Introduction
Esophageal cancer is one of the most common cancers
in the world. With an estimated 456,000 new cases and 400,000
deaths, esophageal cancer was the eighth most common cancer and the
sixth leading cause of cancer-related deaths worldwide in 2012.
There are ~223,000 new cases and 197,200 deaths in China,
accounting for 52.8% of the incidence and 49.3% of the mortality
from this disease worldwide (1).
Although some progress has been made with multidisciplinary
treatment in recent decades, the overall 5-year survival rate is
only ~20% (2). There is a pressing
need to understand more concerning esophageal cancer pathogenesis
and to develop an effective treatment for esophageal cancer. It
would be beneficial for therapeutics of esophageal cancer to target
genes that are either more specifically expressed in esophageal
cancer or involved in multiple pathways, such as cellular
metabolism or nutrient uptake.
Zinc is an essential trace element and a
catalytic/structural component that is used by many metalloenzymes
and transcription factors. Zinc availability is also important for
tumor growth and progression since zinc is a critical component for
many enzymes that are involved in hypoxia, angiogenesis, cell
proliferation and cancer metastasis. Most esophageal cancer cases
arise in the rural countryside of China, which has limited health
resources and an underdeveloped economy. Zou et al reported
that a low nutrient intake was found for zinc, with only 72% of the
recommended daily allowance (RDA) and 62% of the RDA in spring and
autumn, respectively, in high-risk areas (3), which may be one of the high-risk
factors of esophageal cancer. Zinc and zinc transporters have been
shown to have correlations with cancer risk (4–9),
although the role of zinc and zinc transporters in esophageal
cancer progression is largely unknown. Wu et al found that
ZIP6 plays an important role in the prognosis of esophageal
squamous cell carcinoma (ESCC), with a higher expression of ZIP6
protein being correlated with a shorter length of survival in
individuals with advanced ESCC, and that knockdown of ZIP6
expression suppressed the proliferation and invasion of ESCC cells
(7).
ZIP5 plays an important role in maintaining cellular
zinc levels by facilitating the uptake of dietary zinc into
intestinal epithelial cells and releasing zinc from vesicular
compartments. It is primarily expressed in the liver, kidney,
pancreas and small intestine but not in the esophagus (10,11).
Kumar et al found that ZIP5 was differentially expressed
during esophageal tumorigenesis (12). ZIP5 and ZIP6 have very similar
sequences, and they may have various similarities in terms of
function. We hypothesized that ZIP5 plays a role in the development
of esophageal cancer.
In the present study, we detected the expression of
ZIP5 in human esophageal tissues and esophageal cancer cell lines.
A stable ZIP5-silenced cell line was established from the
esophageal cancer cell line KYSE170 by short hairpin RNA using
lentiviral vectors; we then compared the cell proliferation,
migration and apoptosis of the esophageal cancer cell lines to
discover the role of ZIP5 in esophageal cancer progression. We
explored the mechanisms by detecting the expression of significant
genes related to tumor progression to discover new targets for
testing or treatment.
Materials and methods
Human esophageal cancer tissue
specimens
The present study was approved by the Institutional
Human Ethics Committee of Hebei Medical University Fourth Hospital,
and prior informed consent was obtained from all patients. All
specimens were obtained and pathologically confirmed to be ESCC at
the Hebei Medical University Fourth Hospital, and Tumor Hospital of
Hebei Province. Hebei Province is located in northern China on the
North China Plain; it surrounds, but does not govern, Beijing the
capital of China. The 58 patients from whom the samples were
obtained underwent curative resection without previous radiotherapy
and chemotherapy between 2012 and 2014. The patients, 37 males and
21 females, ranged in age from 44 to 77 years (mean age, 51 years).
There were 39 patients at the early stage and 19 with advanced
cancer. Tumor sizes were divided as ≤5 cm (n=47) and >5 cm
(n=11). Cancer, para-carcinoma and normal tissues were collected
from each patient. The para-carcinoma tissue was obtained 2 cm
distant from the tumor margin, and the normal tissue was located at
the farthest edge from the tumor margin in the surgically resection
tissue. All samples were anonymously coded in accordance with local
ethical guidelines.
Immunohistochemical (IHC) assay
Paraffin sections (4-mm thick) were deparaffinized
and rehydrated, followed by treatment with 0.02 M EDTA buffer (pH
9.0; Gene Tech, USA). Then, the sections were immersed in 3%
H2O2 to quench endogenous peroxidase activity
and blocked with 5% normal goat serum, followed by incubation with
the monoclonal anti-ZIP5 antibody (1:150; Sigma, USA) overnight at
4°C. The antibody was diluted in phosphate-buffered saline (PBS)
buffer containing 5% normal goat serum. The negative control for
each slide was incubated with 5% normal goat serum without the
anti-ZIP5 antibody. The sections were then incubated with
HRP-conjugated anti-rabbit IgG (ZSGB-BIO, China) for 45 min at 37°C
and revealed with diaminobenzidine tetrahydrochloride. The stained
slides were scored by three pathologists who were unaware of the
clinical diagnosis. The indices of ZIP5 labeling were implemented
so that samples were scored according to the percentage and
intensity scores of the positively stained tumor cells.
