Introduction
The most common non-hematologic primary malignancy
of bone, osteosarcoma (OS), mainly occurs in children and
adolescents (1,2). In fact, OS is the third most common
malignancy in adolescents and accounts for over 56% of all bone
sarcomas. OS is a primary sarcoma of adolescents, but it is
believed to present as secondary neoplasms attributed to malignant
transformation of Paget disease in older individuals (3–5). OS is
often located in the metaphysis of long bones, and the most common
sites have been reported to be linked to rapid bone growth in the
younger age group, including the distal femur, proximal tibia and
proximal humerus (4,6,7). Due
to the application of pre-operative and post-operative multiagent
chemotherapy combined with gradually developed surgical techniques
for OS management, the long-term survival rate for localized OS has
improved to ~60% (8), still, ~33%
of OS patients present with recurrent disease and OS patients
rarely achieve recovery status (9).
OS patients commonly succumb to respiratory system failure due to
the highly pulmonary metastatic feature of OS (4). Therefore, more efficient anti-tumor
drugs for OS are urgently needed. Recently, traditional Chinese
medicine (TCM) has received increased attention. A high curative
effect has been noted when combining TCM and chemotherapy agents
for various types of cancers.
Evodiamine (EVO;
8,13,13b,14-tetrahydro-14-methylin-dolo[2′3′-3,4]pyrido[2,1-b]quinazolin-5-[7H]-one),
a quinolone alkaloid isolated from a Chinese herbal medicinal plant
Evodia rutaecarpa (10),
plays multiple roles in biological physiological process, such as
anti-inflammatory (11),
anti-nociceptive (12) and
uterotonic effects (13).
Currently, evidence indicates that EVO possesses anticancer
activities. EVO is reported to hinder tumor development by
inhibiting cancer cell proliferation and inducing cell apoptosis in
various types of cancer cells, such as lung (14), acute leukemia (15–17),
prostate (18–20) and cervical (17). Yang et al reported that EVO
can upregulate the expression of phosphatase shatterproof 1 (SHP-1)
(21), which is the key gene of
IL-6-induced signal transducer and activator of transcription
signaling 3 (STAT3) signaling leading to the suppression of
survival and proliferation in hepatocellular carcinoma cells. Yet,
little is known concerning the possible molecular mechanisms
underlying the EVO-induced apoptosis of OS cells.
Phosphatidylinositol 3 kinase (PI3K)/Akt signaling
dominates many aspects of cell growth, cell apoptosis and cell
cycle progression (22). The
hyperactivated PI3K/Akt pathway has been identified as the most
pivotal impetus in tumor development (23). Although the components of PI3K/Akt
signaling are regulated by a range of factors, phosphatase and
tensin homologue (PTEN), considered as an anti-oncogene (24), is the only known lipid phosphatase
attenuating PI3K signaling transduction (23). Mutations of PTEN occur frequently in
many types of carcinomas, such as prostate cancer (25) and human glioma (26). PTEN serves as a phosphatase for the
lipid-signaling second messenger
phosphatidylinositol-3,4,5-trisphosphate (PIP3) (27). The 3′-phosphate on PIP3 is
hydrolyzed to generate PIP2 by PTEN, thereby, PTEN can directly
antagonize PI3K/Akt signaling transduction (28).
In the present study, we investigated the possible
mechanisms involved in the anti-proliferative and
apoptosis-inducing effects of EVO in OS cells. Our data
demonstrated that EVO inhibits the proliferation of OS cells and
the antitumor effect of EVO is mediated by attenuating the PI3K/Akt
signaling pathway through downregulation of the expression of
PTEN.
Materials and methods
Cell culture and agents
The human OS cell line 143B was purchased from the
American Type Culture Collection (ATCC) and EVO was obtained from
Hao-xuan Bio-tech Co., Ltd. (Xi'an, China). EVO was dissolved in
dimethyl sulfoxide (DMSO) for the in vitro experiment.
VO-OHpic was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). EVO was prepared with 0.4% carboxymethylcellulose sodium
(CMC-Na) as suspension for the in vivo experiments. All
other reagents were purchased from Sigma-Aldrich or Fisher
Scientific, unless otherwise indicated. Cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine
serum (FBS), 100 U/ml of penicillin and 100 µg/ml of
streptomycin at 37°C in 5% CO2.
