Introduction
Cardiac myxoma (CM) is mostly sporadic in heart
diseases (1,2), and the clinical manifestation and
long-term complications include the recurrence, sudden death, heart
failure and potential coronary embolisms (3,4). Due
to scarcity of studies indicating the molecular and signaling
mechanisms in the CM development, it is very hard to realize the
target therapy of CM. Generally surgical resection of CM mass is
recommended as the only clinical regimen; however, this option has
severe risk of death and can cause recurrences, infarction and
stroke (5). Thus, it is necessary
to throw light on the molecular mechanisms underlying CM
progression, by which we can develop some new and useful targets
agents.
It should be noted that astrocyte elevated gene-1
(AEG-1) can be produced in primary human fetal astrocytes (6). Recently, some studies proved that
AEG-1 plays a crucial role in the pathogenesis, progression and
invasion in different tumors (7–9). AEG-1
was also reported to induce the expression of E-cadherin and
vimentin (10,11), suggesting that AEG-1 may be involved
in the regulation of epithelial-to-mesenchymal transition (EMT)
(12). Moreover, CCR5, a
G-protein-coupled receptor, activates cellular signaling cascades
by binding to its ligand CCL3 (13,14).
CCL3/CCR5 axis promotes tumor development in various ways,
including angiogenesis, modulation of extracellular matrix
(15). However, the underlying
mechanisms of CCL3/CCR5 in CM and the signaling pathways are not
well known.
In the present study, we investigated and analyzed
the biological roles of AEG-1 in CCL3/CCR5-induced EMT process
using immunohistochemistry, immunoblotting, siRNA transfection and
proliferation assay. Based on our results, we concluded that AEG-1
mediates CCL3/CCR5-induced EMT of CM via Erk1/2 instead of Akt
signaling pathway, which indicates that CCL3/CCR5-AEG-1-Erk1/2-EMT
pathway could be indicative of a useful target to benefit CM
patients.
Materials and methods
Patients and tissues
Thirty left atrial myxomas were enrolled, including
14 male and 16 female patients (mean age, 53±5.8) who underwent
surgery between August 2001 and August 2014. Gross assessment of CM
tissues was carried out in order to measure the basic parameters
such as tumor size. All pathological tissues were subjected to
formalin fixation and paraffin-embedded. Prior to our medical
research, the patients consent was obtained from the Institute
Research Ethics Committee of Qilu Hospital of Shandong
University.
CM cell culture
The culture of CM cells was conducted as previously
mentioned in a published study (16). In vivo CM tissue was obtained
from a 45-year-old patient who was diagnosed with sporadic CM,
during surgical operation at Qilu Hospital. The patients gave
informed consent before the operation, and the study design was
approved and supported by the Qilu Hospital Medical Ethics
Committee. The CM cells were harvested and separated by enzymatic
digestion using collagenase and then were maintained in Dulbecco's
modified Eagle's medium (DMEM). Prior to in vitro assays,
the DMEM medium was changed with serum-free DMEM for another 24 h,
and then changed into fresh DMEM with the indicated reagents.
Reagents
Recombinant human CCL3 was purchased from Sigma (St.
Louis, MO, USA). Antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The specific antibodies
included anti-p-Erk1/2, anti-t-Erk1/2, anti-p-Akt, anti-AEG-1,
anti-N-cadherin, anti-MMP2 and anti-α-tubulin antibody (Santa Cruz
Biotechnology). All experiments were conducted in the absence of
fetal bovine serum (FBS).
Immunohistochemistry
All sections were dewaxed in xylene and rehydrated
in graded ethanol, followed by incubating in 3% hydrogen peroxide
for 10 min to quench endogenous peroxides. Samples were heated in
0.01 mol/l citrate buffer for 15 min at 100°C, and then were placed
at room temperature for 30 min. After cooled, samples were blocked
with 2% normal goat serum in phosphate-buffered saline (PBS) for 30
min to block antigenic epitopes, then incubated with primary
antibody (1:100 dilution; Santa Cruz Biotechnology) at 4°C
overnight. They were washed with PBS for 3 times, and then the
samples were incubated with system-labeled HRP anti-mouse secondary
antibody (Dako, Denmark) at room temperature for 20 min. Next, the
sections were incubated in DAB and counterstained in Mayer's
hematoxylin, dehydrated in alcohol and xylene. The positive-stained
slices (Santa Cruz Co.) were assessed from and PBS was used as
negative control. Under the microscope, the positive areas appeared
as brown yellow granules.