Western blotting
Cells were lysed with ice-cold lysis buffer (Keygen
biotech, China) for 30 min on ice. Cell lysates were then collected
after centrifugation at 12,000 rpm for 5 min at 4°C. Sixty
micrograms of lysate protein was loaded, and the total cellular
protein was separated by 12% SDS-PAGE and then transblotted
overnight at 4°C onto PVDF membranes. The membranes were incubated
with antibody (anti-ZIP5, 1:1,500; anti-GAPDH, 1:2,500; both from
Sigma) at 4°C overnight, washed three times with TBST with 0.1%
Tween, and incubated with a horseradish peroxidase-linked secondary
antibody (1:2,000) for 1 h at room temperature. The membranes were
washed three times with TBST with 0.1% Tween, and the
immunoreactive bands were detected using an Enhanced
Chemiluminescent Plus reagent kit.
RNA isolation and real-time qPCR
Total RNA was extracted using TRIzol reagent
according to the manufacturer's protocol (Invitrogen, USA). The
cDNA was synthesized by reverse transcription. The mRNA levels of
ZIP5 were analyzed by real-time reverse transcriptase PCR. Briefly,
real-time PCR was performed using the GoTaq qPCR Master Mix kit.
PCR included 100 nmol/l of each primer, diluted cDNA templates and
the mix, and was run for 35 cycles at 95°C for 30 sec, 58°C for 45
sec and 72°C for 30 sec. PCR efficiency was examined by serially
diluting the template cDNA, and the melting curve data were
collected to determine PCR specificity. Each cDNA sample was run in
triplicate, and the corresponding no-reverse transcriptase mRNA
sample was included as a negative control. The β-actin primer was
included in every plate to avoid sample variations. The relative
mRNA level was presented as unit values of 2[Ct (β-actin) - Ct
(gene of interest)]. The sequence of the forward primer for
ZIP5 used in the present study was 5′-CTCATGCTTGCCATAACC-3′ and the
sequence of the reverse primer was 5′-AATCCTATTGCTCCTACTGG-3′.
Cell lines
Human esophageal cancer cell line KYSE170 was
obtained from the MD Anderson Cancer Center, USA and was cultured
in RPMI-1640 medium with 10% fetal bovine serum (FBS) (both from
Gibco, USA). The cell line Eca109 was maintained in our laboratory.
The cell lines KYSE70, KYSE150, KYSE180, KYSE510, EC9706 and CaES17
were purchased from Sangon Biotech (China). The cell line HEK 293T
was obtained from the State Key Laboratory of Natural and
Biomimetic Drugs of Peking University in China and was cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco) with 10% FBS.
Lentiviral vector packages and stable
transduction
The potential target sequences were found using
Ambion online tools and retrieval from the GenBank, and the siRNA
was designed according to general design principles. The plasmid
containing the specific shRNA was inserted into the PSD31 plasmid
to obtain the lentiviral vector lenti-plasmid. The lentiviral
vectors were jointly transfected into 293T cells to obtain the
viral solution. The 293T cells were cultured in DMEM with sodium
pyruvate and 10% FBS and were transfected with 2 µg of the
shRNA-lenti-plasmid, 2 µg of pMDL, 1 µg of pRSV and 1
µg of VSVG using the transfection reagent Lipofectamine 2000
(Invitrogen) for 24 h. After the A medium (DMEM without sodium
pyruvate with 3% fetal calf serum) was changed at 6 h, the
lentiviruses were harvested at 48 h and filtered with a 0.45-mm
filter. Experiments for stable lentivector transduction were
performed as follows: KYSE170 cells were seeded in a 6-well cell
culture dish and transduced with the lentiviruses for 24 h.
Selection was performed starting at 48 h using 0.6 µg/ml
puromycin until all parental cells from a parallel experiment died.
The viable cells were the stable esophageal cancer cell lines with
knockdown of ZIP5. The sequence of the ZIP5 shRNA used in the
present study was 5′-CCUGCUGAGCAGGAGCAGAACCAUUACCU-3′. The stable
cell line expressing ZIP5 shRNA was named KYSE170K, and the stable
cell line expressing the empty vector was named KYSE170S.
MTT and CCK-8 assays
Esophageal cancer cells and the cells with knockdown
of ZIP5 were seeded in 96-well plates (5×103
cells/well). Cell proliferation was assessed at 12, 24, 36 and 48
h. Then, 20 µl of MTT (0.5%) reagent was added to each well,
incubated at 37°C and 5% Co2 for 4 h, and then
transduced with 150 µl of dimethylsulfoxide (DMSO).
