Introduction
Breast cancer is the most common cancer in women,
accounting for 23% of all female cancers around the globe, and its
incidence is rising particularly in developing countries (1). Worldwide, it is estimated that more
than one million women are diagnosed with breast cancer every year,
and more than 400,000 will succumb to the disease (2).
Breast cancer is a heterogeneous disease, and risk
factors may be differentially associated with the development of
distinct tumor subtypes that manifest different biological
behaviors and progression (3). In
support of this research, there is growing evidence that known
breast cancer risk factors vary according to hormone receptor
status and perhaps other pathological characteristics of the
disease (4–6).
One major way of defining the type of breast cancer
is whether or not it is endocrine receptor (estrogen or
progesterone receptor)-positive, HER2-positive, triple-negative
(not positive to receptors for estrogen, progesterone or HER2) or
triple-positive (positive for estrogen receptors, progesterone
receptors and HER2) (7,8). These classifications provide
oncologists with valuable information about how the tumor acts and
what type of treatments may work best. Generally, surgical and
radiation treatments are similar for these different types of
breast cancer. Drug treatments, such as chemotherapy, endocrine
therapies and other medications, usually vary. Drug treatments are
targeted to the specific type of cancer (9,10).
Some breast cancers (estimates range between 10 and
17%) are known as ‘triple-negative’ since they lack estrogen and
progesterone receptors and do not overexpress the HER2 protein
(11). The majority of breast
cancers associated with the gene known as BRCA1 are triple-negative
(12). Overall, however, they have
a poorer prognosis than other types of breast cancers. To date, no
targeted therapies such as tamoxifen or Herceptin have been
developed to help prevent recurrence in women with triple-negative
breast cancer (13). Cancer experts
are studying several promising targeted strategies aimed at
triple-negative breast cancer.
Even though surgical intervention combined with
radiotherapy, chemotherapy and endocrine therapy has achieved
beneficial results in many breast cancer treatments; for some
patients, particularly estrogen receptor-negative breast cancer
patients, the combined treatment is not as promising as treatment
for other types of breast cancers. ER-negative breast cancer
accounts for approximately one-third of breast cancers, and has a
high invasiveness among tumor subtypes and a high relapse rate
(14–16). Unfortunately, ER-negative breast
cancer is not very sensitive and often responds poorly to endocrine
therapy and chemotherapy; thus, the prognosis is significantly
worse compared to ER-positive patients (17–19).
Sodium/iodide symporter
(Na+/I− symporter, NIS), an intrinsic plasma
membrane protein, mediates active iodide transport into the thyroid
gland and several extra-thyroidal tissues (19). The uptake of iodine by NIS serves as
the basis for thyroid hormone biosynthesis, diagnostic thyroid
radionuclide imaging as well as treatment of hyperthyroidism and
thyroid cancer by radioactive iodine (19,20).
NIS is also detected in the mammary gland, placenta, salivary and
digestive gland as well as other types of tissues (19,21–23).
Among them, the high expression of NIS in breast cancer is
attracting more attention (24,25).
However, the relationship between the expression of NIS in
ER-negative breast cancer and its clinical significance remains
elusive.
The ability of cancerous thyroid cells to actively
transport iodine via NIS provides a unique and effective delivery
system to detect and target these cells for destruction with
therapeutic doses of radioiodide, largely without harming other
tissues. Therefore, it seems feasible that radioiodide could be a
diagnostic and therapeutic tool for the detection and destruction
of other cancers in which NIS is functionally expressed (19).
Radioisotopes (131I) have been
successfully used for several decades to treat thyroid cancer after
residues and metastasis (26). The
prerequisite for this radioiodide therapy is the existence of NIS,
which facilitates the uptake of radioiodide in thyroid cancer cells
resulting in β-ray emissions which cause irreversible DNA damage,
leading to cell death (19). With
the success of NIS gene cloning and the use of gene transfer
technology it has become possible to introduce the NIS gene into
non-thyroid tumor cells, so that it has a polyiodides function
similar to thyroid tissues (23).
An important recent discovery was that NIS is functionally
expressed in vivo in transgenic mouse mammary tumors and is
immunohistochemically detected in over 80% of human breast cancers
(21), raising the possibility of
using radioiodide as a novel therapy for breast cancer. Other
iodide-transporting tissues also may upregulate NIS in the process
of malignant transformation. It is therefore arguable that
extra-thyroidal NIS-expressing cancers could be targeted with
131I, if NIS is present and functional.
The cloning of rat NIS (rNIS) (27) and human NIS (hNIS) cDNAs (28), and subsequent generation of anti-NIS
antibodies (Abs), have made it possible to examine NIS expression
in human tissues and correlate it with I-uptake (20,29,30).
Eventually, the radioactive iodine treatment extended to
non-thyroid tumors such as breast and colon cancers and
malignant-targeted radiation therapy may provide new modalities in
cancer therapy (19,23). In view of this, we further explored
NIS-mediated 131I irradiation in ER-negative breast
cancer treatment.
In order to study the effect of NIS-mediated
radioiodide therapy in ER-negative breast cancers, we constructed a
recombinant lentivirus plasmid encoding the hNIS gene. Since the
iodine treatment for breast cancer requires high expression of NIS,
we constructed ER-negative breast cancer cell lines by transfecting
MDA-MB-231 cells with the recombinant lentivirus stably and
efficiently expressing the functional NIS gene. A further analysis
of tissue-specific NIS gene expression was carried out by
fractionation of the cells into cell membrane and cytoplasm
fractions. A western blot analysis carried out with these separated
fractions showed that NIS was abundantly overexpressed (~3-fold) on
the cell membrane compared to the cytoplasm. We further
characterized the iodine uptake by these cell lines at different
time-points and the effect of NIS overexpression on 131I
sensitivity in these cancer cells.
Materials and methods
Breast cancer tissue sample collection
and preparation
Tissue and archived paraffin-embedded samples were
obtained from patients diagnosed with breast cancer who underwent
surgical resection at the Department of Breast Surgery, The First
Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
All studies were approved by the Institute Research Medical Ethics
Committee of Sun Yat-sen University. All individuals provided
informed consent prior to their inclusion in the study.
Immunohistochemical (IHC) analysis of NIS
expression in the breast cancer tissues
IHC staining was carried out on formalin-fixed,
paraffin-embedded micro-tissue sections (4- μm thick) which
were deparaffinized in xylene and rehydrated in decreasing
concentrations of ethanol and rinsed in phosphate-buffered saline.