Evaluation of immunohistochemistry
staining
The score of the immunohistochemistry staining was
evaluated by one investigator who was blinded to the present study.
The sections were scored based on the positive percentage and
staining intensity. Sections were defined as positive if there were
substantial amount of brown yellow granules in the plasma of the
cells. The intensity of plasma staining was scored and graded as: 0
(0% cells); 1 (0–25% cells); 2 (25–50% cells); and 3 (>50%
cells). Staining intensity was also evaluated semi-quantitatively
as: 0 (none), 1 (mild), 2 (moderate), 3 (intense). The total score
for each section was then evaluated by multiplying the intensity
and positive percentage score, and was classified respectively into
four levels: 0 (−), 1–3 (+), 4–6 (++) and 7–9 (+++). The score was
considered negative or low expression when the total was <4, and
positive or high expression when it was ≥4.
siRNA transfection
AEG-1 and CCR5 siRNA (a generous gift from Dr Wei
Wang in Chinese Academy Science) transfection was performed as
previously described. Cells were plated in 24-well plates. After 24
h, the cells were transfected with control siRNA or with AEG-1/CCR5
siRNA using siRNA transfection reagent according to the
manufacturer's instructions. The following siRNA sequences were
targeted: AEG-1-siRNA positive sense, 5′-GGCAGGTATCTTTGTAA CTA-3′
and antisense, 5′-GCTGACTGATTCTGGTTCAT-3′; CCR5 siRNA positive
sense, 5′-GUUCAGAAACUACCUCU UAGUCUUCUUC-3′ and antisense,
3′-UUCAAGUCUUUGA UGGAGAAUCAGAAGAAG-5′; control siRNA-positive
sense, 5′-CAACCUUGCGGCCUUAGGGTT-3′ and antisense,
5′-UUGGCCCAAUUUCCCGGGCTT-3′.
Immunoblotting
Total cell protein was extracted using a commercial
kit, and the protein concentration was assessed by the BCA protein
assay. Subsequently 30 μg of denaturalized protein was
separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE),
and then transferred into polyvinylidene fuoride membranes
(Millipore). The protein was blocked with 5% skim milk in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 2 h at
room temperature. The protein was treated overnight with primary
antibodies at 4°C. After washed in TBST 3 times, the polyvinylidene
fuoride membrane was incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody for another 2 h. Finally, the
protein bands were measured using the ECL detection system.
Cell cycle distribution analysis
After cells were seeded for 24 h, the cells reached
~75% confluence and were then treated with CCL3 at concentrations
of 30 μM for 24 h. Following the treatment, cells were
detached and fixed with 70% ethanol at −20°C overnight.
Subsequently, the cells were collected by centrifugation at 250 × g
for 5 min. Then, the cells were washed with PBS and incubated with
25 μg/ml RNase A and 50 μg/ml PI for 30 min in the
dark. A total number of 1×104 cells were subject to cell
cycle analysis using a flow cytometry (Becton-Dickinson
Immunocytometry Systems, San Jose, CA, USA).
Proliferation assays
The proliferation assays were performed as reported
previously (16). Cells were seeded
into plastic wells and grown for 48 h in DMEM with 10% FBS. After
24 h, cells were treated with different IGF-1 treatment for 48 h
with high serum conditions, and then exposed to DMEM containing 1
mCi [3H]-thymidine for the next 24 h. The cells were
washed, obtained and counted in a liquid scintillation counter.
Statistical analysis
Data are expressed as the mean ± standard error
(SEM) of repeated assays. The correlation between CCR5 and AEG-1
was analyzed using Spearman's test. Significant differences between
the two groups were assessed using χ2 analysis,
Student's t-test and one-way ANOVA. In the present study, the
p<0.05 was considered to indicate a statistically significant
result.
