Introduction
Although significant progress has been made in lung
cancer therapy in the past two decades, lung cancer is still the
most commonly diagnosed type of cancer (1). In addition, lung cancer is the leading
cause of cancer-related mortality, with more than 1,600,000 new
cases diagnosed and more than 1,370,000 deaths since 2008
worldwide, with non-small cell lung cancer (NSCLC) accounting for
the majority (1,2). Cisplatin-based combination
chemotherapy is the most common strategy used to treat advanced
NSCLC. Unfortunately, drug resistance has become the main cause of
chemotherapy failure (3).
Therefore, developing new agents for NSCLC is critical.
Natural products from medicinal plants are important
candidates for anticancer agents. For example, pactaxel is found in
Taxus brevifolia and vincristine is found in Catharanthus
roseus, both of which have been applied as multiple cancer
chemotherapies (4,5). Sanguinarine (SAN) is an alkaloid
isolated from plants of the Papaveraceae family (6). Previous studies have shown that SAN
has antibacterial and anti-inflammatory properties (7–9), and
recently, SAN has been shown to inhibit cell growth and induce
apoptosis in prostate cancer (10),
breast cancer (11), cervical
cancer (12) and osteosarcomas
(13), indicating an anticancer
effect. However, the effects of SAN on lung adenocarcinomas remain
unclear, and the underlying mechanisms remain to be clarified
(14).
Reactive oxygen species (ROS) have multiple
functions and play an important role in determining cell fate
(15). Many chemotherapy agents
depend on the generation of ROS to induce cell death (16,17).
Indeed, ROS have become an anticancer target. Studies reveal that
drugs promote cell death by activating endoplasmic reticulum (ER)
stress-induced signaling pathways, and that triggering ER stress
can be an anticancer strategy (18–20).
However, ROS production may be the result of ER stress (21–23),
and ER stress may be the result of ROS production (24,25).
Taken together, the role of ROS and ER stress in cell fate decision
has become an area of interest.
In the present study, we determined the anticancer
effects of SAN in lung cancer using the human lung adenocarcinoma
cell line SPC-A1, and investigated whether ROS production and ER
stress are involved in cell fate decision.
Materials and methods
Reagents and antibodies
SAN was supplied by Professor Chao-Sheng Li
(Institute of Resource, Jilin Academy of Chinese Medicine Sciences,
Changchun, China) and dissolved in dimethyl sulfoxide (DMSO). Fetal
bovine serum (FBS) and Roswell Park Memorial Institute (RPMI)-1640
culture medium were purchased from Invitrogen (Carlsbad, CA, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
tauroursodeoxycholic acid (TUDCA), N-acetylcysteine (NAC) and
Hoechst 33258 were purchased from Sigma (St. Louis, MO, USA). The
ROS indicator 5-(and
-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl
ester (CM-H2DCFDA) was purchased from Molecular Probes
(Eugene, OR, USA). Enhanced chemiluminescence (ECL) reagents were
from Thermo Scientific (Rockford, IL, USA). Anti-CCAAT/enhancer
binding protein homologous protein (CHOP), anti-glucose-regulated
protein 78 (GRP78), anti-p-protein kinase R (PKR)-like ER kinase
(PERK), anti-p-eukaryotic translation initiation factor 2α (eIF2α),
anti-activating transcription factor 4 (ATF4), anti-Bax and
anti-caspase-3 antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA), and anti-β-actin and
horseradish peroxidase-conjugated anti-rabbit and anti-mouse
immunoglobulin were purchased from Proteintech (Chicago, IL, USA).
All reagents and antibodies were purchased from the Changchun
Baoxin Biotechnology Company (Changchun, China).
Cell culture
Human lung adenocarcinoma SPC-A1 cells were obtained
from the Chinese Academy of Medical Sciences and Peking Union
Medical College. Cell lines were cultured at 37°C in 5% (v/v)
CO2 in RPMI-1640 culture medium supplemented with 10%
(v/v) FBS. Media were changed at 2-day intervals, and cells were
split twice a week by trypsinization. All experiments were
performed when cells reached 70% confluency.
Cell viability assays
The cells were plated in 96-well plates at a density
of 1×104 cells/well in 200 µl of complete medium.