Viability assay of the cell cultures
Cell viability was determined using the crystal
violet assay, and it was conducted as previously described
(29). In brief, 143B cells were
plated in a 24-well plate and treated with predesigned
concentrations of EVO. The cells were washed carefully with 4°C
phosphate-buffered saline (PBS) and stained with 0.5% crystal
violet formalin solution to assess the cell viability at room
temperature for 20–30 min. For quantification and imaging, a 1 ml
aliquot of 20% acetic acid was added to the well to dissolve the
crystal violet and the plate was shaken for 20 min at room
temperature. The absorbance was estimated at 570 nm. Each test was
conducted in triplicate.
Flow cytometric analysis of cell cycle
distribution and apoptosis
The cells were plated into 6-well plates. For cell
cycle analysis, the cells were treated with the indicated
concentrations of EVO or DMSO for 48 h. Then, the cells were washed
with PBS (4°C), washed and collected with cold 70% ethanol (4°C)
followed by washing with 50% and 30% ethanol and PBS. After the
above operation, the cells were incubated with 1 ml of 20 mg/ml
propidium iodide (PI) which contained RNase (1 mg/ml) in PBS for 30
min followed by fluorescence-activated cell sorting (FACS) assay.
For the apoptosis assay, the cells were collected after treatment
with the indicated concentrations of EVO for 48 h. The cells were
washed with cold (4°C) PBS, followed by incubation with PI and
Annexin V-EGFP according to the kit procedures (KeyGen Biotech Co.
Ltd., Nanjing, China). Then, the processed cells were inspected
using FACS assay.
Construction of the recombinant
adenoviruses
Recombinant adenoviruses expressing PTEN (Ad-PTEN),
GFP (Ad-GFP) and small interfering RNA (siRNA) fragments
transfection were performed for the detection of PTEN (Ad-siPTEN).
All these recombinant adenovirus were generated previously using
the AdEasy technology, as described (30).
Reverse transcription and polymerase
chain reaction analysis (RT-PCR)
Human OS 143B cells were seeded in T25 flasks and
treated with the indicated concentrations of EVO or solvent for 24
and 48 h. Total RNA was extracted using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) and used to obtain cDNA templates by RT
reaction. Then, the cDNAs were used as templates for determining
the expression of target genes by PCR. The primers were: GAPDH
forward, 5′-CAACGAATTTGGCTACAGCA-3′ and reverse,
5′-AGGGGAGATTCAGTGTGGTG-3′; PTEN forward,
5′-TAAAGGCACAAGAGGCCCTA-3′ and reverse,
5′-CGCCACTGAACATTGGAATA-3′.
Western blot analysis
Subconfluent 143B cells were plated in 6-well plates
and treated with the indicated concentrations of EVO or DMSO. At
the pre-designed time points (24 and 48 h), the cells were washed
with cold (4°C) PBS followed by being lysed with 300 µl
lysis buffer. Then, the lysates were boiled for 10 min. Extracted
total proteins were separated by SDS-PAGE and then transferred onto
polyvinylidene difluoride (PVDF) membranes. Bovine serum albumin
(BSA) (10%) was used to block the membranes at room temperature for
1 h, and then the membranes were blotted with primary antibodies.
Finally, the images of the target proteins were detected with
SuperSignal West Pico Chemiluminescent substrate.
Xenograft tumor model of human
osteosarcoma
All animal experiments abided by the guidelines of
the Institutional Animal Care and Use Committee of Chongqing
Medical University (IACUC, Chongqing, China) and were approved by
the IACUC. Athymic nude mice (female, 4- to 6-weeks old, 5/group)
were obtained from the Animal Center of Chongqing Medical
University (Chongqing, China). The cells (143B) were washed and
resuspended in cold (4°C) PBS. Then the cells were injected into
the backs of nude mice by subcutaneous injection
(2.5×107 cells/injection). Three days after injection,
the athymic nude mice were treated with different doses of EVO (20
and 50 mg/kg) or solvent by intragastric administration once a day.
After a 4-week intragastric administration, the mice were
sacrificed and the sarcoma samples were retrieved for histological
evaluation.
Immunohistochemical staining and
histological evaluation
Formulin (10%) was used to stain the retrieved tumor
samples and paraffin was used to embed the slides respectively
(31). For the immunohistochemical
staining, the processed slides were deparaffinized and then
rehydrated in a graduated manner. The deparaffinized sections were
subjected to antigen retrieval. Then the background was blocked and
the slides were incubation with a primary anti-proliferating cell
nuclear antigen (PCNA) antibody, or mouse IgG as the control group.
Finaly, the primary PCNA was contrasted by 3,3′-diaminobezidine
(DAB) staining (31).