Results
Expression of CCR5 and AEG-1 in the CM
tissue and cells
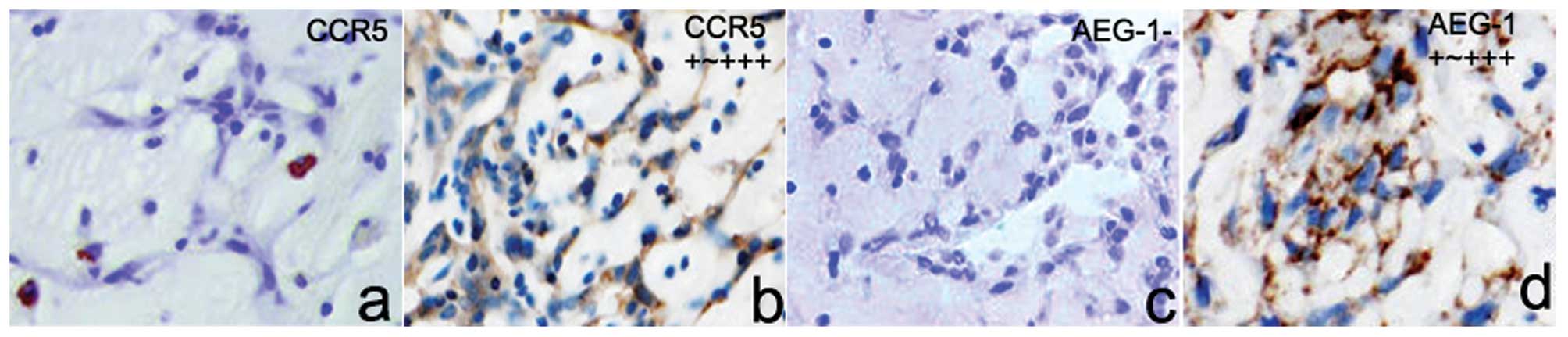
To identify the potential CCR5-AEG-1-EMT pathway in
CM tissues, we introduced immunohistochemistry to investigate the
expression level of CCR5 and AEG-1 in 30 cases of CM tissues. As
shown in Fig. 1, we found that 22
cases in all 30 CM tissues highly expressed CCR5 protein, and the
expression rate was 73.3%. However, CCR5 protein was rarely stained
in surrounding non-tumor samples. Similar with CCR5 expression
model, 23 cases of CM tissues expressed AEG-1 protein and the
expression ratio was 76.7%. We also demonstrated that AEG-1 protein
was not constitutively express in surrounding tissues. Under the
microscope, CCR5 and AEG-1 protein was mainly located at the cell
membrane and cytoplasm. Based on the Spearman's correlation
analysis, high CCR5 expression was closely correlated to high AEG-1
expression in CM tissues (r=0.919, p=0.001).
Correlations between CCR5, AEG-1
expression and clinicopathology
We analyzed the correlation between CCR5, AEG-1
expression and clinicopathology. As described in Table I, tumor size (>5 cm in diameter)
was significantly associated with high-CCR5 expression (p=0.024),
yet age and location had no significant association with the CCR5
expression. Likewise, tumor size (>5 cm in diameter) was also
closely associated with high AEG-1 expression (p=0.003), however,
age, gender and tumor location had no discernible associations with
the AEG-1 expression.
 | Table ICorrelation of CCR5, AEG-1 expression
with clinicopathological features of cardiac myxoma. |
Table I
Correlation of CCR5, AEG-1 expression
with clinicopathological features of cardiac myxoma.