Absorbance was recorded at 450 nm with a universal microplate
reader. CCK-8 was analyzed with a simple test in which 10 µl
of CCK-8 reagent was added to each well and incubated at 37°C and
5% CO2 for 2–4 h, and the absorbance was recorded at 450
nm.
Transwell assays
For the Transwell migration assay, 2×104
cells were plated in the top chamber with a non-coated membrane
(24-well insert; pore size, 8-µm; BD Biosciences). For the
invasion assay, 2.5×104 cells were plated in the top
chamber with a Matrigel-coated membrane (24-well insert; pore size,
8-µm). In both assays, the cells were plated in medium
without serum, and medium supplemented with serum was used as a
chemoattractant in the lower chamber. The cells were incubated for
24 h, and cells that did not migrate or invade through the pores
were gently removed by a cotton swab. Cells on the lower surface of
the membrane were fixed and stained with Giemsa crystal violet
solution and counted under light microscopy magnification.
Flow cytometry (FCM) assays
Cells were harvested and washed twice with FBS and
then fixed in a 70% ethanol solution for 24 h at 4°C. Then, the
fixed cells were washed once with PBS and resuspended as a
single-cell suspension. The suspension was incubated with 500
µl of propidium iodide (PI) (50 µg/ml) for 30 min and
tested by FCM according to standard procedures.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software (SPSS, Inc., USA). Quantitative results are shown as
mean ± SD. The Student-Newman-Keuls test was used for statistical
analyses between the groups. P<0.05 was considered to indicate a
statistically significant result.
Results
ZIP5 is overexpressed in human esophageal
cancer tissue specimens and cell lines
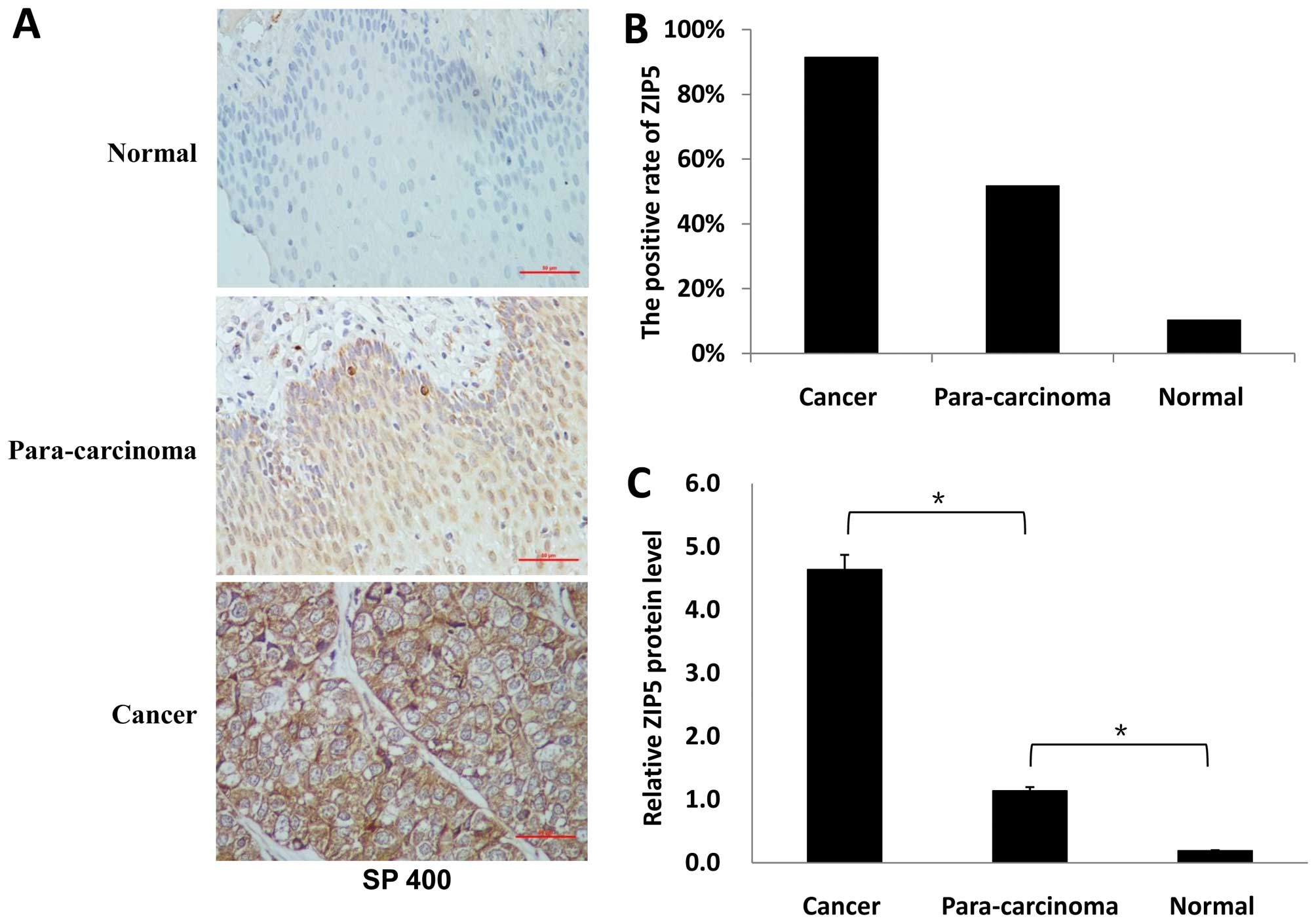
According to the IHC and western blotting results,
ZIP5 protein was overexpressed in the human esophageal cancer
tissue samples compared with levels in the para-carcinoma and
normal samples. The IHC of ZIP5 protein in the tissues of the
esophageal cancer samples (n=58 normal-para-carcinoma-cancer
groups) was examined. In normal esophageal epithelia, ZIP5 labeling
was weak, as only 6 (10.3%) normal samples were stained. In the
para-carcinoma samples, 30 (51.7%) were stained (P<0.01). ZIP5