Statistical analysis
All the experiments were performed at least twice
independently and the results were repeated in triplicate. The data
are represented as mean ± standard deviation. Statistics from
Microsoft Excel software were analyzed using the Student's t-test
among different groups. A P<0.05 was judged to be statistically
significant.
Results
Evodiamine inhibits the proliferation of
human osteosarcoma cells
Previous research has reported the antitumor effect
of EVO on digestive system tumors. To explore whether EVO has the
potential to be a novel pharmacotherapy for OS patients, we used
crystal violet staining to evaluate the inhibitory effect of EVO on
OS cell proliferation. The results revealed that EVO inhibited the
proliferation of 143B cells in a concentration- and time-dependent
manner (Fig. 1A and B). As shown in
Fig. 1C, the activity of PCNA,
which plays a key role in the cell cycle (32), was obviously suppressed. Data
clearly indicated that EVO is capable of inhibiting the
proliferation of 143B cells.
Evodiamine induces apoptosis in human
osteosarcoma cells
We aimed to explore whether EVO induces apoptosis in
143B cells. We first employed FACS and western blot analysis to
estimate the effect of EVO on the apoptosis induction in 143B
cells. The results demonstrated that EVO induced the apoptosis of
the 143B cells in a time- and concentration-dependent manner
(Fig. 2A and B). For further
investigation, Annexin V-EGFP staining was performed. Cells (143B)
were treated with the indicated concentrations of EVO for 24 h
followed by staining with Annexin V-EGFP fusion protein. The
results demonstrated that EVO induced the apoptosis of 143B cells
in a concentration-dependent manner (Fig. 2C). These results strongly indicate
EVO can induce apoptosis in human OS 143B cells.
Evodiamine upregulates the expression of
PTEN and arrests the cell cycle at G1 phase
PTEN has been proven to possess antitumor activity
in many carcinomas for its vital role in the inhibition of cell
proliferation (33,34). Therefore, we investigated whether
PTEN exhibited anti-proliferative activity in 143B cells. The
experimental results of the PCR and western blot analyses (Fig. 3B and C) demonstrated that EVO
concentration dependently suppressed the expression of PTEN in the
treatment group compared to the control. To detect whether these
characteristics are linked with the cell cycle arrest, we analyzed
the cell cycle data in the presence of EVO and/or solvent, and
found that the percentage of cells in the G1 phase was
significantly increased (Fig. 3A)
compared to this percentage in the control. Through further