| Indicators | | CCR5
| | AEG-1
| |
|---|
| Case | High | Low | P-value | High | Low | P-value |
|---|
| Age (years) | | | | | | | |
| <60 | 10 | 8 | 2 | 0.559 | 8 | 2 | 0.760 |
| ≥60 | 20 | 14 | 6 | | 15 | 5 | |
| Gender | | | | | | | |
| Male | 14 | 10 | 4 | 0.825 | 11 | 3 | 0.818 |
| Female | 16 | 12 | 4 | | 12 | 4 | |
| Location | | | | | | | |
| Left atrium | 26 | 20 | 6 | 0.257 | 20 | 6 | 0.933 |
| Right atrium | 4 | 2 | 2 | | 3 | 1 | |
| Tumor size (cm) | | | | | | | |
| <5 | 16 | 9 | 7 | 0.024 | 9 | 8 | 0.003 |
| ≥5 | 14 | 13 | 1 | | 14 | 0 | |
CCL3/CCR5 activates AEG-1 signaling and
the EMT process in cultured CM cells in a time- and dose-dependent
manner
To investigate the potential CCL3/CCR5 signaling
pathway in the EMT process, we cultured CM cells with different
CCL3 concentrations (0, 5, 10, 20, 30 and 40 ng/ml), and then
carried out immunoblotting to assess the expression status of the
downstream signaling protein p-Erk1/2, p-Akt and AEG-1. As shown in
Fig. 2a, immunoblotting results
identified that the expression of p-Erk1/2, p-Akt and AEG-1 was
highly upregulated in CCL3-induced CM cells in a dose-dependent
manner. Besides, we also assessed the EMT biomarkers vimentin,
N-cadherin and MMP2. Our results showed that the protein expression
of vimentin, N-cadherin and MMP2 was aberrantly increased in CCL3
treatment, which was also in a dose-dependent manner. In addition,
we assessed the appropriate time when the downstream protein and
EMT biomarkers in the cells can be activated. We found that CCL3
activates cells time-dependently and 30 min treatment is better
than the other time points. The treatment of 30 and 40 min showed
no differences (p=0.356) (Fig. 2b).
These results indicate that CCL3 activates AEG-1 signaling and the
EMT process in CM cells, and the detailed pathways should be
identified using gene silencing.
 | Figure 2Effects of CCL3/CCR5 axis on AEG-1
signaling pathways and EMT. (a) Cells were maintained in serum-free
medium for 30 min, and were then treated with human recombinant
CCL3 at different concentrations. After 30 min treatment, cells
lysates were subjected to immunoblotting. Subsequently, CCL3
signaling pathway-related molecules and EMT biomarkers were
assessed. Protein expression level was calculated using ImageJ Pro
software. α-tubulin was used as a normalization control. (b) Cells
were maintained in serum-free medium for 30 min, and then treated
with human recombinant CCL3 using 30 ng/ml of CCL3. Then, cells
lysates were subjected to immunoblotting at different time points
(0. 5, 10, 20, 30 and 40 min). Subsequently, CCL3 signaling
pathway-related molecules and EMT biomarkers were assessed. Protein
expression level was calculated using ImageJ Pro software.
α-tubulin was used as a normalization control. Each bar represents
the mean ± SEM of 3 independent experiments;
*p<0.001, compared with si-control. AEG-1, astrocyte
elevated gene-1; EMT, epithelial-to-mesenchymal transition. |
Knockdown of CCR5 abrogates CCL3-induced
AEG-1 signaling and EMT
To further investigate the potential CCL3/CCR5
signaling pathway in the EMT process we cultured CM cells with CCR5
siRNA, then treated with CCL3 treatment (30 ng/ml), and then
carried out immunoblotting to assess the expression status of the
downstream signaling protein p-Erk1/2, p-Akt and AEG-1. As shown in
Fig. 3, immunoblotting results
identified that the expression of p-Erk1/2, p-Akt was obviously
inhibited in CCR5 siRNA-induced CM cells. Besides, we also assessed
the EMT biomarkers vimentin, N-cadherin and MMP2. Our findings
showed that the protein expression of vimentin, N-cadherin and MMP2
is obviously affected. These results indeed indicate that CCL3
activates AEG-1 signaling and the EMT process in cultured CM
cells.