was highly expressed in the cancer samples with 53 (91.4%) of the
cancer samples showing strong immunoreactivity to human ZIP5 Ab
(P<0.01). Based on the percentage and intensity of the
positively stained cells, the immunoreactive score for normal
samples was 0.19±0.66. The score for the para-carcinoma samples was
1.14±1.29 (P<0.01), and the score for the cancer samples was
4.64±3.67 (P<0.01) (Fig.
1A–C).
The protein expression of ZIP5 was measured in 18
groups of samples by western blotting. Using the expression of
normal tissue as a standard, the relative quantity was 5.87±0.20 in
cancer tissues (P<0.01) and 3.42±0.92 in the para-carcinoma
tissues (P<0.01). The results were consistent with the preceding
results, demonstrating that the expression of ZIP5 in esophageal
cancer tissues was higher (Fig. 1D and
E).
The mRNA expression of ZIP5 was also measured in 58
groups of tissues by quantitative RT-PCR (qRT-PCR). With the
expression of normal tissue as a standard, the relative quantity
was 14.83±4.63 in the cancer tissues (P<0.01) and 4.17±0.79 in
the para-carcinoma tissues (P<0.01) (Fig. 1F and G).
We examined the mRNA expression of ZIP5 in eight
types of esophageal cancer cell lines, and the expression levels
were high in all with the expression levels in KYSE170 and KYSE180
being the highest (Fig. 1H).
Knockdown of ZIP5 inhibits the
proliferation, migration and invasion of esophageal cancer
cells
To study the potential functions of ZIP5 in
esophageal cancer, the stable ZIP5 knockdown cell line (KYSE170K)
was established from the esophageal cancer cell line KYSE170 using
a lentiviral vector. Stable cells containing empty vectors were
also established from the KYSE170 cells to serve as controls
(KYSE170S).
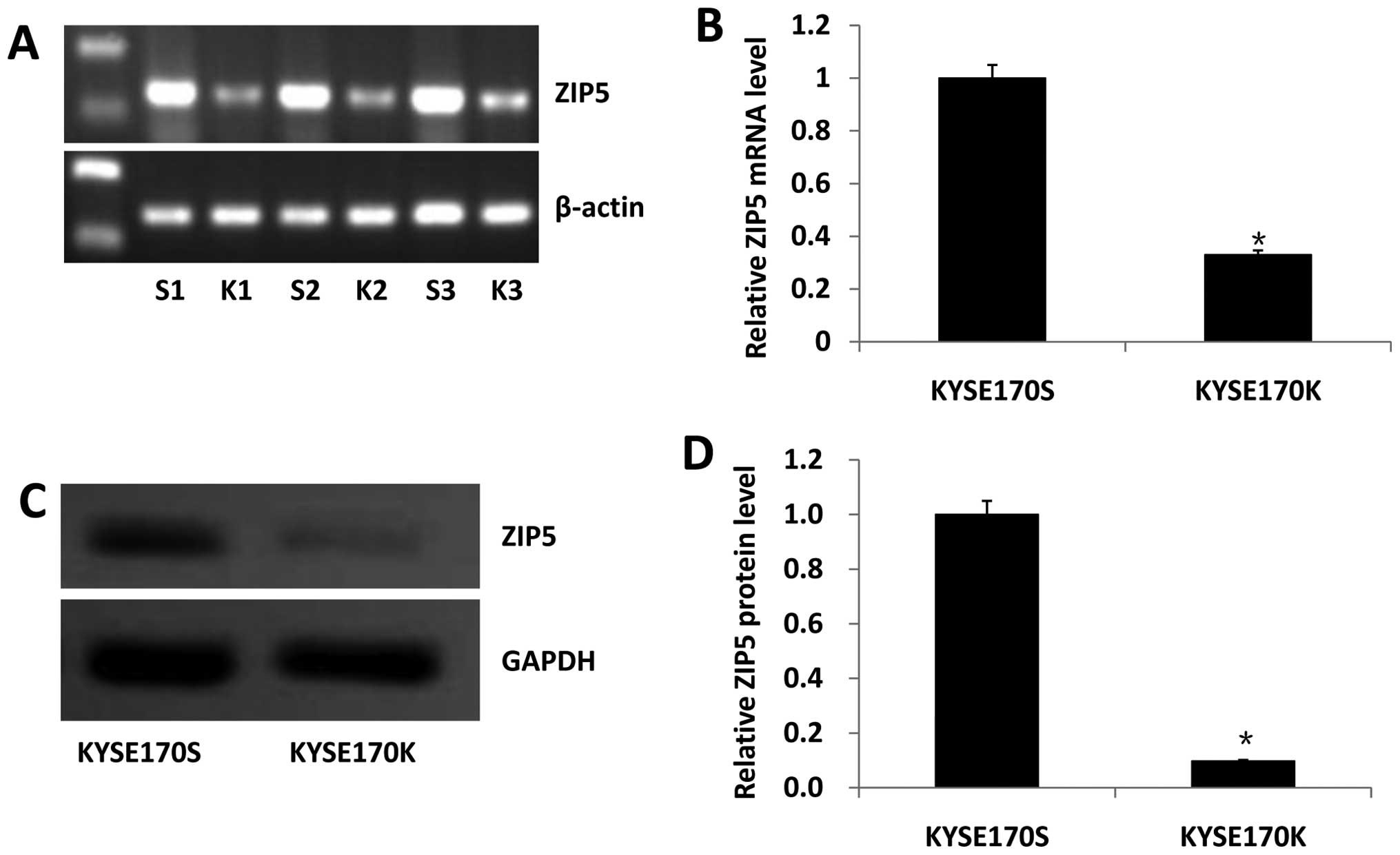
ZIP5 mRNA was measured by qRT-PCR. With the
expression of this mRNA in the corresponding cell line (KYSE170S)
as a standard, the relative quantity was 0.29 in the KYSE170K cells
(P<0.01) and the efficiency of knockdown of ZIP5 mRNA was ~71%
(Fig. 2A and B).
We measured ZIP5 protein expression by western
blotting. Using the ZIP5 expression in the KYSE170S cell line as a
standard, the relative quantity was 0.10 in the KYSE170K cells
(P<0.01), and the efficiency of knockdown of ZIP5 protein