investigation, we also found that over-expression of PTEN
potentiated the anti-proliferative effect of EVO, while knockdown
of PTEN attenuated this effect of EVO in the 143B cells (Fig. 5A and B). Furthermore, PTEN inhibitor
(VO-OHpic) reversed the anti-proliferative effect of EVO in the
143B cells (Fig. 5C and D). Based
on these results, PTEN is thought to be a key gene involved in the
anti-proliferative effect by EVO on human OS cells.
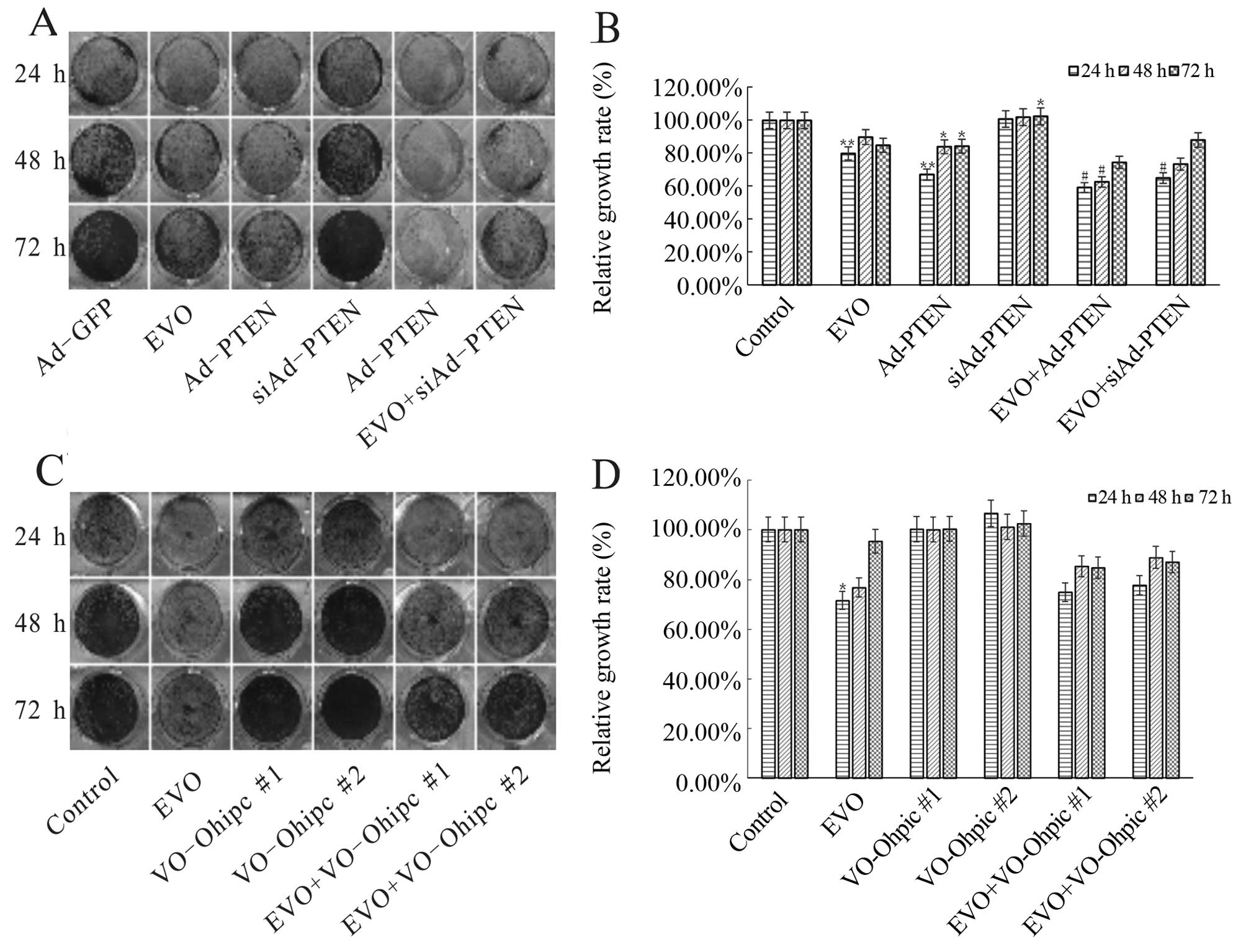 | Figure 5EVO targets PI3K/Akt signaling
resulting in the inhibitory effect on human OS cell proliferation.
(A and B) The effect of PTEN on the inhibition of proliferation of
143B cells mediated by EVO. 143B cells were seeded in 24-well
plates and infected with Ad-PTEN or Ad-siPTEN in the presence or
absence of 1 µM EVO. At 24, 48 and 72 h after treatment, the
cells were stained with crystal violet and the growth rate was
quantified. The assay was performed in triplicate.
*P<0.05, compared with the control;
**P<0.01, compared with the control;
#P<0.05, compared with EVO. (C and D) The effect of
specific PTEN inhibitor (VO-Ohipc) on the proliferation-reducing
effects of EVO on 143B cells. The 143B cells were seeded in 24-well
plates and treated with the indicated concentrations of VO-Ohipc
(#1, 2 nM; #2, 4 nM) in the presence or absence of 1 µM EVO.
At 24, 48 and 72 h after treatment, the cells were stained with
crystal violet and the growth rate was quantified. The assay was
performed in triplicate. *P<0.05, compared with the
control; **P<0.01, compared with the control;
#P<0.05, compared with EVO. OS, osteosarcoma; EVO,
evodiamine; PTEN, phosphatase and tensin homolog. |
Evodiamine attenuates PI3K/Akt signaling
transduction in 143B cells via upregulation of PTEN
The PI3K/Akt signaling pathway, which is involved in
many physiological and pathological processes of tumors, is
consistently upregulated in various malignant diseases (35,36).
We explored whether PI3K/Akt signaling transduction participates in
the EVO-induced inhibition of proliferation in the 143B cells. The
results demonstrated that EVO downregulated the phosphorylation
level of Akt1/2 in a concentration-dependent manner (Fig. 4A). The phosphorylation level of
Akt1/2 was further decreased when the 143B cells were treated with
a combination of EVO and exogenous expression of PTEN (Fig. 4C). Notably, the effect of EVO on the
phosphorylation of Akt1/2 was reversed following knockdown of PTEN
in the 143B cells (Fig. 4B). These
results indicate that PI3K/Akt signaling transduction was
attenuated by PTEN during the the EVO-induced inhibition of 143B
cell proliferation.