The antigen was retrieved with microwave treatment in 10 mM citrate
buffer (pH 6.0). IHC staining was carried out using the EnVision™
kit (Dako) following the manufacturer’s instructions. The
endogenous peroxidase activity was quenched by 3% hydrogen peroxide
for 10 min. The sections were incubated in the primary polyclonal
rabbit anti-NIS antibody (Santa Cruz Biotechnology) at a dilution
of 1:200 for 30 min at room temperature. In the negative controls,
the primary antibody was substituted by normal goat serum. Then the
tissue sections were sequentially incubated with ready-to-use
horseradish peroxidase (HRP)-immunoglobulin (Evision™) for 30 min
and then were developed with 3′3′-diaminobenzidine (DAB) as a
chromogen substrate. The nuclei were counterstained with Meyer’s
hematoxylin (23).
RNA extraction and quantitative real-time
polymerase chain reaction (qRT-PCR)
Total RNA from the breast samples were extracted by
TRIzol (Invitrogen). RNA samples from breast cancer and normal
breast tissues were pooled in equal amounts in single tubes. The
mRNA levels of NIS were examined by real-time PCR. Briefly,
complementary DNAs were prepared from the total RNA (10 ng) using
the QuantiTect Reverse Transcription kit (Qiagen Inc., Valencia,
CA, USA) according to the manufacturer’s instructions. Then the RT
reaction mixture (1 ml) was subjected to real-time PCR analyses
using CFX-96 (Bio-Rad Laboratories, Hercules, CA, USA) according to
the manufacturer’s instructions. The thermal cycle profile used was
incubation at 50°C for 2 min and denaturing at 95°C for 10 min,
followed by 40 cycles of the amplification step. The primer sets
used were: forward, 5′-CCATCCTGGATGACAACTTGG-3′ and reverse,
5′-AAAAACAGACGATCCTCATTGGT-3′; QuantiTect Primer assay, QT00044723
for hNIS and QT00079247 for human glyceraldehyde 3-phosphate
dehydro-genase (GAPDH) (both from Qiagen Inc.).
Western blot analysis
Proteins were extracted from the breast cancer and
normal breast samples. Relative expression levels of NIS and
tubulin were detected by SDS-PAGE with the following antibodies
according to the manufacturer’s instructions: NIS (catalog bs0448R;
Bioss, Beijing, China) and tubulin (catalog 1879-1; Cell Signaling
Technology, Inc., Danvers, MA, USA) which was used as a loading
control. HRP-conjugated anti-rabbit immunoglobulin (catalog
A00098l; GenScript USA Inc., Piscataway, NJ, USA) was used as the
secondary antibody. Finally, protein bands were imaged on X-ray
film (Eastman Kodak Co., Rochester, NY, USA) after incubating PVDF
membranes (Millipore Corp., Bedford, MA, USA) with enhanced
chemiluminescence (ECL) detection reagent (Forevergen Inc,
Guangzhou, China).
Construction of the lentiviral vectors
overexpressing hNIS
To generate the recombinant lentivirus plasmid
encoding the hNIS gene, the hNIS gene (Genebank, NM_000453) was
cloned into the lentivirus vector [CS-CMVsr39tk-I-firefly
luciferase (Fluc)] (kindly provided by Professor Irene L. Wapnir of
Stanford University) at the 5′ Nhe1 and 3′ BamH1
restriction sites. The NIS gene fragment was ligated to the vector
CS-CMVsr39tk-I-Fluc using T4 DNA ligase, generating the
CS-CMV-hNIS-I-Fluc plasmid.
Lentivirus infection of ER-negative
breast cancer cell lines
The CS-CMV-hNIS-I-Fluc plasmid and packaging helper
plasmids, pCMV.R 8.2 and pMD.G, were mixed in a ratio of 9:8:1 and
then transfected into 293T cells. The supernatants were collected
after 72 h and assayed for its viral titer. Breast cancer
MDA-MB-231 cells (HER2/neu-negative) were seeded in 6-well plates
at least 24 h before infection. Purified virus solution 5
μl/ml of medium was mixed with Polybrene to a final
concentration of 10 μg/ml and incubated for 4 h at 37°C. The
medium was exchanged with fresh medium for an additional 48 h of
incubation. After 48 h of transfection, the expression of
fluorescence was determined to evaluate the infection efficiency.
The single clones expressing the NIS gene determined by its
fluorescence were subcultured to continue the expansion culture.
The cells highly expressing hNIS in the membrane were selected for
further studies on the radioiodide uptake and cytotoxicity
assays.
In vitro iodide uptake studies
The ER-negative breast cancer cells [MDA-hNIS and
MDA (control)] were seeded at a concentration of 5×104
cells in 12-well plates. After an 18- to 24-h incubation period at
37°C with 5% CO2, the medium was aspirated and washed
with B-HBSS (buffered Hank’s balanced salt solution). Iodide uptake
was initiated by adding 500 μl HBSS containing 5
μCi/ml Na131I (Shanghai Xinke), and incubated for
different time-points from 5 min to 1 h. At various time-points,
the reactions were rapidly terminated by pipetting the radioactive
B-HBSS off and washing the cells twice with ice-cold HBSS. Cells
were then solubilized by incubation for 20 min with 1% NP-40 cell
lysates in B-HBSS, and accumulated iodide and its radioactivity was
measured using a γ-counter (Shanghai Rihuan). The radioactivity was
normalized to the cell number at the time of assay.
Cytotoxic clonogenic assay in
131I-treated MDA-MB231 cells
Each group of cells (containing 2×105),
respectively, was exposed to 30 μCi/ml Na 131I
and incubated in 5% CO2 at 37°C for 2 and 6 h,
respectively. The reaction was terminated by removing the medium
containing Na 131I and washing the cells twice with
HBSS. The cells then were trypsinized, counted and subsequently
cultured with growth medium in 6-well plates, and the colony
formation was assessed after 10 days. Uptake of 131I was
confirmed by Geiger Mueller counter before plating. Cells were then
fixed in 70% ethanol and stained with Giemsa staining, and the
number of macroscopic colonies was counted. The survival rate was
calculated as the percentage of cell colonies in plates treated
with 131I compared with those with only HBSS, and the
cell survival curves were plotted.
Statistical analysis
All statistical analyses were carried out using the
SPSS 13.0 statistical software package. Data are represented as
means ± standard deviation (SD). P<0.05 was considered to
indicate a statistically significant result.