Each treatment was repeated in 6 separate wells. The cells were
treated as indicated for 24 h, after which 20 µl of 5 mg/ml
MTT reagent in phosphate-buffered saline (PBS) was added to each
well and incubated for 4 h. Formazan crystals were dissolved in 150
µl DMSO. Absorbance was recorded at a wavelength of 490 nm
using a microplate reader (Bio-Rad, Hercules, CA, USA). Cell
viability was calculated as: Viability (%) = absorbance of
experimental group/absorbance of control group × 100%. In each
experiment, we calculated the mean value of 6 wells/treatment
group.
Apoptosis analysis by Hoechst 33258
staining
Apoptotic morphological alterations in nuclear
chromatin were detected by Hoechst 33258 staining. Briefly, the
SPC-A1 cells were cultured in 24-well plates and treated as
indicated for 24 h. The cells were washed with ice-cold PBS and
fixed with 4% (w/v) paraformaldehyde overnight. The plates were
then incubated with 10 µM Hoechst 33258 staining solution
for 10 min. The cells were visualized under a fluorescence
microscope (IX-71; Olympus).
RT2 Profiler PCR Array
system
The Human Unfolded Protein Response RT2
Profiler™ PCR Array (SABiosciences, Qiagen, Chicago, IL, USA)
profiles the expression of 84 key genes involved in unfolded
protein accumulation in the ER. Total cellular RNA was extracted
from the cultured cells according to the manufacturer’s
instructions. Single-stranded cDNA was obtained by reverse
transcription of 1 µg of total RNA using the SABiosciences
RT2 First Strand kit (SABiosciences). Real-time qPCRs
were performed using Applied Biosystems 7300 Fast with SYBR-Green
Fluorophore. The reactions were carried out using the
RT2 SYBR-Green Master Mix. cDNA was used as a template
and cycling parameters were 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 1 min. Fluorescence intensities
were analyzed using the manufacturer’s software, and relative
quantification was calculated using the 2™ΔΔCt method.
Change in the expression of the 84 genes was shown by heat imaging.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a
reference gene.
Western blot analysis
Cells were washed with PBS twice and harvested by
scraping into 300 µl of Radio-Immunoprecipitation Assay
(RIPA) lysis buffer. Cell lysates were ultrasonicated for 15 sec on
ice and then lysed at 4°C for 45 min and centrifuged at 12,000 × g
for 10 min. Protein concentrations in the supernatants were
determined using the Bio-Rad reagent (Bio-Rad, Hercules, CA, USA).
Equal amounts of protein samples (30 µg) were separated by
SDS-polyacrylamide gel electrophoresis in duplicate and blotted
onto polyvinylidene fluoride membranes (Millipore, Billerica, MA,
USA). Transfer efficiency was assessed with Ponceau staining. The
blots were blocked in Tris-buffered saline containing 5% (w/v)
nonfat dry milk and probed with specific primary antibodies
overnight at 4°C. The membranes were washed with PBS-Tween-20 and
then incubated with a peroxidase-conjugated secondary antibody for
2 h at room temperature. Duplicated membranes were probed for
β-actin expression to ensure equal input of cell lysate proteins.
The final dilutions and incubation times suggested by the
manufacturer were used for each antibody. Immunodetection was
performed using the ECL reagents and images were captured using
Syngene Bio Imaging (Synoptics, Cambridge, UK).
Detection of ROS production
SPC-A1 cells were seeded onto glass culture slides
(BD Biosciences, Bedford, MA, USA) and treated with the indicated
drugs. At various time-points, the cells were loaded with 1
µM CM-H2DCFDA in PBS for 10 min at 37°C in the
dark followed by a PBS washing step. DCF-dependent fluorescence was
examined by laser-scanning confocal microscopy (FV 1000;
Olympus).
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD) of repeated experiments, as indicated in the figure
legends. Data are representative of three independent experiments
performed in triplicate. Statistical analysis of the data was
performed using one-way ANOVA. The Tukey’s post hoc test was used
to determine the significance for all pairwise comparisons of
interest. Differences were considered statistically significant at
P<0.05.
