Introduction
FUS1 (TUSC2, tumor suppressor candidate 2) is a
tumor-suppressor gene identified in the human chromosome 3p21.3
region in which allele losses and genetic alterations occur early
and frequently in many human cancers, including breast and lung
(1–10). Loss or reduction of FUS1 expression
has been detected in 100% of small-cell lung cancer (SCLC) and 82%
of non-small cell lung cancer (NSCLC) cases. Meanwhile, its loss or
reduction is associated with significantly worse overall patient
survival in NSCLCs (11). FUS1
deficiency causes increased susceptibility to certain types of
tumors and results in defects in natural killer (NK) cell
maturation coupled with IL-15 insufficiency (12). NK cells can rapidly recognize and
destroy infected or malignant cells and play a decisive role in the
regulation of adaptive immunity by stimulating other components of
the antitumor immune response (13). Numerous studies have emphasized the
major role of IL-15 in NK cell development (14–16).
Moreover, overexpression of FUS1 was found to significantly inhibit
tumor growth and progression in mouse models (17). However, it remains unknown whether
FUS1 regulates glioblastoma cell proliferation, invasion and
metastasis, and the role of FUS1 in microRNA (miRNA) regulation
remains undetermined.
MicroRNAs (miRs) are small non-coding RNAs that
control mRNA stability and the translation of target mRNAs by
binding to regulatory sites which are mostly located in the
3′-untranslated region (3′UTR) of the transcript (18). Recently, the involvement of miRNAs
in phenotypic modulation of human glioma has been reported. For
example, miRNA-21 knockdown was found to disrupt glioma growth
in vivo and to display synergistic cytotoxicity with neural
precursor cell delivered S-TRAIL in human gliomas (19); miRNA-34a is tumor suppressive in
brain tumors and glioma stem cells (20); and miRNA-181a was found to sensitize
human malignant glioma U87MG cells to radiation by targeting Bcl-2
(21).
In the present study, we firstly showed that high
expression of FUS1 was detected in low-grade human glioma and that
FUS1 inhibited the proliferation, migration and invasion of human
glioblastoma cells. Moreover, FUS1 overexpression upregulated
miR-197 expression in the glioblastoma cells. Subsequent studies
showed that miR-197 suppressed the proliferation, migration and
invasion in the cells. Silencing of miR-197 partly reduced the
effects of FUS1. Finally, using human glioblastoma tissue samples,
we demonstrated that miR-197 expression was negatively associated
with metastasis. All the results demonstrated that FUS1 acts as a
tumor-suppressor gene by at least partly upregulating miR-197 in
human glioblastoma and implied that restoration of FUS1 and miR-197
could be new therapeutic targets for glioblastoma.
Materials and methods
Immunohistochemistry
Tissues of human glioma were obtained from the
Yishui Central Hospital, Linyi People’s Hospital, Hubei Cancer
Center and Tianyou Hospital (29, WHO grade I; 27, WHO grade II; 32,
WHO grade III; and 27, WHO grade IV); 46 patients were females. The
mean age was 55 years (range, 30–74). Immunohistochemisty was
performed using standard techniques. Antigen retrieval was
performed by autoclaving. Incubation with 10% normal goat serum in
phosphate-buffered saline was performed for 18 min to eliminate
non-specific staining. Incubation with the anti-FUS1 antibody
(Abcam, Cambridge, MA, USA) was carried out. Finally, sections were
lightly counterstained with 10% Mayer’s hematoxylin, dehydrated,
mounted and observed. Staining was evaluated by a neuropathologist
and an investigator blinded to the diagnosis. Sections were
classified: − (negative), + (focal and weak immunoreactivity), ++
(diffuse and weak or focal and intense immunoreactivity) and +++
(diffuse and intense immunoreactivity). The data were analyzed by
SPSS 19.0 statistical package. Quantitative image analysis was
performed as previously described (22). The comparison of high expression
rates was by Chi-square test. The use of the human tissue samples
followed internationally recognized guidelines as well as local and
national regulations. Research carried out on humans followed
international and national regulations. The medical ethics
committees of the participating institutions approved the
experiments undertaken.