Results
The level of NIS expression in the breast
cancer tissues compared to the normal breast tissues
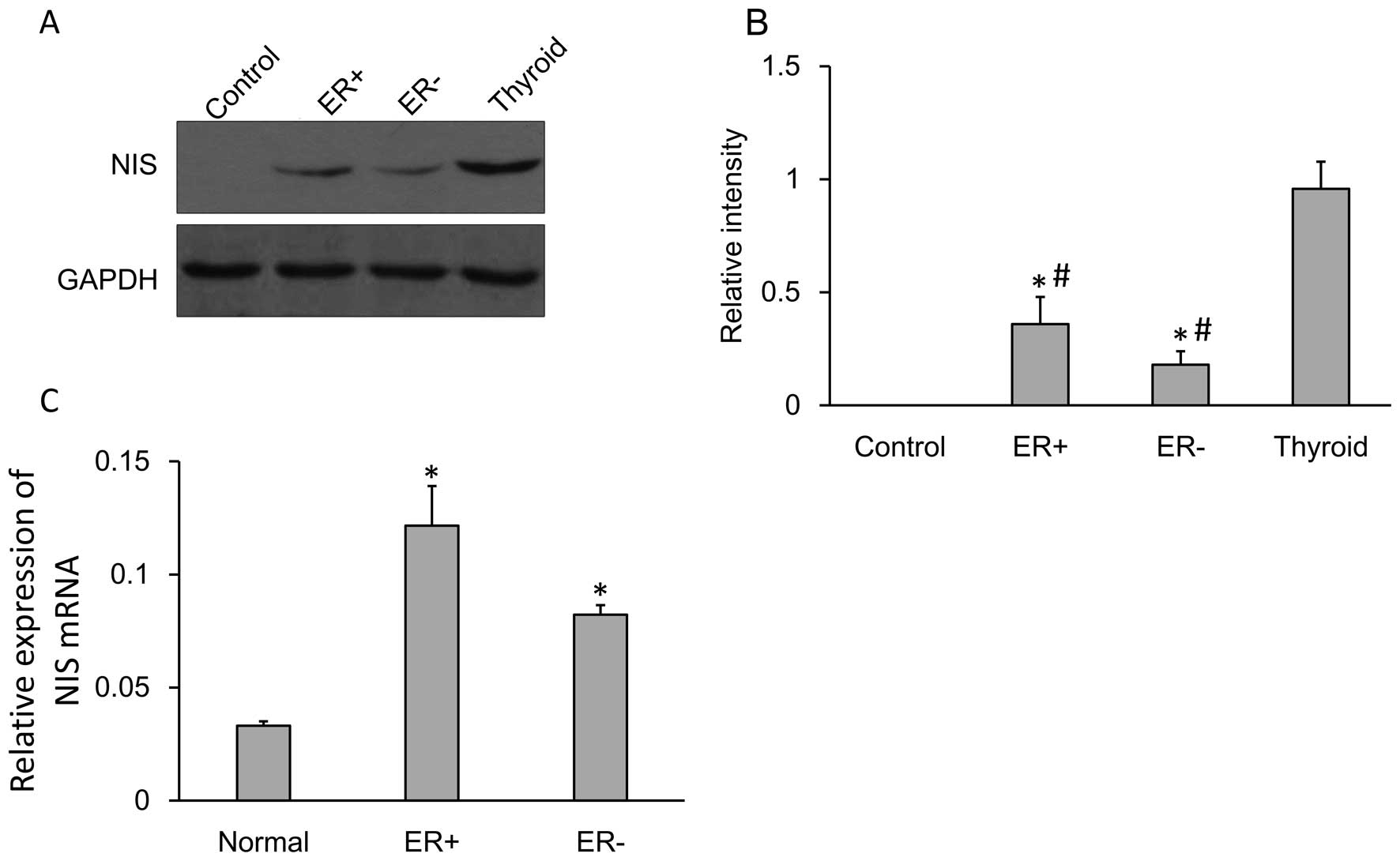
The NIS protein and mRNA expression levels were
confirmed by performing western blot analysis and qRT-PCR. NIS
protein (~75 kDa) was expressed in the normal breast tissues,
ER-positive and ER-negative breast cancer tissues as well as the
thyroid tissues. Tubulin (~55 kDa) was used as an internal control.
The NIS protein expression was quantified (Fig. 1A) and the expression of NIS protein
in the breast cancer tissues (both in ER-negative or ER-positive)
was significantly higher compared to that in the adjacent tissues
while significantly lower compare to that in the thyroid tissues
(Fig. 1B).
Relative expression of NIS mRNA showed significantly
increased expression in the ER-negative cells compared to that in
the normal cells. The level of expression of NIS mRNA between
normal cells and ER-negative cells was significant (Fig. 1C).
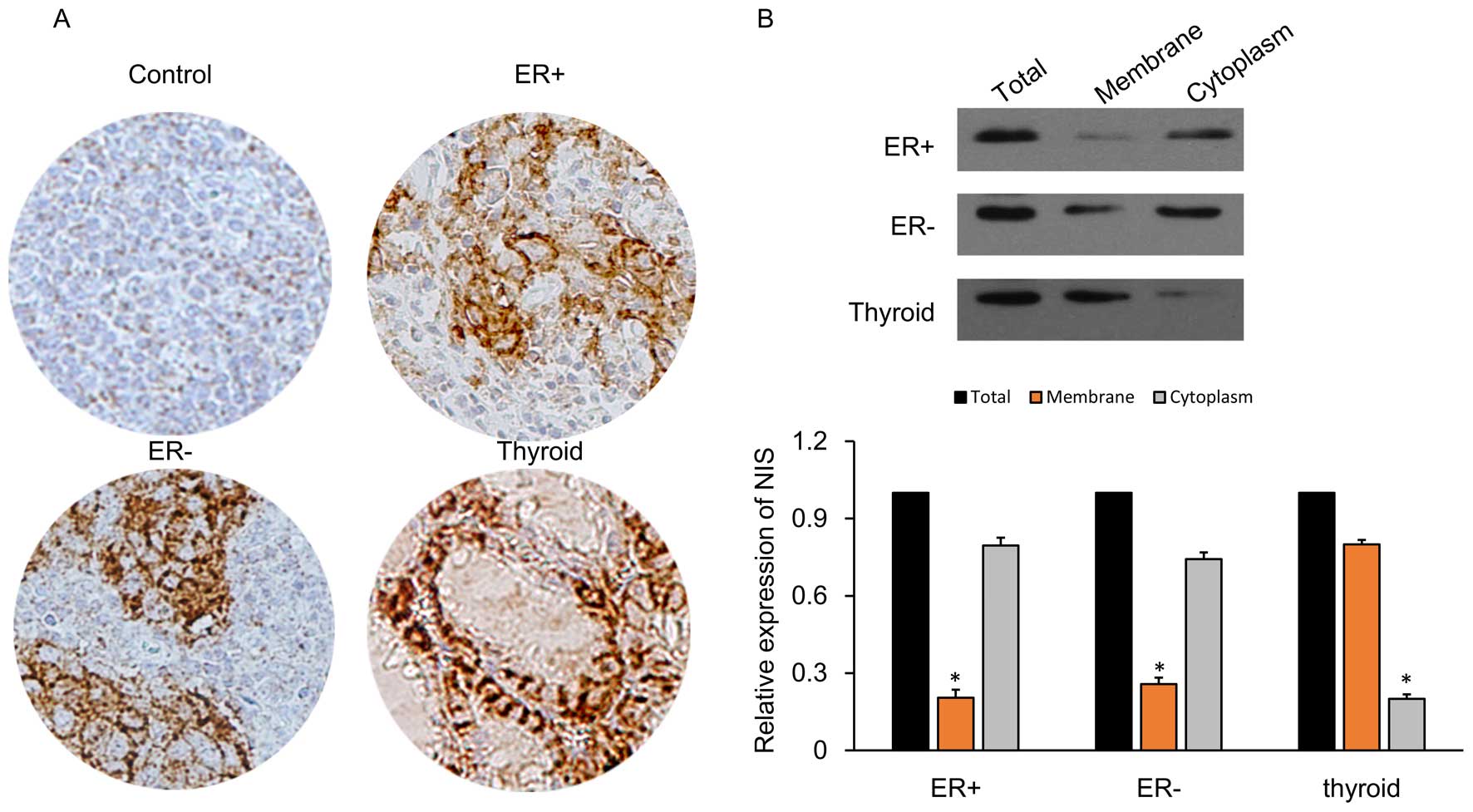
NIS protein expression in the breast
cancer tissues is localized in the cytoplasm
IHC detection of cells was carried out for NIS
expression in various tissues including ER-positive, ER-negative,
thyroid and normal control cells (adjacent tissues) as described
previously (43). Compared to the
control cells, ER-positive and ER-negative breast cancer tissues
showed expression of NIS proteins (brown-colored particles after
staining) which was mainly concentrated in the cytoplasm (Fig. 2A). In the thyroid tissues, the NIS
proteins were expressed mainly in the cell membrane. The
Na+/I− symporter (NIS) is an integral plasma
membrane glycoprotein that mediates active iodine transport into
thyroid follicular cells, the first step in thyroid hormone