Results
SAN inhibits the growth and triggers
apoptosis in lung cancer cells
To measure the effects of SAN on the growth of lung
cancer cells, we treated SPC-A1 cells with different doses (0.5, 1,
2 and 4 µM) of SAN for 24 h and detected cell viability
using the MTT assay. The results revealed that treatment with SAN
inhibited cell growth in a dose-dependent manner (Fig. 1A). To investigate whether inhibition
of cell growth by SAN is related to cell apoptosis, we monitored
apoptosis using Hoechst 33258 staining. After treatment with 0.5,
1, 2 and 4 µM SAN for 24 h, the cells showed obvious
features of apoptosis: chromatin condensation and nuclear
fragmentation (Fig. 1B). We
monitored the expression levels of the pro-apoptotic proteins Bax
and cleaved caspase-3 to confirm SAN-induced apoptosis. As shown in
Fig. 1C, SAN treatment upregulated
the expression of Bax and cleaved caspase-3, suggesting that
inhibition of growth by SAN involves apoptosis. Overall, these
results indicate an anticancer role of SAN in SPC-A1 cells.
SAN-induced ROS production is involved in
the regulation of apoptosis
Classically, chemotherapeutic agent-induced
apoptosis was thought to be associated with ROS production
(24,26,22).
In the present study, we aimed to determine whether ROS were
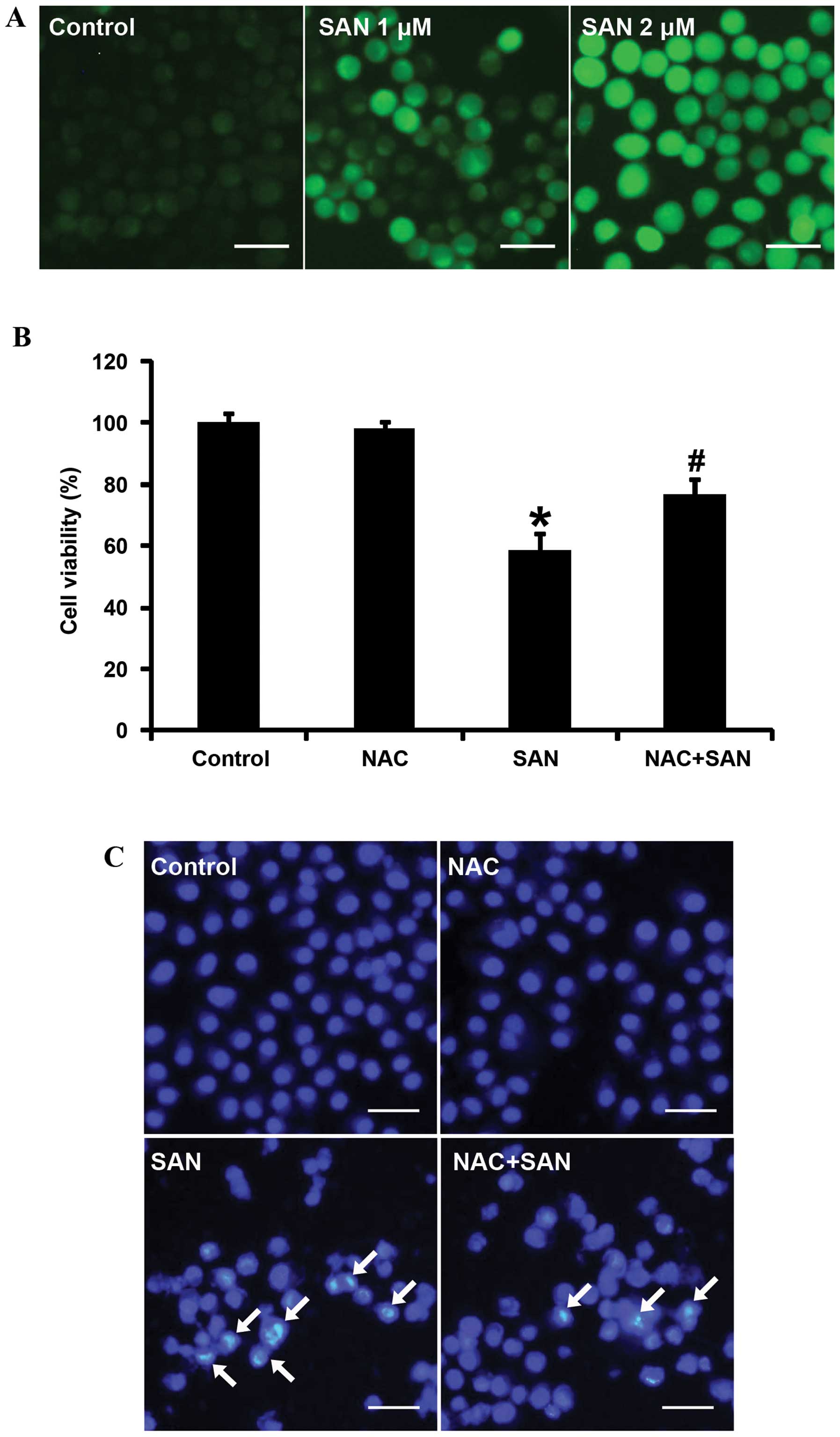
involved in SAN-induced SPC-A1 cell death. After treatment with 1
and 2 µM SAN for 24 h, ROS production was detected using
CM-H2DCFDA staining and laser-scanning confocal
microscopy. The results showed that ROS were significantly elevated
in a dose-dependent manner (Fig.