Cell line and transfection
Human glioblastoma U87MG cells were obtained from MD
Anderson Cancer Center (Houston, TX, USA). The cells were cultured
in a complete medium [RPMI-1640 supplement with 10% fetal calf
serum (FCS); gibco, grand Island, NY, USA]. Cells were maintained
in a humidified atmosphere containing 5% CO2 at 37°C.
FUS1-expressing plasmids, empty vector (pcDNA3.1), FUS1-sh-RNA and
the scramble were purchased from Tiangene (Tianjin, China).
Pre-miR-197 and control-miR were purchased from Ambion (Austin, TX,
USA). A final concentration of 50 nm of pre-miR-197/anti-miR-197
and its respective negative control (control-miR/scramble) were
used for each transfection. For transfection experiments, the cells
were cultured in serum-free medium without antibiotics at 60%
confluency for 24 h, and then transfected with transfection reagent
(Lipofectamine 2000; Invitrogen, Carlsbad, CA, USA) according to
the manufacturer’s instructions. After incubation for 6 h, the
medium was removed and replaced with normal culture medium for 48
h, unless otherwise specified.
Examination of cell proliferation with
MTT assay
Examination of cell proliferation with the MTT assay
was performed as previously described (23). The effect on the cell proliferation
was assessed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay (MTT
assay; Sigma, St. Louis, MO, USA). Absorbance was directly
proportional to the number of surviving cells.
BrdU proliferation analysis
Cell proliferation was assessed using a colorimetric
BrdU proliferation kit according to the manufacturer’s instructions
(Roche, Indianapolis, IN, USA). The transfected cells were labeled
with BrdU for 4 h. The genomic DNA was fixed and denatured, and
then incubated with peroxidase-conjugated anti-BrdU antibody for
100 min. A substrate for the conjugated peroxidase was then added,
and the reaction product was quantified by measuring the
absorbance. The results were then normalized by the number of total
viable cells.
Migration and invasion assays
Migration and invasion assays were performed as
previously described (24). For the
Transwell migration assays, 5×104 cells were plated in
the top chamber with a non-coated membrane (24-well insert; pore
size, 8-mm; BD biosciences, Lincoln, NE, USA). For the invasion
assays, 1.5×105 cells were plated in the top chamber
with a Matrigel-coated membrane (24-well insert; pore size, 8-mm;
BD Biosciences). In both assays, the cells were plated in medium
without serum, and medium supplemented with serum was used as a
chemoattractant in the lower chamber. The cells were incubated for
24 h, and cells that did not migrate or invade through the pores
were removed by a cotton swab. Cells on the lower surface of the
membrane were stained with the Diff-Quick Stain Set (Dade) and
counted.
Western blot analysis
Western blot analysis was performed as previously
described (23). Mainly, after
incubation with the primary antibody anti-FUS1 (1:500), anti-c-myc
(1:500), anti-PCNA (1:500), anti-Ki67 (1:500), anti-RB (1:500) or
anti-β-actin (1:500) (all from Abcam) overnight at 4°C,
IRDye™-800-conjugated anti-rabbit secondary antibodies (LI-COR
Biosciences, Lincoln, NE, USA) were used for 30 min at room
temperature. The specific proteins were visualized by Odyssey™
Infrared Imaging System (gene Company, Lincoln, NE, USA).
miRNA microarray
Total RNA from cultured cells, with efficient
recovery of small RNAs, was isolated using the mirVana miRNA
Isolation kit (Ambion). cRNA for each sample was synthesized using
the 3′ IVT Express kit (Affymetrix, Santa Clara, CA, USA) according
to the manufacturer’s protocols. The purified cRNA was fragmented
by incubation in fragmentation buffer (provided in the 3′IVT
Express kit) at 95°C for 35 min and chilled on ice. The fragmented
labeled cRNA was applied to microRNA2.0 array and hybridized in the
GeneChip Hybridization Oven 640 (both from Affymetrix) at 45°C for
18 h. After washing and staining in GeneChip Fluidics Station 450,
the arrays were scanned using GeneChip Scanner 3000 (both from
Affymetrix). The gene expressions levels of samples were normalized
and compared using Partek GS 6.5 (Partek Inc., St. Louis, MO, USA).
Average-linkage hierarchical clustering of the data was applied
using Cluster (http://rana.lbl.gov) and the results
were displayed using TreeView (http://rana.lbl.gov) (both from Eisen et al,
Stanford University, Stanford, CA, USA).