biosynthesis (31). NIS-mediated
thyroidal iodine transport from the bloodstream to the colloid is a
vectorial process made possible by the selective targeting of NIS
to the basolateral membrane (19).
In addition, we subjected the breast cancer cell
lines (ER-positive and ER-negative) to cell fractionation and
analyzed the NIS expression using western blot analysis (Fig. 2B). NIS protein expression in the
breast cancer cells was localized poorly in the membrane (≤25%),
while in the thyroid tissue membranes it was highly concentrated
(≥70%). These results suggest that NIS is mainly expressed in the
cytoplasm, so that breast cancer may not be as effective as the
thyroid in regards to the uptake of radioactive iodine.
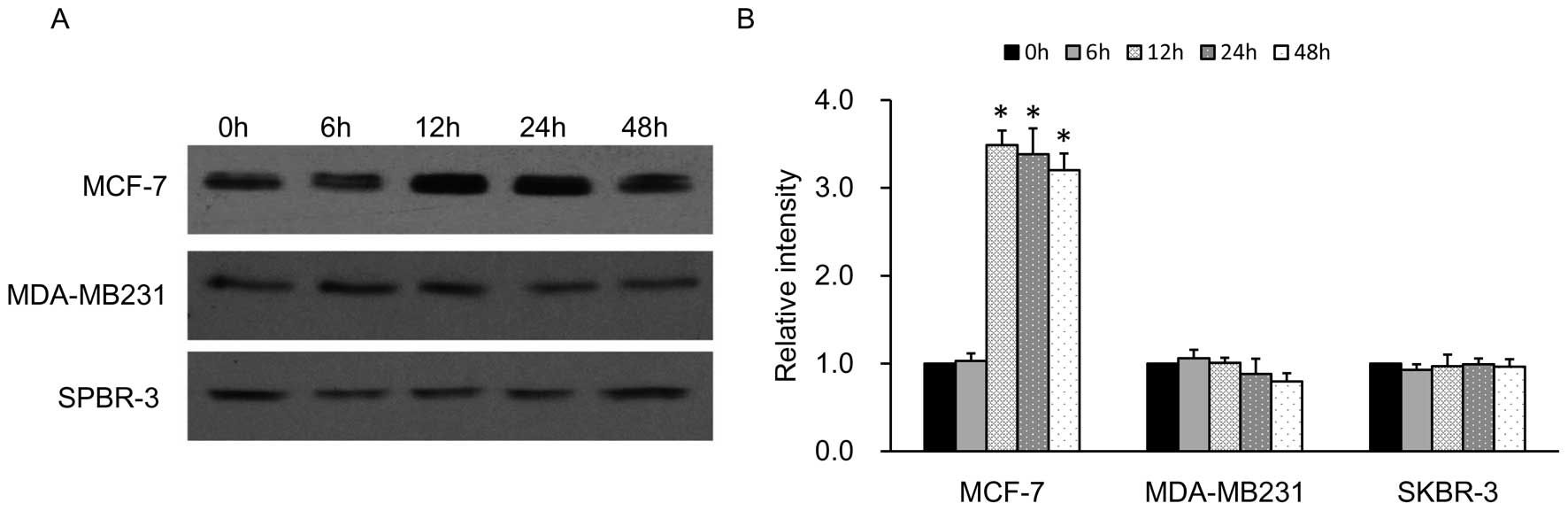
Breast cancer cell lines overexpressing
NIS protein
Patients with breast cancer may benefit from
radioiodine therapy if NIS expression/activity can be increased in
the malignant tissues to levels sufficient for therapy (32). Findings (33) have shown that retinoic acid (RA)
induces the endogenous NIS expression in many malignant cells,
particularly in ER-positive breast cancer cell lines such as MCF-7.
In our study, we found the results of NIS protein expression in
ER-positive and ER-negative cell lines in accordance with the
previous studies. The levels of expression of NIS in the
ER-negative cell lines MDA-MB-231 and SPBR-3 were very low compared
with the high expression levels of RA-induced NIS protein in the
MCF-7 cells at different time-points from 0 to 48 h (Fig. 3). Therefore, we constructed a
lentivirus vector to introduce the exogenous hNIS gene into the
MDA-MB-231 cell line which can upregulate the NIS expression more
efficiently.