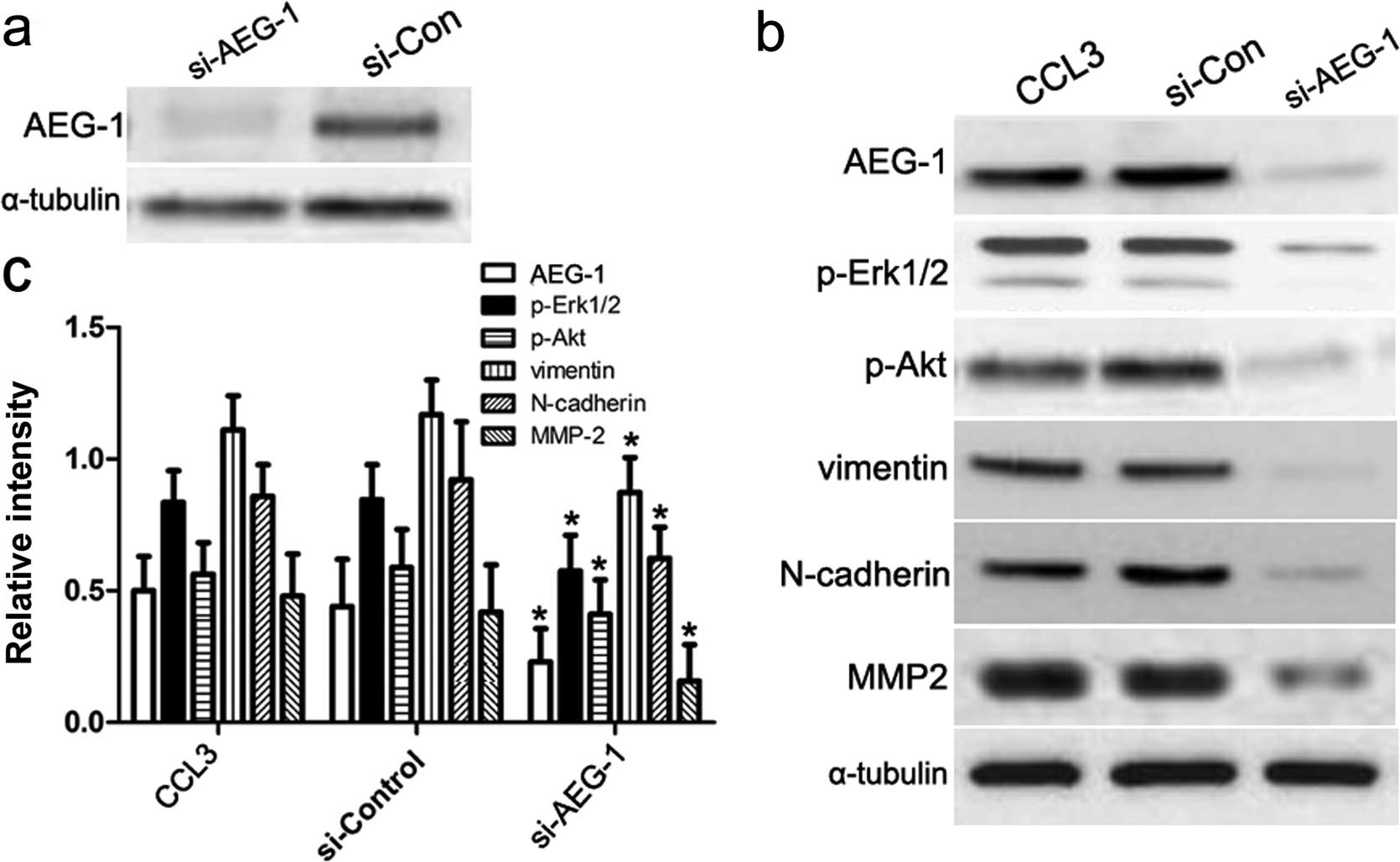
Knockdown of AEG-1 abrogates CCL3-induced
EMT
Based on the above studies, we assumed that AEG-1
played a key role in regulating CCL3-induced EMT. To this end, we
introduced AEG-1 siRNA to silence the mRNA transcription of AEG-1
gene (Fig. 4a), and then performed
immunoblotting assay to assess downstream signaling molecules in
the CCL3 pathway as well as EMT biomarkers as previously mentioned.