Real-time PCR for miRNA
Total RNA from cultured cells, with efficient
recovery of small RNAs, was isolated using the mirVana miRNA
Isolation kit. Detection of the mature form of miRNAs was performed
using the mirVana qRT-PCR miRNA detection kit, according to the
manufacturer’s instructions (Ambion). The U6 small nuclear RNA was
used as an internal control.
Results
Low-expression of FUS1 is detected in
high-grade human glioma
To show the general importance of FUS1 in the
pathogenesis of glioblastoma, we applied human glioma specimens to
detect FUS1 expression. Staining was evaluated by a
neuropathologist and an investigator blinded to the diagnosis. All
the tissue sections expressed FUS1 protein, thus there was no
tissue section that could be classified as − (negative). Positive
staining for FUS1 was observed in the tumor cells (data not shown).
Tissue sections of glioma for each grade were divided into two
groups (+ and ++/+++) (Table I).
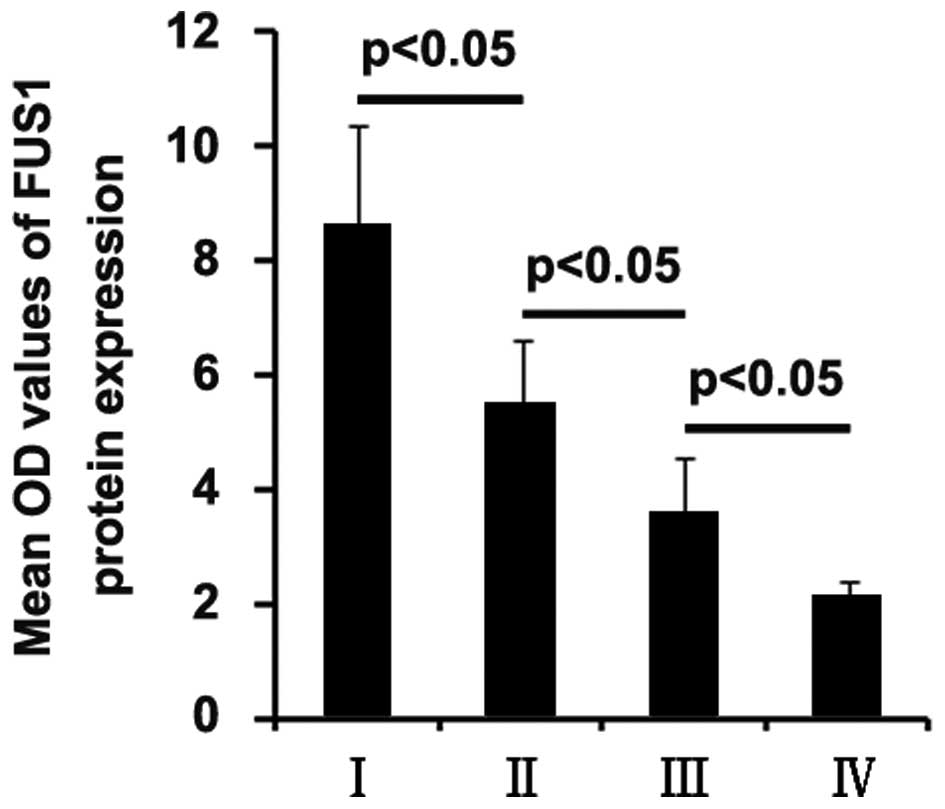
The expression rates of the FUS1 protein in the ++/+++ group in
glioblastoma of grades I, II, III and IV were 83, 66, 34 and 22%,
respectively, and compared with the + group (Table I).
 | Table IFUS1 expression in human glioma. |
Table I
FUS1 expression in human glioma.
| Grade | n | FUS1
|
|---|
| + | ++/+++ (%) |
|---|
| I | 29 | 5 | 24 (83) |
| II | 27 | 9 | 18 (66) |
| III | 32 | 21 | 11 (34) |
| IV | 27 | 21 | 6 (22) |
In order to further confirm that low expression of
FUS1 is associated with high-grade human glioma, quantitative image
analysis was performed to analyze FUS1 protein expression in the
tissue sections. We found that FUS1 expression was negatively
associated with the grade of glioma (Fig. 1).