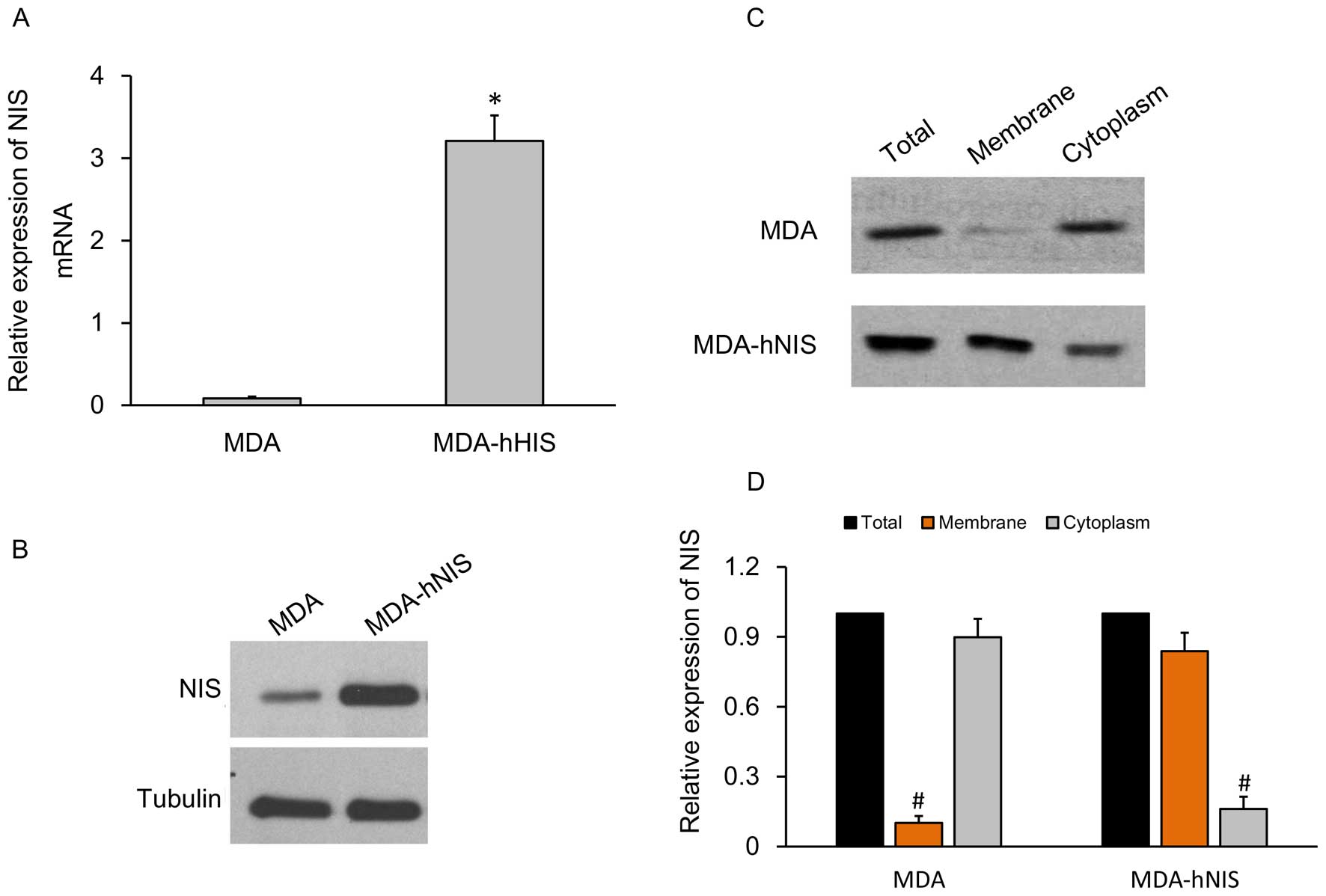
Construction of ER-negative breast cancer
cell line, MDA-hNIS stably expressing the NIS gene
To characterize and identify the capacity of the
MDA-hNIS cell line to overexpress the NIS gene, qRT-PCR to
determine the mRNA expression level and western blot analysis to
determine protein expression level were carried out, and the
results were compared with the control cell lines.
The relative expression levels of hNIS mRNA showed a
10-fold higher expression in the MDA-hNIS cells when compared with
the control cells without the endogenous NIS gene (Fig. 4A). The hNIS protein expression level
was also significantly higher in the MDA-hNIS cells compared to
that in the control cells (Fig.
4B).
A further analysis of tissue-specific NIS gene
expression was carried out by fractionation of the cells into cell
membrane and cytoplasm portions. Western blot analysis (Fig. 4C) and relative expression of the NIS
gene (Fig. 4D) carried out with
these separated fractions showed that hNIS was abundantly
overexpressed on the cell membrane compared to the cytoplasm. The
results prompted us to efficiently express the functional hNIS
gene.
Effect of NIS overexpression on
131I uptake in the MDA-hNIS cells
The functional activity of the NIS protein
expression was evident by its cellular uptake of iodine. The
estrogen ER-negative cell line, MDA-hNIS overexpressing the NIS
protein and control cells MDA-MB-231 (MDA) were cultured in 12-well
plates and were subjected to 500 μl HBSS containing 5
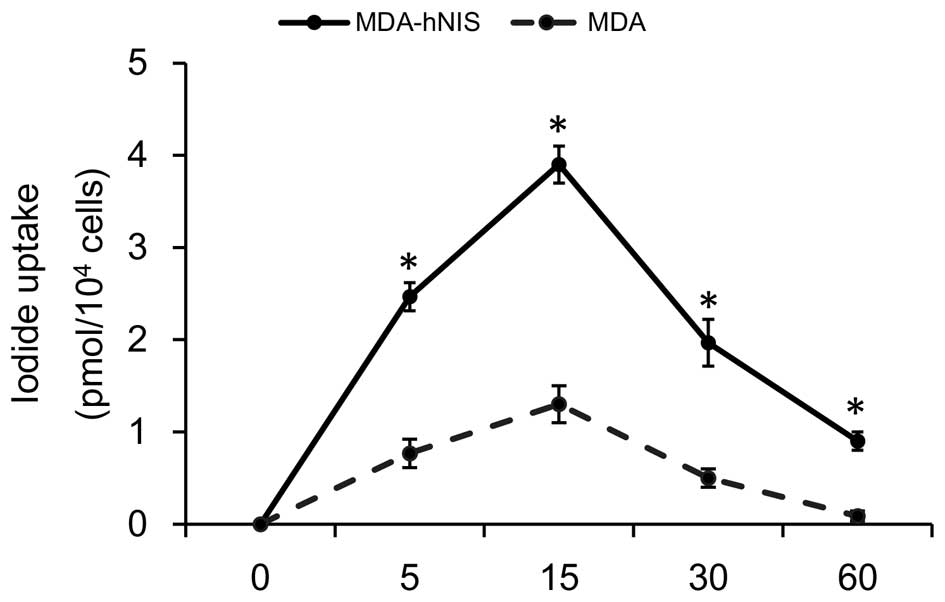
μCi/ml Na131I. As shown in Fig. 5, iodine uptake into the MDA-hNIS
cells was rapid, reaching a maximum after 15 min, followed by a
decline (half-life, 3.2 h). At 60 min after the addition of
131I, the uptake level was maintained at 25% of the peak
activity. These results show that NIS overexpression in MDA-hNIS
cells can increase the uptake of radioiodide compared to the
control cells with low NIS expression, and thus validates the
functional NIS expression in an ER-negative cell line.
