an animal model of CIA (collagen induced arthritis) showed
a dramatic improvement following the administration of the
anti-CX3CL1 antibody. In this case, inflammatory cells permeated
into the synovium and bone destruction were controlled (29).
The progression of multiple myeloma requires that
the cells adhere to the extracellular matrix components such as
fibronectin and VCAM-1 in the bone marrow. Many signaling pathways
are activated when multiple myeloma adheres to ECM, resulting in
upregulation of cell cycle regulating and anti-apoptotic proteins
(9,21). In the present study, we showed that
the CX3CL1/CX3CR1 axis aids in the co-operation between multiple
myeloma and osteoclast cells. Treatment with CX3CL1-induced
adhesion of multiple myeloma cells to bone ECM and also induced
osteoclast differentiation by a secretion factor produced by
multiple myeloma cells (Figs. 4 and
5). We previously reported that the
CX3CL1/CX3CR1 axis also plays an important role in osteoclast
differentiation. Osteoclast precursors selectively expressed
CX3CR1, whereas CX3CL1 was expressed by osteoblasts (22,23).
The demonstration of CX3CR1 expression in multiple myeloma and
CX3CL1 in osteoclast precursors observed in the previous, and the
present study strongly indicates that the CX3CL1/CX3CR1 axis may be
an attractive therapeutic target for prevention of the progression
of multiple myeloma in bone microenvironments. Future studies
should be aimed at investigating the blocking of this axis as a
means of inhibiting myeloma progression, as well as suppressing the
adverse skeletal-related events common in multiple myeloma.
Acknowledgments
We would like to thank Ms. Toyomi Kozawa and Mr.
Yoshihiro Kuwabara for their technical assistance. The present
study was supported by a Grant-in-Aid for Scientific Research (C)
(no. 22501042).
References
|
1
|
Kyle RA and Rajkumar SV: Multiple myeloma.
N Engl J Med. 351:1860–1873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kristinsson SY, Landgren O, Dickman PW,
Derolf AR and Björkholm M: Patterns of survival in multiple
myeloma: A population-based study of patients diagnosed in Sweden
from 1973 to 2003. J Clin Oncol. 25:1993–1999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner H, Gondos A and Pulte D: Recent
major improvement in long-term survival of younger patients with
multiple myeloma. Blood. 111:2521–2526. 2008. View Article : Google Scholar
|
|
4
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar
|
|
5
|
Baggiolini M: Chemokines and leukocyte
traffic. Nature. 392:565–568. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raffaghello L, Cocco C, Corrias MV,
Airoldi I and Pistoia V: Chemokines in neuroectodermal tumour
progression and metastasis. Semin Cancer Biol. 19:97–102. 2009.
View Article : Google Scholar
|
|
7
|
Pistoia V, Corcione A, Dallegri F and
Ottonello L: Lymphoproliferative disorders and chemokines. Curr
Drug Targets. 7:81–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trentin L, Miorin M, Facco M, Baesso I,
Carraro S, Cabrelle A, Maschio N, Bortoli M, Binotto G, Piazza F,
et al: Multiple myeloma plasma cells show different chemokine
receptor profiles at sites of disease activity. Br J Haematol.
138:594–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vallet S, Pozzi S, Patel K, Vaghela N,
Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT,
et al: A novel role for CCL3 (MIP-1α) in myeloma-induced bone
disease via osteocalcin downregulation and inhibition of osteoblast
function. Leukemia. 25:1174–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bazan JF, Bacon KB, Hardiman G, Wang W,
Soo K, Rossi D, Greaves DR, Zlotnik A and Schall TJ: A new class of
membrane-bound chemokine with a CX3C motif. Nature.
385:640–644. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umehara H, Bloom ET, Okazaki T, Nagano Y,
Yoshie O and Imai T: Fractalkine in vascular biology: From basic
research to clinical disease. Arterioscler Thromb Vasc Biol.
24:34–40. 2004. View Article : Google Scholar
|
|
12
|
Fong AM, Robinson LA, Steeber DA, Tedder
TF, Yoshie O, Imai T and Patel DD: Fractalkine and
CX3CR1 mediate a novel mechanism of leukocyte capture,
firm adhesion, and activation under physiologic flow. J Exp Med.