As shown in Fig. 4b and c, our
results showed the expression of p-Erk1/2, p-Akt, vimentin,
N-cadherin and MMP2 increased in CM cells treated with control
siRNA or CCL3 alone, which was consistent with our previous
conclusion. By contrast, once we transfected AEG-1 siRNA into CM
cells, the expression of p-Erk/2, p-Akt, vimentin, N-cadherin and
MMP2 was obviously decreased. These results suggest that AEG-1 acts
as a key mediator in CCL3-induced Erk1/2 and Akt signaling, and
then regulates the progression of EMT.
Silencing of either AEG-1 or CCR5 affects
CCL3-induced CM cell cycle
Based on the above studies, we further assessed the
effects of AEG-1 or CCR5 on cell cycle and the expression of cell
cycle regulators, including CDK2, cyclin D1 in CM cells using flow
cytometry and western blotting. Incubation of CM cells with
si-AEG-1 or si-CCR5 resulted in the inhibition of G1 phase entry
into S phase (Fig. 5a). At the same
time, the expression of CDK2 and cyclin D1 in CM cells was
obviously suppressed in comparison with the control (Fig. 5b), indicating that AEG-1 is a key
regulator in G1-to-S phase transition.
Depletion of either AEG-1 or CCR5 affects
CCL3-induced CM cell proliferation
To assess the impact of AEG-1 and CCR5 on
proliferation behavior of CM cells, we investigated the potential
roles of AEG-1 and CCR5 in CCL3-induced CM cell behavior using
proliferation assay. In the present study, CCL3 treatment caused a
2- to 5-fold increase in [3H]-thymidine incorporation in
a concentration-dependent manner (p<0.05). Unfortunately, this
increase induced by CCL3 stimulation was abolished by knockdown of
AEG-1 or CCR5. AEG-1 depletion decreased the constitutive
proliferation of CM cells in a dose-dependent manner (p<0.05)
(Fig. 6).
Discussion
In the published studies, CCL3/CCR5 axis has been
reported to be involved in CM development. Moreover, AEG-1 is also
reported to promote tumor progression, and involved in various
growth factors, chemokines-induced signaling pathways (17,18).
In the present study, we tried to elucidate the role of AEG-1 in
activation and regulation of CCL3-induced EMT pathway. Firstly,
based on IHC results, high expression of CCR5 and AEG-1 protein was
stained in major CM tissues. Pathological results proved that CCR5
and AEG-1 protein was significantly correlated with tumor size. In
accordance with our results, the published studies identified that
the CCR5 and AEG-1 were highly expressed in many other tumors, such
as hepatocellular carcinoma (19)
and also closely associated with the tumor size. All things
considered, we assumed that CCR5-AEG-1 pathway was implicated in
the progression of CM.
The EMT process was reported to play a key role in
the tumorigenesis. The EMT process is characterized as the
deregulation of cell-to-cell link systems and the consolidation of
cellular migration and motility, making abnormally proliferating
cells detached from the original epithelial tissues (20,21).
Recent studies showed that it is the high N-cadherin expression
that impairs E-cadherin regulated intercellular adhesion, and
results in lack of epithelial barrier and deregulation of
extracellular matrix (20). The
multiple molecules involved in EMT exert a useful effect on cancer
cell invasion, migration and dissemination. However the detailed
molecular mechanisms of EMT have not been absolutely elucidated,
thus we detailed the effects of CCR5-AEG-1 pathway on the EMT
processes. Our findings showed that AEG-1, p-Erk1/2, p-Akt,
vimentin, N-cadherin and MMP2 were highly expressed once stimulated
by CCL3. In contrast, when CM cells were transfected with AEG-1
siRNA, the expression of p-Erk/2, p-Akt, vimentin, N-cadherin and
MMP2 was obviously inhibited. These results indicate that CCL3
activates AEG-1 signaling and the EMT process via Erk1/2 and Akt
pathways.
The mitogen-activated extracellular signal-regulated
kinase Erk1/2 pathway is mostly featured as key signaling mediator
in the signaling transduction of tumor biology. The Erk1/2 pathway
can be activated by various factors, such as chemokines and
inflammation mediators. More and more studies found the dysfunction
of Erk1/2 signaling is the most common cause of cancer cell
proliferation, which greatly promotes malignancy transformation
(22). These kinases in EMT process
offer some novel targets (23). As
reported, the abnormal activation of AEG-1-Erk1/2 pathway in human
sacral chondrosarcoma mediated cell proliferation and EMT
progression (24). Moreover, the
effects of PI3K/Akt pathway on chemokine-induced EMT have been
focused on (25,26). However, few studies described the
role of AEG-1 in Akt-induced EMT. In the present study, our results
suggest that AEG-1 acts as a key mediator in CCL3-induced Erk1/2
pathway and the EMT process, as well as in Akt signaling.