Effect of NIS overexpression on
131I sensitivity in the MDA-hNIS cells
The in vitro therapeutic effect of
radioiodide was estimated by determining the survival of cells in a
cytotoxic clonogenic assay. MDA-hNIS and control cells (MDA) at a
concentration of 2×105 cells were incubated for 2 and 6
h with 30 μCi/ml Na131I. We used a previously
established assay (34) to
investigate whether 131I had selective cytotoxic
activity upon NIS overexpression in the MDA-hNIS cells compared
with the control cells. Cells were exposed to 30 μCi/ml
Na131I for 2 and 6 h, and colony formation was assessed
after 10 days.
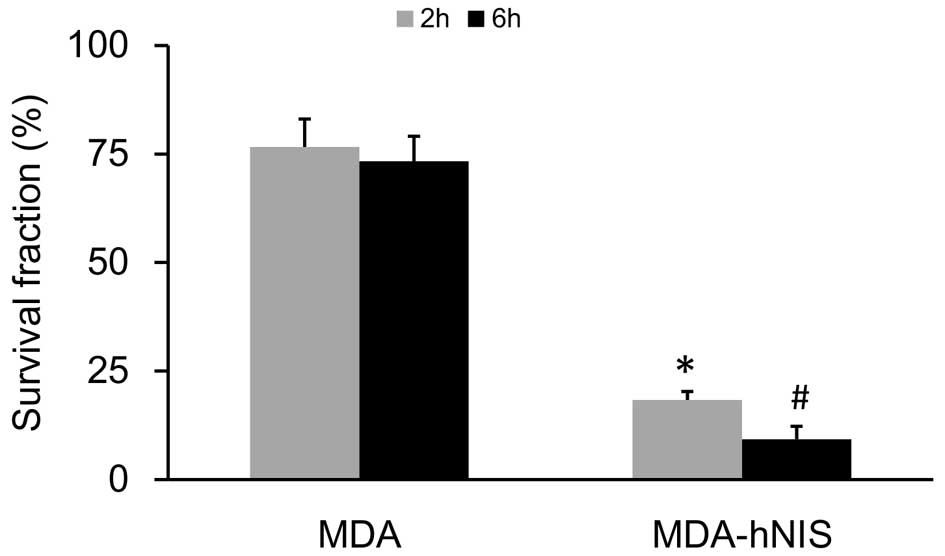
As shown in Fig. 6,
the survival rate, based on the clono-genic assay, was markedly
reduced in response to 131I (P<0.05). Exposure of the
MDA-hNIS cells to 131I resulted in a time-dependent
reduction in colony formation of 58% at 2 h and 64% at 6 h,
compared with the survival of the control cells (MDA). These
results showed that NIS overexpression enhanced the sensitivity of
ER-negative breast cancer cells to 131I.
Discussion
ER-negative breast cancer comprises 15–30% of all
breast tumors (depending on the population), and has an earlier age
at onset and a worse prognosis compared with ER-positive disease
(35). NIS has been widely explored
as a potential therapeutic gene for many invasive and malignant
cancers. The transfer of the NIS gene using many human and nonhuman
vectors into a variety of tumors, including breast (36) and cervical cancer (37) and prostate carcinoma has shown the
capacity to confer radioiodide uptake which has emerged as an
important radiation therapy for many cancers.
In a preliminary study, we investigated the
endogenous NIS gene expression and evaluated its tissue- and
site-specific expression by the IHC analysis of tumor tissue
samples obtained from patients diagnosed with breast cancer in our
hospital. Type-specific breast cancer samples were analyzed for
differential expression of the NIS protein. We found significantly
higher levels of NIS expression in ER-negative tissues compared to
that in the normal tissues and significantly lower levels than
thyroid tissues. ER-negative breast cancer tissues showed
expression of NIS proteins which was mainly concentrated in the
cytoplasm, whereas thyroid cells showed robust expression in the
cell membrane, as uptake of iodine by NIS serves as the basis for
thyroid hormone biosynthesis.
We observed a markedly low expression of NIS protein
in the membrane of breast cancer cells (≤25%), while in the thyroid
tissue membranes it was highly concentrated (≥70%). These results
suggest that NIS is mainly expressed in the cytoplasm, so that
breast cancer may not be as effective as the thyroid in uptake of
radioactive iodine. The likelihood of iodine transport activity is
enhanced whenever NIS is immunohistochemically demonstrated in the
plasma membrane. Cell membrane immunoreactivity was not observed in
other normal or benign breast tissues, with the exception of
gestational or lactating samples (23).
Other reasons may also be anticipated for the
reduced uptake of iodine in breast cancer cells, due to reduced
expression of NIS in breast cancer cells. In a previous study, NIS
expression was demonstrated by RT-PCR in 2 of 2 fibroadenomata and
6 of 7 breast carcinoma messenger ribonucleic acid isolates. The
authors also demonstrated a significantly higher mean total tissue
iodine level (80.9±9.5 ng I/mg protein) in 23 benign tumors
(fibroadenomata) than those in 19 breast cancers taken from either
the tumor (18.2±4.6 ng I/mg) or morphologically normal tissue taken
from within the tumor-bearing breast (38). These authors suggested that
antibody-mediated inhibition played a major role in the inhibition
of 125I uptake into NIS-transfected CHO cells, as these
responses were mediated by serum from breast cancer patients
compared to normal female controls.
We hypothesized that the low expression of NIS
protein in the cell membrane of ER-negative cancer cells may be a
possible reason for the reduced susceptibilty to radioiodide
therapy in this subgroup of cancer patients. Hence, we constructed
a lentivirus vector to introduce the exogenous hNIS gene into the
ER-negative cell line MDA-MB-231 which can upregulate NIS
expression. The relative expression levels of hNIS mRNA showed a
3-fold higher expression in the MDA-hNIS cells compared with the
control cells without the endogenous NIS gene. The NIS protein was
also significantly higher and concentrated in the cell membrane
compared to the cytoplasm.
Functional NIS activities following 131I
uptake in MDA-hNIS cells when analyzed, showed a remarkable
increase in uptake, reaching a maximum within 15 min, followed by a
decline in 1 h. After 1 h of addition of 131I, the
uptake level was maintained at 25% of the peak activity. These
results showed that NIS overexpression in the MDA-hNIS cells
increased the uptake of radioiodide compared to the control cells
with low NIS expression and thus validates the functional NIS
expression in ER-negative cell lines. Finally, the exposure of
MDA-hNIS cells to 131I resulted in a time-dependent
reduction in colony formation by 58% at 2 h and 64% at 6 h,
compared with the survival of the control cells (MDA) which
indicated that NIS overexpression enhanced the sensitivity to
ER-negative breast cancer cells.