188:1413–1419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imai T, Hieshima K, Haskell C, Baba M,
Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ,
et al: Identification and molecular characterization of fractalkine
receptor CX3CR1, which mediates both leukocyte migration
and adhesion. Cell. 91:521–530. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shulby SA, Dolloff NG, Stearns ME, Meucci
O and Fatatis A: CX3CR1-fractalkine expression regulates cellular
mechanisms involved in adhesion, migration, and survival of human
prostate cancer cells. Cancer Res. 64:4693–4698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andre F, Cabioglu N, Assi H, Sabourin JC,
Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C,
et al: Expression of chemokine receptors predicts the site of
metastatic relapse in patients with axillary node positive primary
breast cancer. Ann Oncol. 17:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marchesi F, Piemonti L, Fedele G, Destro
A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi
P, et al: The chemokine receptor CX3CR1 is involved in the neural
tropism and malignant behavior of pancreatic ductal adenocarcinoma.
Cancer Res. 68:9060–9069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andréasson U, Ek S, Merz H, Rosenquist R,
Andersen N, Jerkeman M, Dictor M and Borrebaeck CA: B cell
lymphomas express CX3CR1 a non-B cell lineage adhesion
molecule. Cancer Lett. 259:138–145. 2008. View Article : Google Scholar
|
|
18
|
Ferrer A, Ollila J, Tobin G, Nagy B,
Thunberg U, Aalto Y, Vihinen M, Vilpo J, Rosenquist R and Knuutila
S: Different gene expression in immunoglobulin-mutated and
immunoglobulin-unmutated forms of chronic lymphocytic leukemia.
Cancer Genet Cytogenet. 153:69–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakayama T, Hieshima K, Izawa D, Tatsumi
Y, Kanamaru A and Yoshie O: Cutting edge: Profile of chemokine
receptor expression on human plasma cells accounts for their
efficient recruitment to target tissues. J Immunol. 170:1136–1140.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hojo S, Koizumi K, Tsuneyama K, Arita Y,
Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H,
et al: High-level expression of chemokine CXCL16 by tumor cells
correlates with a good prognosis and increased tumor-infiltrating
lymphocytes in colorectal cancer. Cancer Res. 67:4725–4731. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manier S, Sacco A, Leleu X, Ghobrial IM
and Roccaro AM: bone marrow microenvironment in multiple myeloma
progression. J Biomed Biotechnol. 2012:1574962012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saitoh Y, Koizumi K, Sakurai H, Minami T
and Saiki I: RANKL-induced down-regulation of CX3CR1 via PI3K/Akt
signaling pathway suppresses Fractalkine/CX3CL1-induced cellular
responses in RAW264.7 cells. Biochem Biophys Res Commun.
364:417–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koizumi K, Saitoh Y, Minami T, Takeno N,
Tsuneyama K, Miyahara T, Nakayama T, Sakurai H, Takano Y, Nishimura
M, et al: Role of CX3CL1/fractalkine in osteoclast differentiation
and bone resorption. J Immunol. 183:7825–7831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
International Myeloma Working Group:
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: A report of the
International Myeloma Working Group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma. Correlation of measured myeloma cell
mass with presenting clinical features, response to treatment, and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eychène A, Rocques N and Pouponnot C: A
new MAFia in cancer. Nat Rev Cancer. 8:683–693. 2008. View Article : Google Scholar
|
|
27
|
Morito N, Yoh K, Maeda A, Nakano T, Fujita
A, Kusakabe M, Hamada M, Kudo T, Yamagata K and Takahashi S: A
novel transgenic mouse model of the human multiple myeloma
chromosomal translocation t(14;16)(q32;q23). Cancer Res.
71:339–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murphy G, Caplice N and Molloy M:
Fractalkine in rheumatoid arthritis: A review to date.
Rheumatology. 47:1446–1451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nanki T, Urasaki Y, Imai T, Nishimura M,
Muramoto K, Kubota T and Miyasaka N: Inhibition of fractalkine
ameliorates murine collagen-induced arthritis. J Immunol.
173:7010–7016. 2004. View Article : Google Scholar : PubMed/NCBI
|