Overexpression of FUS1 in human
glioblastoma U87MG cells inhibits cell proliferation, migration and
invasion
In an attempt to identify the role of FUS1 in
regulating the proliferation of U87MG cells, the cells were
transfected with FUS1-expressing plasmids. After stable
transfection, FUS1 protein expression was detected by western
blotting. The results showed that the FUS1-expressing plasmids
evidently increased FUS1 protein expression in the U87MG cells
(Fig. 2A). Moreover, the
proliferation rates of the U87MG cells were tested by MTT assay.
The results showed that FUS1 inhibited the proliferation of the
U87MG cells and the decrease in cell proliferation was
dose-dependent (Fig. 2B). This was
further revealed by BrdU incorporation analysis showing that
transfection of the U87MG cells with the FUS1-expressing plasmids
resulted in decreased DNA synthesis activity/viable cells (Fig. 2C). To further confirm that FUS1
regulates proliferation of the cells, we performed western blotting
to detect expression of the proliferation-associated markers,
c-myc, PCNA, Ki67 and RB. The results showed that c-myc, PCNA and
Ki67 expression levels were downregulated in the cells transfected
with the FUS1-expressing plasmids (Fig.
2D). All of the results demonstrated that FUS1 inhibits the
proliferation of U87MG cells.
Given that the invasive ability of glioma is found
to be closely correlated with their pathological grade (25) and FUS1 expression is associated with
the grade of glioma, we next sought to determine whether FUS1 has
any impact on the migration and invasion of U87MG cells. The cell
migration and invasion assays showed that overexpression of FUS1
not only suppressed the migration of the U87MG cells, but also
inhibited cell invasion (Fig.
2E).
Knockdown of FUS1 promotes the
proliferation, migration and invasion of U87MG cells
To provide further evidence that FUS1 is involved in
the proliferation, migration and invasion of U87MG cells, we
studied the effects of an inhibitor of FUS1. After transfection,
FUS1 expression was detected by western blotting, and the
proliferation rate of the U87MG cells was tested by MTT assay. The
results showed that FUS1-sh-RNA significantly decreased FUS1
expression in the U87MG cells, and proliferation of the cells
transfected with FUS1-sh-RNA was found to be higher than that of
the cells transfected with the scramble (Fig. 3A and B). Consistent with the MTT
assay, BrdU incorporation analysis demonstrated that DNA synthesis