Evodiamine inhibits human osteosarcoma
growth in a xenograft tumor model
The results of the in vitro experiments
demonstrated the anti-osteosarcoma effects of EVO and the
corresponding molecular mechanisms. We estimated the
anti-osteosarcoma effects of EVO with an in vivo experiment.
To construct the xenograft tumor model, 143B cells were injected
into the backs of athymic nude mice by subcutaneous injection.
Three days after the injection, the athymic nude
mice were treated with different doses of EVO (20 and 50 mg/kg) or
solvent via intragastric administration once a day for four weeks.
The result revealed that the treatment groups exhibited significant
suppression of tumor growth in a dose-dependent manner, compared to
the control group (Fig. 6A).
Hematoxylin and eosin (H&E) staining results showed that the
EVO-treated groups exhibited a decreased cellularity in the tumor
masses. The H&E histologic evaluation confirmed that the
EVO-treated groups showed more necrotic cells than that in the
control group (Fig. 6C). In
addition, we also determined the expression of PCNA (Fig. 6B), and the results revealed that EVO
significantly inhibited the proliferation of 143B cells in the EVO
treatment group. Thus, these in vivo experimental results
further demonstrated that EVO effectively inhibits OS growth.
Discussion
OS is the most frequent cancer of the bone (37). OS has a bimodal distribution; the
first presentation may occur in adolescents and the second
occurence is in the elderly. To date, combination therapy of
multi-antitumor drugs has been introduced for OS treatment for the
past 40 years, yet ~80% of patients with local early stage tumors
progress to a metastatic status (2,38), and
recurrent and/or metastatic OS tumors are extremely invasive and
even resistant to traditional antitumor strategies (2). Therefore, identification of a novel
treatment modality with more efficacy for OS patients is vital.
EVO, (Chinese name, Wu-Chu-Yu), a quinolone
alkaloid, is the essential component extracted from the fruit of
Evodia rutaecarpa (39,40).
The role of EVO in the inhibition of tumor cell proliferation has
been well-documented in a number of studies (13–20),
while our knowledge of the antitumor effects of EVO on OS cells
remains rather sparse. To the best of our knowledge, this is the
first study to explore whether EVO possesses anticancer activities
in OS cells, and the possible molecular mechanism involved. Based
on the data presented in the present study, we found that EVO
significantly inhibited the proliferation and induced the apoptosis
in 143B cells in a time- and concentration-dependent manner, even
at a concentration of 0.5 µM (Fig. 1A and B). More importantly, it has
been reported that EVO exhibits low cytotoxicity to human
peripheral blood cells (41) and
increasing evidence suggests that EVO has strong specificity to
tumor cells. Mechanistically, our results also showed that
overexpression of PTEN is responsible for the anti-proliferation
effect of EVO on 143B OS cells. Furthermore, PI3K/Akt signaling
transduction is inhibited via EVO-induced upregulation of PTEN.
According to previous research, EVO was found to
strongly inhibit tumor progression by reducing the proliferation
rate and inducing apoptosis in a variety of tumor cells (13–20). A
series of analyses attempted to explain the underlying molecular
mechanisms. A large number of trials have shown that EVO can cause
cell cycle arrest at the G2/M phase in a majority of cancer cells
(42), yet research on the cell
cycle regulation by EVO in OS cells has not been carried out. Kan
et al found that EVO activated the Cdc2/cyclin B complex to
regulate cycle cycle arrest (G2/M) in human prostate cancer cell
lines DU145 and PC3 (18).
Conversely, EVO developed atypical apoptosis in murine fibrosarcoma
L929 cells by cell cycle arrest at the G0/G1 phase (15). Our research obtained similar results
(Fig. 3A), suggesting that the cell
cycle regulation by EVO in 143B cells may be different from that in
other types of tumor cells. In addition, Takada et al found
that EVO exerts apoptotic activity by regulating NF-κB activation,
resulting in the inhibition of NF-κB-controlled downstream genes,
including cyclin D1, c-Myc, survivin, X chromosome-linked IAP
(XIAP), Bcl-2 and Bcl-Xl (43).
