Introduction
In the United States, prostate cancer is the most
commonly diagnosed cancer in males (1). Treatment strategies for PCa are based
on androgen deprivation, but in the late hormone-refractory stage,
it is still necessary to develop improved treatment strategies.
TNF-related apoptosis-inducing ligand (TRAIL), a new member of the
tumor necrosis factor (TNF) family, was shown to possess the
ability to induce apoptosis in a wide range of human cancer cell
lines without significant cytotoxicity towards normal cells
(2,3). Yet, previous studies show that both
intrinsic and acquired resistance to TRAIL poses a huge problem in
establishing clinically efficacious TRAIL therapies. Combinatorial
therapy represents a promising strategy for treating cancers that
are resistant to TRAIL (4–12).
It is increasingly evident that several
anti-apoptotic pathways including those regulated by nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) are critical in
modulating the effects of TRAIL (4–6).
Constitutive NF-κB activation has been implicated in resistance to
TRAIL (7). As a member of the TNF
superfamily, upon receptor ligation, TRAIL simultaneously activates
intrinsic (or mitochondrial) and extrinsic cell death pathways
(8), as well as survival signaling
via transcription of NF-κB (9,10). In
the static state, NF-κB stays in the cytoplasm forming a complex
with inhibitor of κB (IκB). Binding of TRAIL (11) to the receptors activates the
inhibitor of κB-kinase (IKK). IκB is phosphorylated by activated
IKK and is then ubiquitinated and targeted for proteolysis. The
degradation of IκB allows NF-κB to translocate to the nucleus where
it binds to NF-κB response elements, which activate transcription
of target genes, such as Bcl-XL (12), carrying out antitumor function.
Casein kinase 2 (CK2), a most highly conserved and
ubiquitous protein serine/threonine kinase, has been recognized as
a key player in cell growth and proliferation as well as in the
regulation of apoptotic activity in cells. Due to the general
prosurvival effects of CK2 activity, its inhibitors are considered
as potential anticancer drugs (13,14).
Their application appears particularly promising in combination
with other anticancer agents whose effective concentrations may be
reduced in this manner. In the case of death ligand TRAIL, its
synergism with CK2 inhibitors has been demonstrated (15,16).
Downregulation of CK2 sensitizes prostate cancer cells to
TRAIL-mediated apoptosis through effects on caspase-3, -8, and -9,
and mitochondrial apoptotic proteins (15). Yet, the effect of CK2 on the
TRAIL-induced NF-κB pathway remains largely unclear.
In the present study, we investigated the effects of
CK2 on TRAIL-mediated NF-κB signaling. We found that under the
stimulation of TRAIL, downregulation of CK2 with a specific
inhibitor (TBB, 4,5,6,7-tetrabromobenzotriazole) or specific small
interfering RNA (siRNA) decreased the phosphorylation of the
subunit of NF-κB, p65, and blocked the translocation of p65 into
the nucleus from the cytosol. These effects inhibited the
activation of p65, and then decreased the expression of downstream
pro-apoptotic genes. Thus, downregulation of CK2 sensitizes
prostate cancer cells to TRAIL-mediated apoptosis through the
suppression of NF-κB signaling.
Materials and methods
Cell line, cell culture and reagents
The prostate cancer cell line PC-3 was purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). PC-3 cells were cultured in RPMI-1640 containing 10% fetal
bovine serum (FBS), 100 μg/ml streptomycin, 100 U/ml
penicillin, and 0.25 μg/ml amphotericin B. Cells were
incubated at 37°C with 5% CO2. TBB was purchased from
Calbiochem (San Diego, CA, USA) and TRAIL was from R&D Systems
(Minneapolis, MN, USA).
Cell transfection
Transfections were performed by electroporation
using an Electro Square Porator ECM 830 (BTX) (17) or by using Lipofectamine 2000
(Invitrogen). siRNA pool specific for CK2α and non-specific (NS)
control siRNAs were purchased from GE Health Dharmacon (Lafayette,
CO, USA). Cells were harvested 48–72 h after transfection.
Approximately 75–90% transfection efficiencies were routinely
achieved.
Western blot analysis
Protein samples were prepared by lysing cells in
modified RIPA buffer [1X PBS, 1% Nonidet P-40, 0.1% sodium dodecyl
sulfate, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis,
MO, USA)]. Lysates (50–100 μg) were separated on a 7.5%
SDS-PAGE gel and transferred to a nitrocellulose membrane. The
membrane was probed with the specific primary antibody and
HRP-conjugated secondary antibody and then visualized by
chemiluminescence. Antibodies against CK2α (C-18) and ERK2 (D-2)
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Antibody against p65 (610868) was purchased from BD
(Biosciences, San Jose, CA, USA). Antibody against β-tubulin was
purchased from Sigma-Aldrich Co. LLC. Antibodies against histone 3
(9715) and phospho-NF-κB p65 (Ser536, 3031) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA).