2A). ROS production is closely related to the induction of
apoptosis. Thus, we investigated whether ROS affect SAN-induced
growth inhibition and apoptosis. Pretreatment of SPC-A1 cells with
the ROS scavenger (NAC, 100 µM for 1 h) significantly
reversed growth inhibition (Fig.
2B) and apoptosis (Fig. 2C)
induced by SAN (2 µM for 24 h). Therefore, these results
suggest that SAN-mediated growth inhibition and apoptosis partially
rely on the generation of ROS.
SAN-induced ER stress is involved in the
regulation of apoptosis
ROS trigger oxidative stress, which may lead to ER
stress through the accumulation of unfolded proteins and/or
misfolded proteins. ER stress triggers the unfolded protein
response (UPR), which involves a great variety of molecules. We
monitored changes in the expression of genes associated with the
UPR pathway using the PCR array assay. The results showed that 32
genes were upregulated more than 2-fold after treatment with SAN (2
µM for 24 h) in the SPC-A1 cells, including
glucose-regulated protein 78 (GRP78, also called HSPA5, position
D4), inositol requiring kinase 1α (IRE1α, also called ERN1,
position C2), activating transcription factor 6α (ATF6α, position
A3), activating transcription factor 4 (ATF4, position A2), growth
arrest and DNA-damage-inducible gene 34 (GADD34, also called
PPP1R15A, position E11) and CAAT/enhancer binding protein
homologous protein (CHOP, also called DDIT3, position B2) (Fig. 3A). We next confirmed ER stress by
measuring protein expression by western blotting. Consistently, the
expression of GRP78, p-protein kinase R (PKR)-like ER kinase
(p-PERK), p-eukaryotic translation initiation factor 2α (p-elF2α),
ATF4 and CHOP were significantly increased in a dose-dependent
manner (Fig. 3B). Therefore, SAN
can induce ER stress in SPC-A1 cells.
 | Figure 3SAN induces ER stress. (A) Expression
of UPR pathway genes. SPC-A1 cells were incubated with 2 µM
SAN for 24 h. Changes in gene expression levels were detected using
the PCR array assay. Red, upregulation; green, downregulation. Gene
details not shown. (B) Effects of SAN on ER stress-related
proteins. The protein expression levels of GRP78, p-PERK, p-eIF2α,
ATF4 and CHOP were detected by western blotting. Cells were treated
with different concentrations of SAN (0, 0.5, 1, 2 and 4 µM)
for 24 h. (C) Effects of TUDCA on SAN-induced cell growth
inhibition. Treatment groups: 50 µM TUDCA, 2 µM SAN,
and 50 µM TUDCA plus 2 µM SAN for 24 h. Values
represent the mean ± SD (n=6), *P<0.01 compared with
the control group, #P<0.05 compared with the SAN
group. (D) Effects of TUDCA on SAN-induced apoptosis. Apoptotic
cells were stained with Hoechst 33258. Treatment groups: 50
µM TUDCA, 2 µM SAN, and 50 µM TUDCA plus 2
µM SAN for 24 h. Arrows indicate apoptotic cells. Scale bar,
25 µm. SAN, sanguinarine; UPR, unfolded protein response;
ER, endoplasmic reticulum; TUDCA, tauroursodeoxycholic acid. |
ER stress is thought to play an important role in
the cell pathophysiology process associated with cell death
(27). To confirm the effects of ER
stress on SAN-induced growth inhibition, we blocked ER stress using
50 µM TUDCA for 1 h, followed by treatment with 2 µM
SAN for 24 h, and then detected cell viability using the MTT assay.