in the cells was increased by FUS1-sh-RNA (Fig. 3C). In addition, we also performed
migration and invasion assays and found that FUS1-sh-RNA
significantly increased the migration and invasion of the U87MG
cells (Fig. 3D). FUS1-sh-RNA played
an opposite role when compared with FUS1 in regulating the
proliferation, migration and invasion in the U87MG cells.
FUS1 significantly upregulates miR-197
expression in U87MG cells
Tumor-suppressor genes exerts their functions by
regulating miRNA expression in glioma (26) and miRNA involvement in glioma
pathogenesis and some function as tumor-suppressor genes or
oncogenes (20,27,28).
Thus, we reasoned that FUS1 functions as a tumor suppressor by
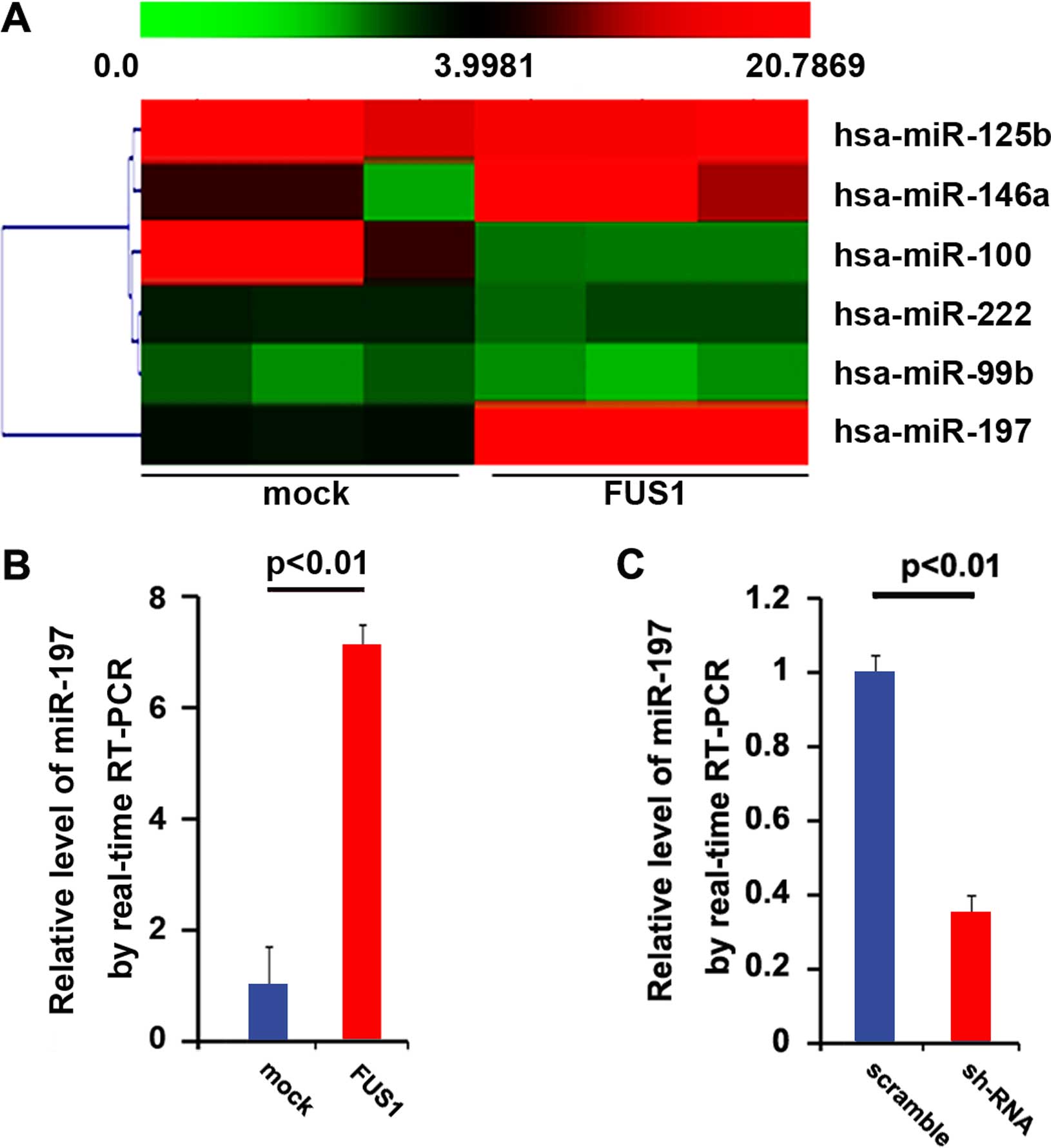
regulating relevant miRNAs. Thus, an miRNA microarray was
performed. RNAs isolated from the U87MG cells were hybridized to a
custom miRNA microarray platform. After three series of
hybridization, quantification and normalization, a number of miRNAs
were found to be altered in the cells. Yet, we were interested in
miR-197, since it was downregulated in glioblastoma (29). The results of the microarray showed
that miR-197 was increased >10-fold (Fig. 4A). To further confirm the regulation
of FUS1, we performed real-time PCR to detect the expression of
miR-197. Consistent with previous studies, the results of the
real-time PCR showed that FUS1 significantly upregulated miR-197
expression (Fig. 4b) and
FUS1-sh-RNA downregulated miR-197 expression (Fig. 4C) in the U87MG cells.
Overexpression of miR-197 inhibits the
proliferation, migration and invasion of U87MG cells
miR-197 expression was found to be downregulated in
glioblastoma (29). Having
demonstrated that FUS1 functions as a tumor-suppressor gene as well
as that miR-197 expression is upregulated by FUS1 in human
glioblastoma U87MG cells, we reasoned that FUS1 suppresses the
proliferation, migration and invasion in U87MG cells by
upregulating miR-197 expression.
In an attempt to identify the role of miR-197 in
regulating the proliferation of U87MG cells, the cells were
transfected with pre-miR-197. After stable transfection, miR-197
expression was detected by real-time PCR and the proliferation
rates of U87MG cells were tested by MTT assay. The results showed
that exogenous miR-197 stably increased its expression in the U87MG
cells (Fig. 5A). Overexpression of
miR-197 significantly reduced the proliferation rate of the U87MG
cells after 48 and 72 h of transfection, and the inhibition of cell
proliferation was time-dependent (Fig.