expression was 90% (Fig. 2C and
D).
We compared the proliferation of KYSE170S and
KYSE170K cells using MTT and CCK-8 assays to investigate whether
the knockdown of ZIP5 affects the proliferation of esophageal
cancer cells, to study the functions of ZIP5 and to determine the
effects of silencing of ZIP5 in vitro. The proliferation of
the KYSE170K cell line was dramatically decreased by 28% when
compared with that of the KYSE170S cell line, according to the MTT
assay results, and by 38% according to the CCK-8 experimental
results (Fig. 3A and B).
To investigate whether knockdown of ZIP5 affects the
migration and invasion of esophageal cancer cells, we performed
Transwell assays. Knockdown of ZIP5 significantly decreased the
cell migratory (Fig. 3C and D) and
invasive (Fig. 3E and F) abilities
of the KYSE170K cell line by 54 and 68%, respectively, compared
with these abilities in the KYSE170S cell line.
The results of the FCM analyses showed that there
was no difference in apoptosis in the two cell lines. However, the
percentage of G1 phase cells (50%) was significantly higher in The
KYSE170K than that in the KYSE170S cells (25%), and the difference
was statistically significant (Fig.
3G).
Knockdown of ZIP5 expression inhibits the
expression of COX2 and cyclin D1 and increases the expression of
E-cadherin
We conducted a gene profiling study using the
KYSE170S and KYSE170K cell lines to detect the expression of
significant genes related to tumor progression, including COX2,
cyclin D1, E-cadherin, bcl-2, bax, MMP-9, MMP-2, VEGF and p53,
using qRT-PCR and western blotting. We found that the expression of
COX2 and cyclin D1 was significantly downregulated in the KYSE170K
cell line compared with the KYSE170S cell line, and the expression
of E-cadherin was significantly upregulated in the KYSE170K cell
line. We did not find differences in the expression levels of other
genes (Fig. 4A).
Using the expression level in KYSE170S cells as a
standard, the COX2 mRNA relative quantity was 0.32 in the KYSE170K
cells (P<0.01). With the protein expression in KYSE170S cells as
a standard, the relative quantity was 0.25 in the KYSE170K cells
(P<0.01). Knockdown of ZIP5 suppressed 68% of COX2 at the mRNA
level and 75% at the protein level (Fig. 4).