Since functional analysis demonstrates the real
effects of AEG-1, we conducted proliferation assays, and observed
that CCL3 caused an obvious increase in [3H]-thymidine
incorporation in a concentration-dependent mode, and then
[3H]-thymidine incorporation was affected by knockdown
of AEG-1. AEG-1 depletion decreased the constitutive proliferation
of CM cells. These results indicate that AEG-1 interferes with
CCL3-induced CM cell invasion and migration. In agreement with our
studies, some studies on tumor biology also testified the
significance of AEG-1 in the cell growth of tumor cells (27,28).
In conclusion, our results suggest that AEG-1
mediates CCL3/CCR5-induced EMT of CM via both Akt and Erk1/2
signaling pathways in CM development, indicating that
CCL3-AEG-1-Erk1/2 and Akt-EMT pathways could be suggested as a
prospective target to antagonize the CM progression, which can
benefit CM patients in the clinical practice.
Acknowledgments
We greatly thank other members of our laboratory for
valuable suggestions and writing.
References
|
1
|
Lee HS, Kim HK, Park EA, Kim KH, Kim YJ
and Sohn DW: Left atrial intramural hematoma after removal of
atrial myxoma: Cardiac magnetic resonance in the differential
diagnosis of intracardiac mass. J Cardiovasc Ultrasound.
22:205–208. 2014. View Article : Google Scholar
|
|
2
|
Watanabe H, Nara I, Yamaura G, Iino K,
Iino T, Shimbo M, Seki K and Ito H: Blood balloon induced by an
atrial myxoma in the heart. Circulation. 130:2351–2353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anpalakhan S, Ramasamy D and Fan KS: An
unusual presentation of atrial myxoma. Singapore Med J.
55:e156–e158. 2014. View Article : Google Scholar
|
|
4
|
Choi K, Jung D, Hong SW, Jeon Y and Kim
SO: Extra cardiac tumor misdiagnosed as a left atrial myxoma.
Korean J Anesthesiol. 67(Suppl): S67–S68. 2014. View Article : Google Scholar
|
|
5
|
Di Vito A, Mignogna C and Donato G: The
mysterious pathways of cardiac myxomas: A review of histogenesis,
pathogenesis and pathology. Histopathology. 66:321–332. 2015.
View Article : Google Scholar
|
|
6
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoo BK, Emdad L, Su ZZ, Villanueva A,
Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet
JM, et al: Astrocyte elevated gene-1 regulates hepatocellular
carcinoma development and progression. J Clin Invest. 119:465–477.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SG, Jeon HY, Su ZZ, Richards JE,
Vozhilla N, Sarkar D, Van Maerken T and Fisher PB: Astrocyte
elevated gene-1 contributes to the pathogenesis of neuroblastoma.
Oncogene. 28:2476–2484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu G, Chong RA, Yang Q, Wei Y, Blanco MA,
Li F, Reiss M, Au JL, Haffty BG and Kang Y: MTDH activation by 8q22
genomic gain promotes chemoresistance and metastasis of
poorprognosis breast cancer. Cancer Cell. 15:9–20. 2009. View Article : Google Scholar :
|
|
10
|
Liu K, Guo L, Miao L, Bao W, Yang J, Li X,
Xi T and Zhao W: Ursolic acid inhibits epithelial-mesenchymal
transition by suppressing the expression of astrocyte-elevated
gene-1 in human nonsmall cell lung cancer A549 cells. Anticancer
Drugs. 24:494–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Zhang N, Song LB, Liao WT, Jiang LL,
Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS, et al: Astrocyte elevated
gene-1 is a novel prognostic marker for breast cancer progression
and overall patient survival. Clin Cancer Res. 14:3319–3326. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weber CE, Li NY, Wai PY and Kuo PC:
Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound
healing and tissue remodeling after injury. J Burn Care Res.