5B). This was further revealed by BrdU incorporation analysis
showing that transfection of U87MG cells with pre-miR-197 resulted
in reduced DNA synthesis activity/viable cells (Fig. 5C).
We next sought to determine whether miR-197 has any
impact on migration and invasion in the U87MG cells. The cell
migration and invasion assays of the U87MG cells showed that
overexpression of miR-197 not only inhibited the migration of the
U87MG cells, yet also suppressed cell invasion (Fig. 5D).
Knockdown of miR-197 promotes the
proliferation, migration and invasion of U87MG cells
To provide further confirm that miR-197 is involved
in U87MG cell proliferation, migration and invasion, we studied the
effects of an inhibitor of miR-197. After transfection, miR-197
expression was detected by real-time PCR, and the proliferation
rate of the U87MG cells was tested by MTT assay. The results showed
that miR-197 inhibitor (anti-miR-197) decreased miR-197 expression
in the U87MG cells (Fig. 6A), and
the proliferation rate of the U87MG cells transfected with miR-197
inhibitors was found to be higher than that of the cells
transfected with the scramble (Fig.
6B). Consistent with the MTT assay, BrdU incorporation analysis
demonstrated that DNA synthesis was increased by the miR-197
inhibitor in the cells (Fig. 6C).
Finally, we found that miR-197 inhibitos also significantly
increased the migration and invasion of the U87MG cells (Fig. 6D). miR-197 inhibitors play an
opposite role when compared with miR-197 in regulating the
proliferation, migration and invasion of the U87MG cells.
miR-197 knockdown attenuates the
biological functions of FUS1
Having demonstrated that FUSI significantly promotes
miR-197 expression as well as both FUS1 and miR-197 inhibit the
proliferation, migration and invasion, we reasoned that FUS1
functions as a tumor-suppressor gene by regulating miR-197 in the
U87MG cells. Thus, miR-197 knockdown attenuated the biological
functions of FUS1. Anti-miR-197 effectively inhibited FUS1-mediated
miR-197 regulation (Fig. 7A).
Importantly, miR-197 knockdown attenuated the effects of FUS1 in
the U87MG cells not totally but partially (Fig. 7B–D). All the results further
illustrated that FUS1 inhibited the proliferation, migration and
invasion by partly upregulating miR-197 in the U87MG cells.
miR-197 expression is downregulated in
metastatic glioblastoma tissues
We determined miR-197 expression levels in primary
tumor samples from 23 patients with glioblastoma. When compared
with the normal tissues, the miR-197 expression level was lower in
all of the glioblastoma tissues from metastasis-positive patients
(6/6). In contrast, 50% of the metastasis-free patients (9/18) had
elevated miR-197 levels in their primary tumors (P<0.05,
Fig. 8). These results are
consistent with the expression pattern of miR-197 in the cultured
human glioblastoma cells.
Discussion
FUS1 is a novel candidate tumor-suppressor gene
frequently inactivated in lung cancer, and loss of FUS1 expression
has been observed in almost all SCLC cell lines and tumor tissue
specimens (11), suggesting that
FUS1 functions as a tumor suppressor in SCLC. Although the
importance of FUS1 in lung cancer development has been firmly
established, its role in glioma has rarely been addressed.
Consistent with previous studies in lung cancer, we found that FUS1
expression is negatively associated with the grade of glioblastoma.
In vitro, our results for the first time demonstrated that
FUS1, as an antitumor agent, inhibited the proliferation, migration
and invasion of U87MG cells. In addition, consistent with the
results of MTT and BrdU assays, western blotting of proliferation
markers (PCNA, c-myc and Ki67) showed that the proliferation of
U87MG cells was inhibited by FUS1.