Results indicated that TUDCA partly reversed SAN-induced cell
growth inhibition (Fig. 3C). In
addition, the Hoechst 33258 staining assay showed similar results,
with TUDCA inhibiting SAN-induced apoptosis (Fig. 3D). These data suggest that ER stress
is associated with SAN-induced cell growth inhibition and apoptosis
in SPC-A1 cells.
Relationship between ER stress and ROS in
lung cancer cells
To investigate the relationship between ER stress
and ROS, we aimed to determine whether ER stress is involved in the
regulation of ROS production. Notably, pretreatment with 50
µM TUDCA for 1 h, followed by treatment with 2 µM SAN
for 24 h, partially reversed SAN-induced ROS production (Fig. 4A). Next, the expression of the ER
stress-related proteins GRP78 and CHOP were monitored after
blocking ROS with NAC. After pretreatment of SPC-A1 cells with 100
µM NAC for 1 h, followed by treatment with 2 µM SAN
for 24 h, SAN-induced expression of GRP78 and CHOP was markedly
attenuated (Fig. 4B). These data
suggest that blocking ER stress with TUDCA can reduce ROS
production, whereas eliminating ROS by NAC can attenuate the extent
of ER stress. Therefore, ER stress and ROS production promote each
other and form a vicious cycle.
Discussion
Chemotherapy resistance is an important cause of
treatment failure in lung cancer. Therefore, identifying new and
effective agents is of particular importance (28). In the present study, we investigated
the anticancer effects and mechanism of action of SAN in lung
adenocarcinoma cells. We found that: i) SAN inhibit cell growth in
the human lung adenocarcinoma cell line SPC-A1 and induce apoptosis
in a dose-dependent manner; ii) SAN triggered the generation of
ROS, and eliminating ROS led to marked downregulation of
SAN-induced growth inhibition and apoptosis; iii) SAN activated the
ER stress signaling pathway, and blocking ER stress markedly
attenuated SAN-induced growth inhibition and apoptosis; and iv)
inhibition of ER stress can decrease ROS production, whereas
inhibition of ROS production can decrease the extent of ER
stress.
SAN is a benzophenanthridine alkaloid found in
several plants of the Papaveraceae family, including Chelidonium
majus, Bocconia frutescens and Sanguinaria
canadensis. Studies have demonstrated that SAN has anticancer
effects in various types of cancer, including prostate cancer,
osteosar-comas, breast cancer and colorectal cancer (10,13,29,30).
In the present study, SAN decreased cell viability in a
dose-dependent manner in the human lung adenocarcinoma cell line
SPC-A1. Meanwhile, we observed a dose-related induction of
apoptosis, indicating that SAN-induced growth inhibition involved
apoptosis. This evidence suggests that SAN may be a potential
chemotherapeutic candidate for lung cancer.
The most common forms of ROS, including superoxide,
H2O2 and hydroxyl radicals, are primarily
generated during respiratory ATP synthesis in mitochondria
(31). ROS are involved in a
variety of cell signaling processes and have a vital role in the
execution of cell physiological function. However, accumulation of
excessive intercellular ROS can trigger oxidative stress, leading
to apoptosis (32). A previous
study showed that SAN induced apoptosis in the human colorectal
cancer cell line HCT-116 through generation of ROS, followed by
mitochondrial membrane potential collapse (30). Thus, we examined whether SAN-induced
growth inhibition and apoptosis were dependent on ROS production in
lung cancer cell line. Our results showed that SAN triggered a
dose-dependent generation of ROS. Importantly, eliminating ROS
using NAC decreased ROS production, and attenuated SAN-induced
growth inhibition and apoptosis. These data suggest that SAN
induces an anticancer effect that is partially dependent on ROS
production.