The mRNA expression level of cyclin D1 in the
KYSE170K cells was 0.38 times the level in the KYSE170S cells
according to qRT-PCR (P<0.01). With the protein expression level
in KYSE170S cells as a standard, the relative quantity was 0.40 in
the KYSE170K cells (P<0.01). Knockdown of ZIP5 suppressed the
expression level of cyclin D1 by ~60% (Fig. 4).
The mRNA expression of E-cadherin in the KYSE170K
cells was 1.8 times the expression in the KYSE170S cells according
to qRT-PCR (P<0.01), and the protein expression level in
KYSE170K cells was 1.6 times the expression in KYSE170S cells.
Knockdown of ZIP5 expression upregulated the mRNA expression level
of E-cadherin by 80% and the protein expression level by 60%
(Fig. 4).
Discussion
Although the incidence of esophageal adenocarcinoma
in Western countries has been rapidly increasing over the past few
decades (13), the worldwide
incidence of ESCC seems to be relatively stable or slightly
decreased. Esophageal cancer is usually fatal, with an extremely
poor 5-year survival rate of 20.9% in China (2). The main reason is that most cases are
asymptomatic and go undetected until they are at an advanced stage
and no longer amenable to surgical resection. It is important to
determine the underlying mechanisms of esophageal cancer and search
for new biomarkers for early detection and early treatment.
Zinc, an essential trace element, is necessary for
the stabilization and function of numerous metalloenzymes involved
in RNA and DNA and protein synthesis, and catabolism and energy
metabolism (14). The intracellular
zinc concentration is correlated to cell proliferation,
differentiation and apoptosis (15,16).
As zinc treatments were first reported to inhibit tumor growth in
animal models in 1969, there has been considerable interest in the
role of zinc in cancer development and progression. In rodents, the
association of zinc deficiency with the development of esophageal
cancer has been unequivocally demonstrated, and differentially
expressed zinc-regulated genes have been identified using
microarrays (17–20). People who consume large quantities
of whole grains and relatively little meat, which is a common
dietary habit in high-risk areas are likely to be zinc deficient.
Evidence from epidemiological studies suggests that zinc deficiency
is associated with esophageal cancer (3). Abnet et al conducted a
case-control study in a Chinese population that showed that a high
tissue zinc concentration was strongly associated with a reduced
risk of developing ESCC (21).
Zinc homeostasis is essential for maintaining normal
physiological function. There are two important zinc transporter
protein families that are directly involved in the metabolic
homeostasis of intracellular zinc ions, ZIP and ZnT. The ZIP family
contains ZIP1-ZIP14, and the ZnT family contains ZnT1-ZnT10; they
are distributed in different tissues and organs (9,14,22,23).
The intracellular zinc level is determined by a variety of zinc
transporter interactions, and the zinc transporter regulates
intestinal zinc excretion to protect against zinc toxicity
(7). ZIP5 is a central player in
mammalian zinc metabolism and localizes to the basolateral surface
of polarized cells. Some studies have shown that zinc transporters
may play important roles in cancer progression. For example, the
overexpression of ZIP1 promoted prostate cancer progression
(4). Upregulation of ZIP4 was found
to cause or accelerate pancreatic cancer (6). Increased expression of ZIP6 protein
promoted proliferation and invasion in ESCC cells (7). The overexpression of ZIP7 and ZIP10
significantly increased the invasion of breast cancer cells
(8,9,24).
Kumar et al presented a schematic illustration of different
stages of esophageal tumorigenesis in India and identified 19
differentially expressed genes encoding zinc binding or modulating
proteins, including zinc transporters ZnT7 and ZIP5 by microarray.
They found that ZIP5 is affected by an upregulation of zinc
metabolizing protein, suggesting the deregulation of zinc
homeostasis during esophageal tumorigenesis (12), yet the relationship between ZIP5 and
esophageal cancer has not yet been clarified.
The results of the present study illustrated that
there was weak expression of ZIP5 in normal esophageal tissues,
significantly increased expression in the para-cancerous tissues
and significantly higher expression in cancer tissues. The high
expression levels of ZIP5 corresponded to the degree of tumor
malignancy; thus, ZIP5 plays an important role in the development
of esophageal cancer. We found that the severity of hyperplasia and
a high expression level of ZIP5 are consistent with other research
studies on human esophageal cancer tissues, yet the causal
relationship between them is not clear. Therefore, we established
an esophageal cancer cell line with knockdown of ZIP5 expression to
explore their relationship by researching the influence of the
knockdown of ZIP5 on esophageal cancer cells. In this experiment,
we used a lentiviral-mediated RNAi technology, and the silencing
efficiency was 70% at the mRNA level and 90% at the protein level.
We explored cell proliferation using MTT and CCK assays, and the
results showed that the proliferation of the esophageal cancer cell
lines was reduced significantly after ZIP5 knockdown. ZIP5 plays an
important role in the proliferation of esophageal cancer cells.