Numerous experiments have demonstrated that EVO activated initiator
caspases (caspase-8 and 9) and/or effector caspases (caspase-3) to
induce the apoptosis in a variety of tumor cell lines including
human colorectal carcinoma COLO205 and HT-29 cells (44) and human thyroid cancer cell line ARO
(45). In addition, another study
showed that EVO promoted translocation of apoptosis-inducing factor
(AIF) into the nucleus of human leukemia U937 cells (46). Collectively, EVO was found to induce
the apoptosis of many tumor cell lines via caspase-dependent and
caspase-independent signaling pathways (47). Moreover, Liu et al proved
that EVO-mediated autophagy leading to increased apoptosis and
reduction in cell viability was via a calcium-JNK signaling pathway
(48). Further study suggested that
the downregulation of PI3K/Akt/caspase and Fas-L/NF-κB signaling
pathways may be responsible for the apoptosis of A375-S cells
induced by EVO (49). Although a
number of studies have analyzed the possible molecular mechanisms
by which EVO exhibits inhibitory action on tumorigenesis in various
cancer cells, the inhibitory effect of EVO on OS cells and the
possible molecular mechanism of the signaling transduction invovled
in the anticancer activity of EVO remain unclear.
PTEN, a tumor suppressor, is commonly mutated in the
majority of human epithelial cell-derived tumors (50). On account of its phospholipid
phosphatase characteristic, PTEN regulates various cellular
processes and potentially affects the transduction of many other
signaling pathways. PTEN is able to catalyze 3′-phosphate on PIP3
to form PIP2, thereby, PI3K/Akt signaling transduction is directly
abrogated by PTEN (28). Despite
the high aberration rate of PTEN in many types of cancers, it is
controversial that PTEN mutations are linked with OS tumorigenesis.
For example, the biallelic and monoallelic mutation rate of PTEN in
OS samples was found to reach ~15 and 33%, respectively (51). The present results indicated that
both the protein and gene levels of PTEN were increased
concentration-dependently in the EVO-induced 143B cells. Exogenous
PTEN strengthened the effect of EVO in 143B cells, while VO-OHpic
treatment or knockdown of PTEN reversed the inhibitory effect on
proliferation caused by EVO. Further research showed that EVO
upregulated the expression of PTEN through the deactivation of
PI3K/Akt signaling transduction by the reduction of phosphorylated
Akt1/2 in 143B cells. The in vivo experiment results showed
that EVO inhibited tumorgenesis. Collectively, PTEN/PI3K/Akt
signaling was found to participate in the inhibition of
proliferation of human OS 143B cells by EVO.
The present results suggest that EVO may be a
promising antitumor strategy against human OS. Further studies are
warranted to elucidate additional targets of EVO and the specific
molecular mechanisms of the anticancer activity of EVO. In
addition, a number of pre-clinical assessments should be carried
out for the analysis of drug safety.
Acknowledgments
We would like to thank Dr T.C. He (University of
Chicago Medical Center, USA) for generously providing all
recombinant adenoviruses.
References
|
1
|
Graudal N, Hubeck-Graudal T, Tarp S,
Christensen R and Jurgens G: Effect of combination therapy on joint
destruction in rheumatoid arthritis: A network meta-analysis of
randomized controlled trials. PLoS One. 9:e1064082014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang N, Song WX, Luo J, Haydon RC and He
TC: Osteosarcoma development and stem cell differentiation. Clin
Orthop Relat Res. 466:2114–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Price CH: Osteogenic sarcoma; an analysis
of the age and sex incidence. Br J Cancer. 9:558–574. 1955.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weinfeld MS and Dudley HR Jr: Osteogenic
sarcoma. A follow-up study of the ninety-four cases observed at the
Massachusetts General Hospital from 1920 to 1960. J Bone Joint Surg
Am. 44-A. pp. 269–276. 1962
|
|
7
|
Dahlin DC and Coventry MB: Osteogenic
sarcoma. A study of six hundred cases. J Bone Joint Surg Am.
49:101–110. 1967.PubMed/NCBI
|
|
8
|
Ando K, Heymann MF, Stresing V, Mori K,
Redini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar
|
|
9
|
Anderson PM and Pearson M: Novel
therapeutic approaches in pediatric and young adult sarcomas. Curr
Oncol Rep. 8:310–315. 2006. View Article : Google Scholar
|
|
10
|
Lin H, Tsai SC, Chen JJ, Chiao YC, Wang
SW, Wang GJ, Chen CF and Wang PS: Effects of evodiamine on the
secretion of testosterone in rat testicular interstitial cells.
Metabolism. 48:1532–1535. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiou WF, Sung YJ, Liao JF, Shum AY and
Chen CF: Inhibitory effect of dehydroevodiamine and evodiamine on
nitric oxide production in cultured murine macrophages. J Nat Prod.