The NIS gene is well-known for its advantage as a
reporter gene in the early diagnosis of many carcinomas. In a
previous study (39),
131I SPECT revealed a clear image of recombinant
baculovirus-infected tumors in vivo, and uptake of
131I in tumors was quantified which suggests that the
NIS gene would be a promising tool for non-invasive monitoring of
vector-mediated gene expression in vivo. Many studies have
demonstrated a beneficial response to NIS-based radioiodide therapy
in various tumors using tissue-specific promoters, including
various cancer markers such as prostate-specific antigen (40), carcinoembryonic antigen (41) and calcitonin (42). However, some specific promoters
exhibit lower activity levels than those of non-specific promoters,
such as the CMV promoter. We found that following the use of
lentivirus-mediated hNIS gene expression in an ER-negative cell
line (MDA-hNIS), the iodine uptake assay demonstrated robust and
functional NIS activity mediated by the CMV promoter. Moreover, the
increased uptake of radioiodide resulted in a marked reduction in
the survival rate of ER-negative breast cancer cells.
In conclusion, the present study is a novel method
of upregulating the NIS gene expression in ER-negative breast
cancer cells using a mammalian lentiviral vector, in order to
increase the uptake of radioiodide and to reduce the survival rate
of breast tumor cells in ER-negative breast cancer. The potential
advantage of radiation inducible genetic constructs has been
demonstrated in the so called ‘genetic radiotherapy’ strategy. The
use of radioisotopes that accumulate in tumors offers an advantage
for selective induction of exogenous genes. Our research suggests
the development of a genetic radiation therapy by boosting NIS
expression in ER-negative breast cancer tissues to increase the
uptake of radioiodide and increase the susceptibility to radiation
therapy for the treatment of breast cancer. This strategy may also
prevent metastasis at an early stage in ER-negative breast cancer
patients. The clinical applications of hNIS gene transfer is most
promising to facilitate radioiodine ablation of locally invasive
cancer cells that cannot be completely resected surgically.
However, to fullfill this goal many issues need to be resolved,
such as selective cytotoxicity in breast cancer cells by careful
design and performing in vivo assays in experimental animal
models.
Acknowledgments
The authors thank their colleagues who helped with
the outcome data collection. This study was supported by
Specialized Scientific Research Fund for the Young Teachers Program
of the Sun Yat-Sen University (09ykpy45), Guangdong Natural Science
Foundation (S2011010000791) and Guangdong Medical Research
Foundation (A2011026). We are grateful to 91SCI Company for
language editing assistance.
References
|
1
|
El Saghir NS, Khalil MK, Eid T, El Kinge
AR, Charafeddine M, Geara F, Seoud M and Shamseddine AI: Trends in
epidemiology and management of breast cancer in developing Arab
countries: a literature and registry analysis. Int J Surg.
5:225–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tfayli A, Temraz S, Abou Mrad R and
Shamseddine A: Breast cancer in low- and middle-income countries:
an emerging and challenging epidemic. J Oncol. 2010:4906312010.
View Article : Google Scholar
|
|
3
|
Garcia-Closas M and Chanock S: Genetic
susceptibility loci for breast cancer by estrogen receptor status.
Clin Cancer Res. 14:8000–8009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma H, Bernstein L, Pike MC and Ursin G:
Reproductive factors and breast cancer risk according to joint
estrogen and progesterone receptor status: a meta-analysis of
epidemiological studies. Breast Cancer Res. 8:R432006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reeves GK, Beral V, Green J, Gathani T and
Bull D; Million Women Study Collaborators: Hormonal therapy for
menopause and breast-cancer risk by histological type: a cohort
study and meta-analysis. Lancet Oncol. 7:910–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Althuis MD, Fergenbaum JH, Garcia-Closas
M, Brinton LA, Madigan MP and Sherman ME: Etiology of hormone
receptor- defined breast cancer: a systematic review of the
literature. Cancer Epidemiol Biomarkers Prev. 13:1558–1568.
2004.PubMed/NCBI
|
|
7
|
Nakagawa M, Bando Y, Nagao T, Takai C,
Ohnishi T, Honda J, Moriya T, Izumi K, Takahashi M, Tangoku A, et
al: Among triple-negative breast cancers, HER2(0) breast cancer
shows a strong tendency to be basal-like compared with HER2(1+)
breast cancer: preliminary results. Breast Cancer. 19:54–59. 2012.
View Article : Google Scholar
|
|
8
|
Vaklavas C and Forero-Torres A: How do I
treat ‘triple-negative’ disease. Curr Treat Options Oncol.
12:369–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berry DA, Cirrincione C, Henderson IC,
Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB,
Norton L, et al: Estrogen-receptor status and outcomes of modern
chemotherapy for patients with node-positive breast cancer. JAMA.
295:1658–1667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Altman MB, Flynn MJ, Nishikawa RM, Chetty
IJ, Barton KN, Movsas B, Kim JH and Brown SL: The potential of
iodine for improving breast cancer diagnosis and treatment. Med
Hypotheses. 80:94–98. 2013. View Article : Google Scholar
|
|
11
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: a collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med. 7:e10002792010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu KC and Anderson WF: Rates for breast
cancer characteristics by estrogen and progesterone receptor status
in the major racial/ethnic groups. Breast Cancer Res Treat.
74:199–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Argiris A, Wang CX, Whalen SG and
DiGiovanna MP: Synergistic interactions between tamoxifen and
trastuzumab (Herceptin). Clin Cancer Res. 10:1409–1420. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Madsen MW and Briand P: Relationship
between tumorigenicity, in vitro invasiveness, and plasminogen
activator production of human breast cell lines. Eur J Cancer.
26:793–797. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson EW, Paik S, Brünner N, Sommers
CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME,
et al: Association of increased basement membrane invasiveness with
absence of estrogen receptor and expression of vimentin in human
breast cancer cell lines. J Cell Physiol. 150:534–544. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sommers CL, Byers SW, Thompson EW, Torri
JA and Gelmann EP: Differentiation state and invasiveness of human
breast cancer cell lines. Breast Cancer Res Treat. 31:325–335.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skoog L, Humla S, Axelsson M, Frost M,
Norman A, Nordenskjöld B and Wallgren A: Estrogen receptor levels
and survival of breast cancer patients. A study on patients
participating in randomized trials of adjuvant therapy. Acta Oncol.