Silencing of ZIP5 was also associated with a decrease in cell
migration and invasion. The results of the Transwell assays showed
that the cell migratory and invasive abilities of the KYSE170K
cells were decreased by 54 and 68%, respectively, when compared
with the KYSE170S cells which strongly suggests that ZIP5 not only
regulates esophageal cancer cell proliferation but also has an
impact on cancer metastasis.
We detected several target genes related to the
proliferation, apoptosis, migration and invasion of cancer cells.
COX2 is an important inducible enzyme during the development of the
inflammatory reaction, and it is closely related to human tumors
(25,26). COX2 is overexpressed in a variety of
tumors, particularly in digestive system cancer cells and cell
lines (27–29). Upon examination of the COX2
expression of the stable cell lines, we found that the knockdown of
ZIP5 suppressed the expression of COX2 in the esophageal cancer
cells which may indicate that ZIP5 has an effect on the progression
of esophageal cancer by influencing COX2. We also found that the
cyclin D1 expression level in the KYSE170K cells was significantly
lower than that in the KYSE170S cells. Cyclin D1 is a critical
protein for the regulation of the G1 cell cycle phase, and its main
function is to promote cell proliferation; hence, increased
expression of cyclin D1 promotes cell proliferation. Therefore, we
demonstrated that the knockdown of ZIP5 expression suppressed
proliferation by reducing the expression levels of cyclin D1. The
FCM results also supported the observation that increasing cells in
the G1 phase following knockdown of ZIP5 weakened cell
proliferation. Our result showed that the expression of E-cadherin
was increased following knockdown of ZIP5. E-cadherin is a
calcium-dependent cell adhesion molecule, and its main role is in
regulating intercellular adhesion, and adhesion significantly
inhibits the migration and invasion of tumors. Knockdown of ZIP5
upregulated the expression of E-cadherin, which explains the
results in which the silencing of ZIP5 decreased cell migration and
invasion.
In the present study, we explored the role of ZIP5
in esophageal cancer progression through cell experiments, and we
will conduct more in vitro animal experiments to test and
verify these results. We observed the phenomenon that ZIP5 was
overexpressed in human esophageal cancer tissues but did not
investigate whether zinc deficiency was the causen. These issues
will be investigated in the future.
In summary, we discovered that the expression level
of ZIP5 was highest in ESCC, intermediate in para-carcinoma and
lowest in the normal tissue samples. We constructed a stable
knockdown ZIP5 cell line and found that knockdown of ZIP5
expression decreased the proliferation, metastasis and invasion of
esophageal cancer cells. These results establish an experimental
foundation for further study and can help us to discover new
therapies for esophageal cancer.
Acknowledgments
The present study was supported by grants from the
National Natural Scientific Foundation of China (81272682), and the
National Natural Scientific Foundation of Hebei Province
(C2011206058).
References
|
1
|
GLOBOCAN 2012: Estimated Cancer Incidence,
Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
2012
|
|
2
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar
|
|
3
|
Zou XN, Taylor PR, Mark SD, Chao A, Wang
W, Dawsey SM, Wu YP, Qiao YL and Zheng SF: Seasonal variation of
food consumption and selected nutrient intake in Linxian, a high
risk area for esophageal cancer in China. Int J Vitam Nutr Res.
72:375–382. 2002. View Article : Google Scholar
|
|
4
|
Makhov P, Golovine K, Uzzo RG, Wuestefeld
T, Scoll BJ and Kolenko VM: Transcriptional regulation of the major
zinc uptake protein hZip1 in prostate cancer cells. Gene.
431:39–46. 2009. View Article : Google Scholar :
|
|
5
|
Iguchi K, Otsuka T, Usui S, Sugimura Y and
Hirano K: Correlation between ZIP2 messenger RNA expression and
zinc level in rat lateral prostate. Biol Trace Elem Res.
112:159–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H,
Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, et al: Aberrant
expression of zinc transporter ZIP4 (SLC39A4) significantly
contributes to human pancreatic cancer pathogenesis and
progression. Proc Natl Acad Sci USA. 104:18636–18641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu C, Li D, Jia W, Hu Z, Zhou Y, Yu D,
Tong T, Wang M, Lin D, Qiao Y, et al: Genome-wide association study
identifies common variants in SLC39A6 associated with length of
survival in esophageal squamous-cell carcinoma. Nat Genet.
45:632–638. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor KM, Vichova P, Jordan N, Hiscox S,
Hendley R and Nicholson RI: ZIP7-mediated intracellular zinc
transport contributes to aberrant growth factor signaling in
antihormone-resistant breast cancer Cells. Endocrinology.
149:4912–4920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hogstrand C, Kille P, Nicholson RI and
Taylor KM: Zinc transporters and cancer: A potential role for ZIP7
as a hub for tyrosine kinase activation. Trends Mol Med.
15:101–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Kim BE, Petris MJ and Eide DJ: The
mammalian Zip5 protein is a zinc transporter that localizes to the
basolateral surface of polarized cells. J Biol Chem.