The levels of miR-197 were found to be markedly
upregulated in cancer cell lines, suggesting that miR-197 functions
as an oncogene in lung cancer and miR-197 downregulated
tumor-suppressor gene FUS1 expression through targeting its 3′UTR
(30). Yet, in glioblastoma,
miR-197 was downregulated compared with normal tissues (29). We demonstrated that miR-197 was
significantly upregulated by FUS1 in the U87MG cells. Yet, miR-197
did not suppress FUS1 protein expression in U87MG and MDA-MB-468
cells (data not shown). We showed that miR-197 inhibited the
proliferation, migration and invasion as well as was negatively
associated with metastasis in glioblastoma. Thus, miR-197 plays a
completely different role between lung cancer and glioblastoma.
miR-197 knockdown partly reduced FUS1-mediated
proliferation, and partly eliminated the migration and invasion
promoted by it. Thus, except for miR-197, we reasoned that there
are other downstream effectors of FUS1 responsible for its
tumor-suppressing function.
FUS1 deficiency results in increased susceptibility
to a certain range of tumors and causes defects in NK maturation
coupled with IL-15 insufficiency (12). In future studies we will aim to
ascertain whether FUS1 overexpression upregulates IL-15 in
glioblastoma, whether FUS1 is associated with NK cell maturation,
how miR-197 is related to this process, and which downstream target
genes mediate the roles of miR-197.
Consistent with previous studies showing that
miR-197 expression is downregulated in glioblastoma compared with
normal tissues (29), we
demonstrated that FUS1 inhibited U87MG cell proliferation, invasion
and migration, at least in part, by regulating miR-197 expression.
The FUS1 functions in glioblastoma U87MG cells demonstrated in the
present study have potential basic and clinical implications. FUS1
may be a powerful suppressor of proliferation/migration/invasion in
human glioblastoma, and pharmacological restoration of FUS1 may
represent a promising therapeutic strategy. Expression of FUS1
protein is regulated at various levels, leading to loss or greatly
diminished tumor-suppressor function. miR-93, miR-98 and miR-197
negatively regulate the expression of tumor-suppressor gene FUS1 in
SCLC cells (30). Yet, we did not
detect any change in FUS1 in miR-197-transfected U87MG and
MDA-MB-468 cells. Thus, miR-197 appears to negatively regulate the
expression of FUS1 only in SCLC. Mechanisms involved in the
regulation of FUS1 expression and targets of miR-197 continue to be
identified in glioblastoma.
References
|
1
|
Yan PS, Shi H, Rahmatpanah F, Hsiau TH,
Hsiau AH, Leu YW, Liu JC and Huang TH: Differential distribution of
DNA methylation within the RASSF1A CpG island in breast cancer.
Cancer Res. 63:6178–6186. 2003.PubMed/NCBI
|
|
2
|
Zabarovsky ER, Lerman MI and Minna JD:
Tumor suppressor genes on chromosome 3p involved in the
pathogenesis of lung and other cancers. Oncogene. 21:6915–6935.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maitra A, Wistuba II, Washington C,
Virmani AK, Ashfaq R, Milchgrub S, Gazdar AF and Minna JD:
High-resolution chromosome 3p allelotyping of breast carcinomas and
precursor lesions demonstrates frequent loss of heterozygosity and
a discontinuous pattern of allele loss. Am J Pathol. 159:119–130.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller BJ, Wang D, Krahe R and Wright FA:
Pooled analysis of loss of heterozygosity in breast cancer: A
genome scan provides comparative evidence for multiple tumor
suppressors and identifies novel candidate regions. Am J Hum genet.
73:748–767. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Q, Yoshimura G, Mori I, Sakurai T and
Kakudo K: Chromosome 3p and breast cancer. J Hum genet. 47:453–459.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lerman MI and Minna JD: The 630-kb lung
cancer homozygous deletion region on human chromosome 3p21.3:
Identification and evaluation of the resident candidate tumor
suppressor genes. The International Lung Cancer Chromosome 3p213
Tumor Suppressor Gene Consortium. Cancer Res. 60:6116–6133.
2000.PubMed/NCBI
|
|
7
|
Wistuba II, Gazdar AF and Minna JD:
Molecular genetics of small cell lung carcinoma. Semin Oncol.
28(Suppl 4): S3–S13. 2001. View Article : Google Scholar
|
|
8
|
Minna JD, Fong K, Zöchbauer-Müller S and
Gazdar AF: Molecular pathogenesis of lung cancer and potential
translational applications. Cancer J. 8(Suppl 1): S41–S46.