The ER is involved in secretion and
membrane-targeted protein folding and modification. Abnormal
conditions such as oxidative stress, nutrient deprivation and
disruption of Ca2+ homeostasis can lead to misfolded
and/or unfolded protein accumulation in the lumen of the ER,
followed by activation of the ER stress response-UPR (20,27,33).
There are three sensors of ER stress: IRE1, PERK and ATF6, which
are responsible for initiation of UPR signaling (34). The UPR attenuates ER stress by
decreasing general protein translation, promoting the protein
folding capacity of the ER or by enhancing ER-associated
degradation (35). However,
recovery from severe ER stress is difficult and it triggers
apoptosis through activation of CHOP, a pro-apoptotic transcription
factor involved in all three arms of UPR signaling (36–38).
Many studies have found that ER stress is involved in the response
of cancer cells to chemotherapeutic agents (39–41).
In the present study, SAN triggered ER stress, which resulted in
the regulation of growth inhibition and apoptosis in lung cancer
SPC-A1 cells. We found that: i) SAN upregulated the expression of
32 genes involved in the UPR pathway; ii) SAN increased the
expression of ER stress-related proteins, including GRP78, p-PERK,
p-elF2α, ATF4 and CHOP; and iii) blocking ER stress with TUDCA
markedly decreased SAN-induced growth inhibition and apoptosis.
Therefore, these results indicate that SAN-induced growth
inhibition and apoptosis partially depend on ER stress.
Numerous studies have shown that ER stress is
associated with oxidative stress. High levels of ROS can induce the
accumulation of misfolded and/or unfolded proteins in the ER and
lead to ER stress (20,24), whereas continuous ER stress can
promote ROS production (21,26).
Our study showed that SAN increased ROS production, and that
blocking ER stress using TUDCA decreased SAN-induced ROS
production. Notably, eliminating ROS with NAC markedly decreased
the expression of ER stress-associated proteins GRP78 and CHOP.
These data indicate that ROS production and ER stress are mutually
essential and form a vicious cycle (22).
Collectively, we demonstrated that SAN induced cell
growth inhibition and apoptosis in lung adenocarcinoma SPC-A1
cells, and that these effects were partly dependent on ROS
production and ER stress. Our findings may be useful for the
development of SAN as an anticancer agent for lung cancer. However,
the anticancer effects in vivo and the precise mechanism of
action of SAN require further investigation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81272876) and the Development of
Science and Technology Plan Projects of Jilin (no. 2012Z019).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klastersky J and Awada A: Milestones in
the use of chemotherapy for the management of non-small cell lung
cancer (NSCLC). Crit Rev Oncol Hematol. 81:49–57. 2012.
|
|
3
|
Wang G, Reed E and Li QQ: Molecular basis
of cellular response to cisplatin chemotherapy in non-small cell
lung cancer (Review). Oncol Rep. 12:955–965. 2004.Review.
PubMed/NCBI
|
|
4
|
de Weger VA, Beijnen JH and Schellens JHM:
Cellular and clinical pharmacology of the taxanes docetaxel and
paclitaxel - a review. Anticancer Drugs. 25:488–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Wei D, Han X, Zhang W, Fan C,
Zhang J, Mo C, Yang M, Li J, Wang Z, et al: The combinational
effect of vincristine and berberine on growth inhibition and
apoptosis induction in hepatoma cells. J Cell Biochem. 115:721–730.
2014. View Article : Google Scholar
|
|
6
|
Sun M, Lou W, Chun JY, Cho DS, Nadiminty
N, Evans CP, Chen J, Yue J, Zhou Q and Gao AC: Sanguinarine
suppresses prostate tumor growth and inhibits survivin expression.
Genes Cancer. 1:283–292. 2010. View Article : Google Scholar
|
|
7
|
Godowski KC: Antimicrobial action of
sanguinarine. J Clin Dent. 1:96–101. 1989.PubMed/NCBI
|
|
8
|
Eisenberg AD, Young DA, Fan-Hsu J and
Spitz LM: Interactions of sanguinarine and zinc on oral
streptococci and Actinomyces species. Caries Res. 25:185–190. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaturvedi MM, Kumar A, Darnay BG, Chainy
GB, Agarwal S and Aggarwal BB: Sanguinarine (pseudochelerythrine)
is a potent inhibitor of NF-κB activation, IκBα phosphorylation,
and degradation. J Biol Chem. 272:30129–30134. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adhami VM, Aziz MH, Reagan-Shaw SR, Nihal
M, Mukhtar H and Ahmad N: Sanguinarine causes cell cycle blockade
and apoptosis of human prostate carcinoma cells via modulation of
cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery.