60:708–711. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi Y: The nociceptive and
anti-nociceptive effects of evodiamine from fruits of Evodia
rutaecarpa in mice. Planta Med. 69:425–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
King CL, Kong YC, Wong NS, Yeung HW, Fong
HH and Sankawa U: Uterotonic effect of Evodia rutaecarpa alkaloids.
J Nat Prod. 43:577–582. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong JY, Park SH, Min HY, Park HJ and Lee
SK: Anti-proliferative effects of evodiamine in human lung cancer
cells. J Cancer Prev. 19:7–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhang QH, Wu LJ, Tashiro S,
Onodera S and Ikejima T: Atypical apoptosis in L929 cells induced
by evodiamine isolated from Evodia rutaecarpa. J Asian Nat Prod
Res. 6:19–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang YC, Guh JH and Teng CM: Induction of
mitotic arrest and apoptosis by evodiamine in human leukemic
T-lymphocytes. Life Sci. 75:35–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fei XF, Wang BX, Li TJ, Tashiro S, Minami
M, Xing DJ and Ikejima T: Evodiamine, a constituent of Evodiae
Fructus, induces anti-proliferating effects in tumor cells. Cancer
Sci. 94:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kan SF, Yu CH, Pu HF, Hsu JM, Chen MJ and
Wang PS: Anti-proliferative effects of evodiamine on human prostate
cancer cell lines DU145 and PC3. J Cell Biochem. 101:44–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang DM, Guh JH, Huang YT, Chueh SC,
Chiang PC and Teng CM: Induction of mitotic arrest and apoptosis in
human prostate cancer pc-3 cells by evodiamine. J Urol.
173:256–261. 2005. View Article : Google Scholar
|
|
21
|
Yang J, Cai X, Lu W, Hu C, Xu X, Yu Q and
Cao P: Evodiamine inhibits STAT3 signaling by inducing phosphatase
shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett.
328:243–251. 2013. View Article : Google Scholar
|
|
22
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang S and Yu D: PI(3)king apart PTEN's
role in cancer. Clin Cancer Res. 16:4325–4330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahimainathan L and Choudhury GG:
Inactivation of platelet-derived growth factor receptor by the
tumor suppressor PTEN provides a novel mechanism of action of the
phosphatase. J Biol Chem. 279:15258–15268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshimoto M, Cunha IW, Coudry RA, Fonseca
FP, Torres CH, Soares FA and Squire JA: FISH analysis of 107
prostate cancers shows that PTEN genomic deletion is associated
with poor clinical outcome. Br J Cancer. 97:678–685. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishii N, Maier D, Merlo A, Tada M,
Sawamura Y, Diserens AC and Van Meir EG: Frequent co-alterations of
TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human
glioma cell lines. Brain Pathol. 9:469–479. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maehama T and Dixon JE: The tumor
suppressor, PTEN/MMAC1, dephosphorylates the lipid second
messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem.
273:13375–13378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franke TF, Kaplan DR, Cantley LC and Toker
A: Direct regulation of the Akt proto-oncogene product by
phosphati-dylinositol-3,4-bisphosphate. Science. 275:665–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He BC, Chen L, Zuo GW, Zhang W, Bi Y,
Huang J, Wang Y, Jiang W, Luo Q, Shi Q, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He BC, Gao JL, Luo X, Luo J, Shen J, Wang
L, Zhou Q, Wang YT, Luu HH, Haydon RC, et al: Ginsenoside Rg3
inhibits colorectal tumor growth through the downregulation of
Wnt/ss-catenin signaling. Int J Oncol. 38:437–445. 2011. View Article : Google Scholar
|
|
32
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): A key factor in DNA
replication and cell cycle regulation. Ann Bot. 107:1127–1140.
2011. View Article : Google Scholar :
|
|
33
|
Singha PK, Pandeswara S, Geng H, Lan R,
Venkatachalam MA and Saikumar P: TGF-beta induced TMEPAI/PMEPA1
inhibits canonical Smad signaling through R-Smad sequestration and
promotes non-canonical PI3K/Akt signaling by reducing PTEN in
triple negative breast cancer. Genes Cancer. 5:320–336.
2014.PubMed/NCBI
|
|
34
|
Marques RB, Aghai A, de Ridder CM,
Stuurman D, Hoeben S, Boer A, Ellston RP, Barry ST, Davies BR,
Trapman J, et al: High efficacy of combination therapy using
PI3K/AKT inhibitors with androgen deprivation in prostate cancer
preclinical models. Eur Urol. Sep 11–2014.Epub ahead of print.