2002.PubMed/NCBI
|
|
9
|
Sekido Y, Fong KM and Minna JD: Molecular
genetics of lung cancer. Annu Rev Med. 54:73–87. 2003. View Article : Google Scholar
|
|
10
|
Ji L and Roth JA: Tumor suppressor FUS1
signaling pathway. J Thorac Oncol. 3:327–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prudkin L, Behrens C, Liu DD, Zhou X,
Ozburn NC, Bekele BN, Minna JD, Moran C, Roth JA, Ji L, et al: Loss
and reduction of FUS1 protein expression is a frequent phenomenon
in the pathogenesis of lung cancer. Clin Cancer Res. 14:41–47.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ivanova AV, Ivanov SV, Pascal V, Lumsden
JM, Ward JM, Morris N, Tessarolo L, Anderson SK and Lerman MI:
Autoimmunity, spontaneous tumourigenesis, and IL-15 insufficiency
in mice with a targeted disruption of the tumour suppressor gene
Fus1. J Pathol. 211:591–601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Degli-Esposti MA and Smyth MJ: Close
encounters of different kinds: Dendritic cells and NK cells take
centre stage. Nat Rev Immunol. 5:112–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vosshenrich CA, Ranson T, Samson SI,
Corcuff E, Colucci F, Rosmaraki EE and Di Santo JP: Roles for
common cytokine receptor gamma-chain-dependent cytokines in the
generation, differentiation, and maturation of NK cell precursors
and peripheral NK cells in vivo. J Immunol. 174:1213–1221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Williams NS, Klem J, Puzanov IJ, Sivakumar
PV, Schatzle JD, Bennett M and Kumar V: Natural killer cell
differentiation: Insights from knockout and transgenic mouse models
and in vitro systems. Immunol Rev. 165:47–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fehniger TA and Caligiuri MA: Interleukin
15: Biology and relevance to human disease. Blood. 97:14–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji L, Nishizaki M, Gao B, Burbee D, Kondo
M, Kamibayashi C, Xu K, Yen N, Atkinson EN, Fang B, et al:
Expression of several genes in the human chromosome 3p21.3
homozygous deletion region by an adenovirus vector results in tumor
suppressor activities in vitro and in vivo. Cancer Res.
62:2715–2720. 2002.PubMed/NCBI
|
|
18
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corsten MF, Miranda R, Kasmieh R,
Krichevsky AM, Weissleder R and Shah K: MicroRNA-21 knockdown
disrupts glioma growth in vivo and displays synergistic
cytotoxicity with neural precursor cell delivered S-TRAIL in human
gliomas. Cancer Res. 67:8994–9000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guessous F, Zhang Y, Kofman A, Catania A,
Li Y, Schiff D, Purow B and Abounader R: microRNA-34a is tumor
suppressive in brain tumors and glioma stem cells. Cell Cycle.
9:1031–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P
and Hu W: MicroRNA-181a sensitizes human malignant glioma U87MG
cells to radiation by targeting Bcl-2. Oncol Rep. 23:997–1003.
2010.PubMed/NCBI
|
|
22
|
Bacus SS, Zelnick CR, Plowman G and Yarden
Y: Expression of the erbB-2 family of growth factor receptors and
their ligands in breast cancers. Implication for tumor biology and
clinical behavior. Am J Clin Pathol. 102(Suppl 1): S13–S24.
1994.PubMed/NCBI
|
|
23
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar
|
|
24
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohgaki H and Kleihues P: Genetic pathways
to primary and secondary glioblastoma. Am J Pathol. 170:1445–1453.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Novakova J, Slaby O, Vyzula R and Michalek
J: MicroRNA involvement in glioblastoma pathogenesis. Biochem
Biophys Res Commun. 386:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sasayama T, Nishihara M, Kondoh T, Hosoda
K and Kohmura E: MicroRNA-10b is overexpressed in malignant glioma
and associated with tumor invasive factors, uPAR and RhoC. Int J
Cancer. 125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sana J, Hajduch M, Michalek J, Vyzula R
and Slaby O: MicroRNAs and glioblastoma: Roles in core signalling
pathways and potential clinical implications. J Cell Mol Med.
15:1636–1644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du L, Schageman JJ, Subauste MC, Saber B,
Hammond SM, Prudkin L, Wistuba II, Ji L, Roth JA, Minna JD, et al:
miR-93, miR-98, and miR-197 regulate expression of tumor suppressor
gene FUS1. Mol Cancer Res. 7:1234–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|