Mol Cancer Ther. 3:933–940. 2004.PubMed/NCBI
|
|
11
|
Park SY, Jin ML, Kim YH, Lee S-J and Park
G: Sanguinarine inhibits invasiveness and the MMP-9 and COX-2
expression in TPA-induced breast cancer cells by inducing HO-1
expression. Oncol Rep. 31:497–504. 2014.
|
|
12
|
Xu JY, Meng QH, Chong Y, Jiao Y, Zhao L,
Rosen EM and Fan S: Sanguinarine inhibits growth of human cervical
cancer cells through the induction of apoptosis. Oncol Rep.
28:2264–2270. 2012.PubMed/NCBI
|
|
13
|
Park H, Bergeron E, Senta H, Guillemette
K, Beauvais S, Blouin R, Sirois J and Faucheux N: Sanguinarine
induces apoptosis of human osteosarcoma cells through the extrinsic
and intrinsic pathways. Biochem Biophys Res Commun. 399:446–451.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jang BC, Park JG, Song DK, Baek WK, Yoo
SK, Jung KH, Park GY, Lee TY and Suh SI: Sanguinarine induces
apoptosis in A549 human lung cancer cells primarily via cellular
glutathione depletion. Toxicol In Vitro. 23:281–287. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martínez-Reyes I and Cuezva JM: The
H(+)-ATP synthase: A gate to ROS-mediated cell death or cell
survival. Biochim Biophys Acta. 1837:1099–1112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding Y, Zhu W, Sun R, Yuan G, Zhang D, Fan
Y and Sun J: Diphenylene iodonium interferes with cell cycle
progression and induces apoptosis by modulating NAD(P)H
oxidase/ROS/cell cycle regulatory pathways in Burkitt’s lymphoma
cells. Oncol Rep. 33:1434–1442. 2015.PubMed/NCBI
|
|
17
|
Ma J, Yang J, Wang C, Zhang N, Dong Y,
Wang C, Wang Y and Lin X: Emodin augments cisplatin cytotoxicity in
platinum-resistant ovarian cancer cells via ROS-dependent MRP1
downregulation. BioMed Res Int. 2014:1076712014. View Article : Google Scholar
|
|
18
|
Lim YJ, Choi JA, Lee JH, Choi CH, Kim HJ
and Song CH: Mycobacterium tuberculosis 38-kDa antigen induces
endoplasmic reticulum stress-mediated apoptosis via toll-like
receptor 2/4. Apoptosis. 20:358–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanchez-Lopez E, Zimmerman T, Gomez del
Pulgar T, Moyer MP, Lacal Sanjuan JC and Cebrian A: Choline kinase
inhibition induces exacerbated endoplasmic reticulum stress and
triggers apoptosis via CHOP in cancer cells. Cell Death Dis.