PubMed/NCBI
|
|
35
|
Wang J, Chu ES, Chen HY, Man K, Go MY,
Huang XR, Lan HY, Sung JJ and Yu J: microRNA-29b prevents liver
fibrosis by attenuating hepatic stellate cell activation and
inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget.
6:7325–7338. 2014.PubMed/NCBI
|
|
36
|
Yu P, Ye L, Wang H, Du G, Zhang J, Zhang J
and Tian J: NSK-01105 inhibits proliferation and induces apoptosis
of prostate cancer cells by blocking the Raf/MEK/ERK and
PI3K/Akt/mTOR signal pathways. Tumour Biol. 36:2143–2153. 2015.
View Article : Google Scholar
|
|
37
|
Cormier JN and Pollock RE: Soft tissue
sarcomas. CA Cancer J Clin. 54:94–109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gorlick R, Anderson P, Andrulis I, Arndt
C, Beardsley GP, Bernstein M, Bridge J, Cheung NK, Dome JS, Ebb D,
et al: Biology of childhood osteogenic sarcoma and potential
targets for therapeutic development: meeting summary. Clin Cancer
Res. 9:5442–5453. 2003.PubMed/NCBI
|
|
39
|
Wang L, Hu CP, Deng PY, Shen SS, Zhu HQ,
Ding JS, Tan GS and Li YJ: The protective effects of rutaecarpine
on gastric mucosa injury in rats. Planta Med. 71:416–419. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu X, Wu DZ, Yuan JY, Zhang RR and Hu ZB:
Gastroprotective effect of fructus evodiae water extract on
ethanol-induced gastric lesions in rats. Am J Chin Med.
34:1027–1035. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liao CH, Pan SL, Guh JH, Chang YL, Pai HC,
Lin CH and Teng CM: Antitumor mechanism of evodiamine, a
constituent from Chinese herb Evodiae fructus, in human
multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro
and in vivo. Carcinogenesis. 26:968–975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang J and Hu C: Evodiamine: a novel
anti-cancer alkaloid from Evodia rutaecarpa. Molecules.
14:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takada Y, Kobayashi Y and Aggarwal BB:
Evodiamine abolishes constitutive and inducible NF-kappaB
activation by inhibiting IkappaBalpha kinase activation, thereby
suppressing NF-kappaB-regulated antiapoptotic and metastatic gene
expression, upregulating apoptosis, and inhibiting invasion. J Biol
Chem. 280:17203–17212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamine-induced apoptosis and G2/M arrest in human colorectal
carcinoma cells: a structure-activity study of evodiamine. PLoS
One. 9:e997292014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen MC, Yu CH, Wang SW, Pu HF, Kan SF,
Lin LC, Chi CW, Ho LL, Lee CH and Wang PS: Anti-proliferative
effects of evodiamine on human thyroid cancer cell line ARO. J Cell
Biochem. 110:1495–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee TJ, Kim EJ, Kim S, Jung EM, Park JW,
Jeong SH, Park SE, Yoo YH and Kwon TK: Caspase-dependent and
caspase-independent apoptosis induced by evodiamine in human
leukemic U937 cells. Mol Cancer Ther. 5:2398–2407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu H, Jin H, Gong W, Wang Z and Liang H:
Pharmacological actions of multi-target-directed evodiamine.
Molecules. 18:1826–1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu AJ, Wang SH, Chen KC, Kuei HP, Shih
YL, Hou SY, Chiu WT, Hsiao SH and Shih CM: Evodiamine, a plant
alkaloid, induces calcium/JNK-mediated autophagy and
calcium/mitochondria-mediated apoptosis in human glioblastoma
cells. Chem Biol Interact. 205:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang C, Li S and Wang MW:
Evodiamine-induced human melanoma A375-S2 cell death was mediated
by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and
augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro.
24:898–904. 2010. View Article : Google Scholar
|
|
50
|
Ali IU, Schriml LM and Dean M: Mutational
spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid
phosphatase activity. J Natl Cancer Inst. 91:1922–1932. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Freeman SS, Allen SW, Ganti R, Wu J, Ma J,
Su X, Neale G, Dome JS, Daw NC and Khoury JD: Copy number gains in
EGFR and copy number losses in PTEN are common events in
osteosarcoma tumors. Cancer. 113:1453–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|




