33:311–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soria G and Ben-Baruch A: The inflammatory
chemokines CCL2 and CCL5 in breast cancer. Cancer Lett.
267:271–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mencarelli A, Graziosi L, Renga B,
Cipriani S, D'Amore C, Francisci D, Bruno A, Baldelli F, Donini A
and Fiorucci S: CCR5 antagonism by maraviroc reduces the potential
for gastric cancer cell dissemination. Transl Oncol. 6:784–793.
2013. View Article : Google Scholar
|
|
15
|
Sasaki S, Baba T, Shinagawa K, Matsushima
K and Mukaida N: Crucial involvement of the CCL3-CCR5 axis-mediated
fibroblast accumulation in colitis-associated carcinogenesis in
mice. Int J Cancer. 135:1297–1306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakamoto H, Sakamaki T, Kanda T, Tsuchiya
Y, Sato M, Sato H, Oyama Y, Sawada Y, Tamura J, Nagai R, et al:
Vascular endothelial growth factor is an autocrine growth factor
for cardiac myxoma cells. Circ J. 68:488–493. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He W, He S, Wang Z, Shen H, Fang W, Zhang
Y, Qian W, Lin M, Yuan J, Wang J, et al: Astrocyte elevated gene-1
(AEG-1) induces epithelial-mesenchymal transition in lung cancer
through activating Wnt/β-catenin signaling. BMC Cancer. 15:1072015.
View Article : Google Scholar
|
|
18
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Huaier polysaccharides
suppresses hepatocarcinoma MHCC97-H cell metastasis via
inactivation of EMT and AEG-1 pathway. Int J Biol Macromol.
64:106–110. 2014. View Article : Google Scholar
|
|
19
|
Khorramdelazad H, Mortazavi Y, Momeni M,
Arababadi MK, Khandany BK, Moogooei M and Hassanshahi G: Lack of
correlation between the CCR5-Δ32 mutation and acute myeloid
leukemia in Iranian patients. Indian J Hematol Blood Transfus.
31:29–31. 2015. View Article : Google Scholar
|
|
20
|
Shi Y, Wu H, Zhang M, Ding L, Meng F and
Fan X: Expression of the epithelial-mesenchymal transition-related
proteins and their clinical significance in lung adenocarcinoma.
Diagn Pathol. 8:892013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chowdhury I, Thompson WE and Thomas K:
Prohibitins role in cellular survival through Ras-Raf-MEK-ERK
pathway. J Cell Physiol. 229:998–1004. 2014. View Article : Google Scholar
|
|
23
|
Neuzillet C, Tijeras-Raballand A, de
Mestier L, Cros J, Faivre S and Raymond E: MEK in cancer and cancer
therapy. Pharmacol Ther. 141:160–171. 2014. View Article : Google Scholar
|
|
24
|
Wang F, Ke ZF, Wang R, Wang YF, Huang LL
and Wang LT: Astrocyte elevated gene-1 (AEG-1) promotes
osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway.
Biochem Biophys Res Commun. 452:933–939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar
|
|
26
|
Bhat FA, Sharmila G, Balakrishnan S,
Arunkumar R, Elumalai P, Suganya S, Raja Singh P, Srinivasan N and
Arunakaran J: Quercetin reverses EGF-induced epithelial to
mesenchymal transition and invasiveness in prostate cancer (PC-3)
cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 25:1132–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao C, Lv S, Han M, Zhang J, Zhang Y,
Zhang L, Yi R, Zhuang D and Wu J: The association of Crk-like
adapter protein with poor prognosis in glioma patients. Tumour
Biol. 35:5695–5700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Srivastava J, Robertson CL, Rajasekaran D,
Gredler R, Siddiq A, Emdad L, Mukhopadhyay ND, Ghosh S, Hylemon PB,
Gil G, et al: AEG-1 regulates retinoid X receptor and inhibits
retinoid signaling. Cancer Res. 74:4364–4377. 2014. View Article : Google Scholar : PubMed/NCBI
|