4:e9332013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Min KJ, Jung KJ and Kwon TK: Carnosic acid
induces apoptosis through reactive oxygen species-mediated
endoplasmic reticulum stress induction in human renal carcinoma
Caki cells. J Cancer Prev. 19:170–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang X, Xiang X, Xia M, Su J, Wu Y, Shen
L, Xu Y and Sun L: Inhibition of JNK3 promotes apoptosis induced by
BH3 mimetic S1 in chemoresistant human ovarian cancer cells. Anat
Rec. 298:386–395. 2015. View
Article : Google Scholar
|
|
22
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: a vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song B, Scheuner D, Ron D, Pennathur S and
Kaufman RJ: Chop deletion reduces oxidative stress, improves β cell
function, and promotes cell survival in multiple mouse models of
diabetes. J Clin Invest. 118:3378–3389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boussabbeh M, Salem IB, Prola A, Guilbert
A, Bacha H, Abid-Essefi S and Lemaire C: Patulin induces apoptosis
through ROS-mediated endoplasmic reticulum stress pathway. Toxicol
Sci. 144:328–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muchowicz A, Firczuk M, Wachowska M,
Kujawa M, Jankowska-Steifer E, Gabrysiak M, Pilch Z, Kłossowski S,
Ostaszewski R and Golab J: SK053 triggers tumor cell apoptosis by
oxidative stress-mediated endoplasmic reticulum stress. Biochem
Pharmacol. 93:418–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He PX, Zhang J, Che YS, He QJ, Chen Y and
Ding J: G226, a new epipolythiodioxopiperazine derivative, triggers
DNA damage and apoptosis in human cancer cells in vitro via ROS
generation. Acta Pharmacol Sin. 35:1546–1555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malhotra JD and Kaufman RJ: ER stress and
its functional link to mitochondria: Role in cell survival and
death. Cold Spring Harb Perspect Biol. 3:a0044242011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Pan H, Liu D, Mao N, Zuo C, Li L,
Xie T, Huang D, Huang Y, Pan Q, et al: Excision repair cross
complementation group 1 is a chemotherapy-tolerating gene in
cisplatin-based treatment for non-small cell lung cancer. Int J
Oncol. 46:809–817. 2015.
|
|
29
|
Kalogris C, Garulli C, Pietrella L,
Gambini V, Pucciarelli S, Lucci C, Tilio M, Zabaleta ME, Bartolacci
C, Andreani C, et al: Sanguinarine suppresses basal-like breast
cancer growth through dihydrofolate reductase inhibition. Biochem
Pharmacol. 90:226–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han MH, Kim GY, Yoo YH and Choi YH:
Sanguinarine induces apoptosis in human colorectal cancer HCT-116
cells through ROS-mediated Egr-1 activation and mitochondrial
dysfunction. Toxicol Lett. 220:157–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gupta SC, Hevia D, Patchva S, Park B, Koh
W and Aggarwal BB: Upsides and downsides of reactive oxygen species
for cancer: the roles of reactive oxygen species in tumorigenesis,
prevention, and therapy. Antioxid Redox Signal. 16:1295–1322. 2012.
View Article : Google Scholar :
|
|
32
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malhotra JD and Kaufman RJ: The
endoplasmic reticulum and the unfolded protein response. Semin Cell
Dev Biol. 18:716–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mollereau B, Manié S and Napoletano F:
Getting the better of ER stress. J Cell Commun Signal. 8:311–321.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dubey R and Saini N: STAT6 silencing
up-regulates cholesterol synthesis via miR-197/FOXJ2 axis and
induces ER stress-mediated apoptosis in lung cancer cells. Biochim
Biophys Acta. 1849:32–43. 2015. View Article : Google Scholar
|
|
37
|
Jiang T, Wang L, Li X, Song J, Wu X and
Zhou S: Inositol-requiring enzyme 1-mediated endoplasmic reticulum
stress triggers apoptosis and fibrosis formation in liver cirrhosis
rat models. Mol Med Rep. 11:2941–2946. 2015.
|
|
38
|
Feng J, Chen X and Sun X, Wang F and Sun
X: Expression of endoplasmic reticulum stress markers GRP78 and
CHOP induced by oxidative stress in blue light-mediated damage of
A2E-containing retinal pigment epithelium cells. Ophthalmic Res.
52:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang KA, Kim JK, Jeong YJ, Na SY and Hyun
JW: Dictyopteris undulata extract induces apoptosis via induction
of endoplasmic reticulum stress in human colon cancer cells. J
Cancer Prev. 19:118–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Alam GN, Ning Y, Visioli F, Dong
Z, Nör JE and Polverini PJ: The unfolded protein response induces
the angiogenic switch in human tumor cells through the PERK/ATF4
pathway. Cancer Res. 72:5396–5406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mimura N, Hideshima T, Shimomura T, Suzuki
R, Ohguchi H, Rizq O, Kikuchi S, Yoshida Y, Cottini F, Jakubikova
J, et al: Selective and potent Akt inhibition triggers anti-myeloma
activities and enhances fatal endoplasmic reticulum stress induced
by proteasome inhibition. Cancer Res. 74:4458–4469. 2014.
View Article : Google Scholar : PubMed/NCBI
|