279:51433–51441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dufner-Beattie J, Kuo YM, Gitschier J and
Andrews GK: The adaptive response to dietary zinc in mice involves
the differential cellular localization and zinc regulation of the
zinc transporters ZIP4 and ZIP5. J Biol Chem. 279:49082–49090.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar A, Chatopadhyay T, Raziuddin M and
Ralhan R: Discovery of deregulation of zinc homeostasis and its
associated genes in esophageal squamous cell carcinoma using cDNA
microarray. Int J Cancer. 120:230–242. 2007. View Article : Google Scholar
|
|
13
|
Bosetti C, Levi F, Ferlay J, Garavello W,
Lucchini F, Bertuccio P, Negri E and La Vecchia C: Trends in
oesophageal cancer incidence and mortality in Europe. Int J Cancer.
122:1118–1129. 2008. View Article : Google Scholar
|
|
14
|
Gaither LA and Eide DJ: Eukaryotic zinc
transporters and their regulation. Biometals. 14:251–270. 2001.
View Article : Google Scholar
|
|
15
|
Franklin RB and Costello LC: Zinc as an
anti-tumor agent in prostate cancer and in other cancers. Arch
Biochem Biophys. 463:211–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uzzo RG, Crispen PL, Golovine K, Makhov P,
Horwitz EM and Kolenko VM: Diverse effects of zinc on NF-kappaB and
AP-1 transcription factors: Implications for prostate cancer
progression. Carcinogenesis. 27:1980–1990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fong LY, Sivak A and Newberne PM: Zinc
deficiency and methylbenzylnitrosamine-induced esophageal cancer in
rats. J Natl Cancer Inst. 61:145–150. 1978.PubMed/NCBI
|
|
18
|
Fong LY, Lau KM, Huebner K and Magee PN:
Induction of esophageal tumors in zinc-deficient rats by single low
doses of N-nitrosomethylbenzylamine (NMBA): Analysis of cell
proliferation, and mutations in H-ras and p53 genes.
Carcinogenesis. 18:1477–1484. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fong LY and Magee PN: Dietary zinc
deficiency enhances esophageal cell proliferation and
N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor
incidence in C57BL/6 mouse. Cancer Lett. 143:63–69. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fong LY, Nguyen VT and Farber JL:
Esophageal cancer prevention in zinc-deficient rats: Rapid
induction of apoptosis by replenishing zinc. J Natl Cancer Inst.
93:1525–1533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM,
Taylor PR, Dong ZW, Mark SD and Dawsey SM: Zinc concentration in
esophageal biopsy specimens measured by X-ray fluorescence and
esophageal cancer risk. J Natl Cancer Inst. 97:301–306. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palmiter RD and Findley SD: Cloning and
functional characterization of a mammalian zinc transporter that
confers resistance to zinc. EMBO J. 14:639–649. 1995.PubMed/NCBI
|
|
23
|
Liuzzi JP and Cousins RJ: Mammalian zinc
transporters. Annu Rev Nutr. 24:151–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kagara N, Tanaka N, Noguchi S and Hirano
T: Zinc and its transporter ZIP10 are involved in invasive behavior
of breast cancer cells. Cancer Sci. 98:692–697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song X, Lin HP, Johnson AJ, Tseng PH, Yang
YT, Kulp SK and Chen CS: Cyclooxygenase-2, player or spectator in
cyclooxygenase-2 inhibitor-induced apoptosis in prostate cancer
cells. J Natl Cancer Inst. 94:585–591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukazawa EM, Baiocchi G, Soares FA,
Kumagai LY, Faloppa CC, Badiglian-Filho L, Coelho FR, Gonçalves WJ,
Costa RL and Góes JC: COX-2, EGFR, and ERBB-2 expression in
cervical intraepithelial neoplasia and cervical cancer using an
automated imaging system. Int J Gynecol Pathol. 33:225–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dohadwala M, Luo J, Zhu L, Lin Y,
Dougherty GJ, Sharma S, Huang M, Pold M, Batra RK and Dubinett SM:
Non-small cell lung cancer cyclooxygenase-2-dependent invasion is
mediated by CD44. J Biol Chem. 276:20809–20812. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costa C, Soares R, Reis-Filho JS, Leitão
D, Amendoeira I and Schmitt FC: Cyclo-oxygenase 2 expression is
associated with angiogenesis and lymph node metastasis in human
breast cancer. J Clin Pathol. 55:429–434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smyth GP, Stapleton PP, Barden CB, Mestre
JR, Freeman TA, Duff MD, Maddali S, Yan Z and Daly JM: Renal cell
carcinoma induces prostaglandin E2 and T-helper type 2 cytokine
production in peripheral blood mononuclear cells. Ann Surg Oncol.
10:455–462. 2003. View Article : Google Scholar : PubMed/NCBI
|