26:95–100. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sandelin K, Skoog L, Humla S and Farnebo
LO: Oestrogen, progesterone, and glucocorticoid receptors in normal
and neoplastic parathyroid glands. Eur J Surg. 158:467–472.
1992.PubMed/NCBI
|
|
19
|
Dohán O, De la Vieja A, Paroder V, Riedel
C, Artani M, Reed M, Ginter CS and Carrasco N: The sodium/iodide
symporter (NIS): characterization, regulation, and medical
significance. Endocr Rev. 24:48–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung JK: Sodium iodide symporter: its
role in nuclear medicine. J Nucl Med. 43:1188–1200. 2002.PubMed/NCBI
|
|
21
|
Tazebay UH, Wapnir IL, Levy O, Dohan O,
Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG, et
al: The mammary gland iodide transporter is expressed during
lactation and in breast cancer. Nat Med. 6:871–878. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mitchell AM, Manley SW, Morris JC, Powell
KA, Bergert ER and Mortimer RH: Sodium iodide symporter (NIS) gene
expression in human placenta. Placenta. 22:256–258. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wapnir IL, van de Rijn M, Nowels K, Amenta
PS, Walton K, Montgomery K, Greco RS, Dohán O and Carrasco N:
Immunohistochemical profile of the sodium/iodide symporter in
thyroid, breast, and other carcinomas using high density tissue
microarrays and conventional sections. J Clin Endocrinol Metab.
88:1880–1888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Upadhyay G, Singh R, Agarwal G, Mishra SK,
Pal L, Pradhan PK, Das BK and Godbole MM: Functional expression of
sodium iodide symporter (NIS) in human breast cancer tissue. Breast
Cancer Res Treat. 77:157–165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kogai T, Taki K and Brent GA: Enhancement
of sodium/iodide symporter expression in thyroid and breast cancer.
Endocr Relat Cancer. 13:797–826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mazzaferri EL: Carcinoma of the follicular
epithelium. The Thyroid: A Fundamental and Clinical Text. Braverman
LE and Utiger R: 8th. Lippincott Williams and Wilkins;
Philadelphia, PA: pp. 904–930. 2000
|
|
27
|
Dai G, Levy O and Carrasco N: Cloning and
characterization of the thyroid iodide transporter. Nature.
379:458–460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smanik PA, Liu Q, Furminger TL, Ryu K,
Xing S, Mazzaferri EL and Jhiang SM: Cloning of the human sodium
lodide symporter. Biochem Biophys Res Commun. 226:339–345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paire A, Bernier-Valentin F, Selmi-Ruby S
and Rousset B: Characterization of the rat thyroid iodide
transporter using anti-peptide antibodies. Relationship between its
expression and activity. J Biol Chem. 272:18245–18249. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caillou B, Troalen F, Baudin E, Talbot M,
Filetti S, Schlumberger M and Bidart JM:
Na+/I− symporter distribution in human
thyroid tissues: an immunohistochemical study. J Clin Endocrinol
Metab. 83:4102–4106. 1998.PubMed/NCBI
|
|
31
|
Carrasco N: Iodide transport in the
thyroid gland. Biochim Biophys Acta. 1154:65–82. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen DH, Kloos RT, Mazzaferri EL and Jhian
SM: Sodium iodide symporter in health and disease. Thyroid.
11:415–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kogai T, Schultz JJ, Johnson LS, Huang M
and Brent GA: Retinoic acid induces sodium/iodide symporter gene
expression and radioiodide uptake in the MCF-7 breast cancer cell
line. Proc Natl Acad Sci USA. 97:8519–8524. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mandell RB, Mandell LZ and Link CJ Jr:
Radioisotope concentrator gene therapy using the sodium/iodide
symporter gene. Cancer Res. 59:661–668. 1999.PubMed/NCBI
|
|
35
|
Campa D, Barrdahl M, Tsilidis KK, Severi
G, Diver WR, Siddiq A, Chanock S, Hoover RN, Ziegler RG, Berg CD,
et al: A genome-wide ‘pleiotropy scan’ does not identify new
susceptibility loci for estrogen receptor negative breast cancer.
PLoS One. 9:e859552014. View Article : Google Scholar
|
|
36
|
Dwyer RM, Bergert ER, O’connor MK, Gendler
SJ and Morris JC: In vivo radioiodide imaging and treatment of
breast cancer xenografts after MUC1-driven expression of the sodium
iodide symporter. Clin Cancer Res. 11:1483–1489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boland A, Ricard M, Opolon P, Bidart JM,
Yeh P, Filetti S, Schlumberger M and Perricaudet M:
Adenovirus-mediated transfer of the thyroid sodium/iodide symporter
gene into tumors for a targeted radiotherapy. Cancer Res.
60:3484–3492. 2000.PubMed/NCBI
|
|
38
|
Kilbane MT, Ajjan RA, Weetman AP, Dwyer R,
McDermott EW, O’Higgins NJ and Smyth PP: Tissue iodine content and
serum-mediated 125I uptake-blocking activity in breast
cancer. J Clin Endocrinol Metab. 85:1245–1250. 2000.PubMed/NCBI
|
|
39
|
Zhang M, Guo R, Shi S, Miao Y, Zhang Y and
Li B: Baculovirus vector-mediated transfer of sodium iodide
symporter and plasminogen kringle 5 genes for tumor radioiodide
therapy. PLoS One. 9:e923262014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spitzweg C, O’Connor MK, Bergert ER,
Tindall DJ, Young CY and Morris JC: Treatment of prostate cancer by
radioiodine therapy after tissue-specific expression of the sodium
iodide symporter. Cancer Res. 60:6526–6530. 2000.PubMed/NCBI
|
|
41
|
Scholz IV, Cengic N, Baker CH, Harrington
KJ, Maletz K, Bergert ER, Vile R, Göke B, Morris JC and Spitzweg C:
Radioiodine therapy of colon cancer following tissue-specific
sodium iodide symporter gene transfer. Gene Ther. 12:272–280. 2005.
View Article : Google Scholar
|
|
42
|
Cengic N, Baker CH, Schütz M, Göke B,
Morris JC and Spitzweg C: A novel therapeutic strategy for
medullary thyroid cancer based on radioiodine therapy following
tissue-specific sodium iodide symporter gene expression. J Clin
Endocrinol Metab. 90:4457–4464. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Knostman KA, Cho JY, Ryu KY, Lin X,
McCubrey JA, Hla T, Liu CH, Di Carlo E, Keri R, Zhang M, et al:
Signaling through 3′,5′-cyclic adenosine monophosphate and
phosphoinositide-3 kinase induces sodium/iodide symporter
expression in breast cancer. J Clin Endocrinol Metab. 89:5196–5203.
2004. View Article : Google Scholar : PubMed/NCBI
|