Nuclear cytoplasmic fractionation
Cell fractionation was performed as described
previously (18). Cells were washed
with PBS twice, then lysed in cold buffer A (10 mM HEPES pH 7.9, 10
mM KCl, 1.5 mM MgCl2, 0.5 mM DTT). Complete Mini (Roche)
protease inhibitor (PIC) was added fresh containing 0.1% NP-40 for
10 min on ice. The pellet was washed 3 times with buffer A after
centrifugation at 6,500 rpm for 3 min. The supernatant (cytoplasmic
fraction) was transferred to a new tube and centrifuged for 10 min
at high speed to clear debris/membranes. The crude nuclear fraction
pellet was washed with 1 ml of buffer A for 3 times. The pellet was
resuspended in an equal volume of cold buffer B (20 mM HEPES pH
7.9, 0.42 sM NaCl, 25% glycerol, 1.5 mM MgCl2, 0.2 mM
EDTA, 0.5 mM DTT; PIC added fresh). Samples were rotated at 4°C for
30 min; centrifuged for 20 min at high speed, and the supernatant
was transferred to a new tube (nuclei). Histone 3 and β-tubulin
were used as nuclear and cytoplasmic markers, respectively.
Semi-quantitative and real-time
RT-PCR
Total RNA was isolated with TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). CDNA was synthesized using
SuperScript II reverse transcriptase (Invitrogen). Quantitative
real-time PCR was performed with cDNA samples using the IQ
SYBR-Green Supermix and ABI Prism 7900 platform (Bio-Rad, Hercules,
CA, USA) according to the manufacturer's instructions. The
2−ΔΔCt method was used to calculate the relative
expression level by normalization at glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) levels. The following primer sequences
were used: BCL-XL, 5′-gatctggtcccttgcagctagttt-3′ and
5′-ctaaggggtaagggttgcaccaat-3′; TRAF2,
5′-agaacattgtctgcgtcctgaacc-3′ and 5′-tctccaagaccttctgctccaagt-3′;
and GAPDH, 5′-acccactcctccacctttgac-3′ and
5′-tgttgctgtagccaaattcgtt-3′.
Luciferase reporter assay
For luciferase reporter assays, the cells were
harvested 24 h after transfection, and firefly and Renilla
luciferase activities in cell lysates were measured using a
Dual-Luciferase kit (Promega). Renilla luciferase activity
of the cells was used as internal controls. Cell transfection was
performed as described previously. Approximately 75–90%
transfection efficiencies were routinely achieved.
Cell viability assay
Cell viability was monitored by absorbance using the
MTS assay according to the manufacturer's instructions (Promega).
Briefly, PC-3 cells were plated in 96-well plates at a density of
2,500 cells/well and treated with 60 μM of TBB for 3 h, and
then treated with or without 10 ng/ml of TRAIL. At 0, 24 and 48 h
after the TBB treatment, 20 μl of CellTiter 96R AQueous One
Solution reagent (Promega) was added to the cells. After incubation
for 90 min at 37°C in a cell incubator, cell viability was measured
in a microplate reader at 490 nm.
Statistical analysis
All values are expressed as means ± SD. Comparison
between two mean values was made by an independent-samples t-test.
Statistical significance was set at P<0.05.
Results
Downregulation of CK2 combined with a
sub-dose of TRAIL suppresses p65 phosphorylation
In a previous study (15), we clarified that CK2 suppressed
TRAIL-induced apoptosis via its effects on the activation of
caspases, DNA fragmentation, and also by blocking the mitochondrial
apoptosis machinery. Here, we wanted to ascertain whether CK2 has
an effect on the TRAIL-induced NF-κB pathway. Since we wanted to
ascertain the mechanism involved in the increased sensitivity of
PCa cells to the antitumor effect of TRAIL, we used TRAIL at
concentrations that did not induce significant apoptosis alone (10
ng/ml), in the PC-3 cells (15).
Firstly, we employed an MTS assay to assess the
effects of a sub-dose of TRAIL and TBB on prostate cancer cell
viability. As shown in Fig. 1A,
cell viability was significantly reduced following the combination
therapy of sub-dose TRAIL and TBB, but almost no change was noted
following treatment of a sub-dose TRAIL or TBB alone. This
confirmed that suppression of CK2 sensitized the PCa cells to the
antitumor effect of TRAIL.
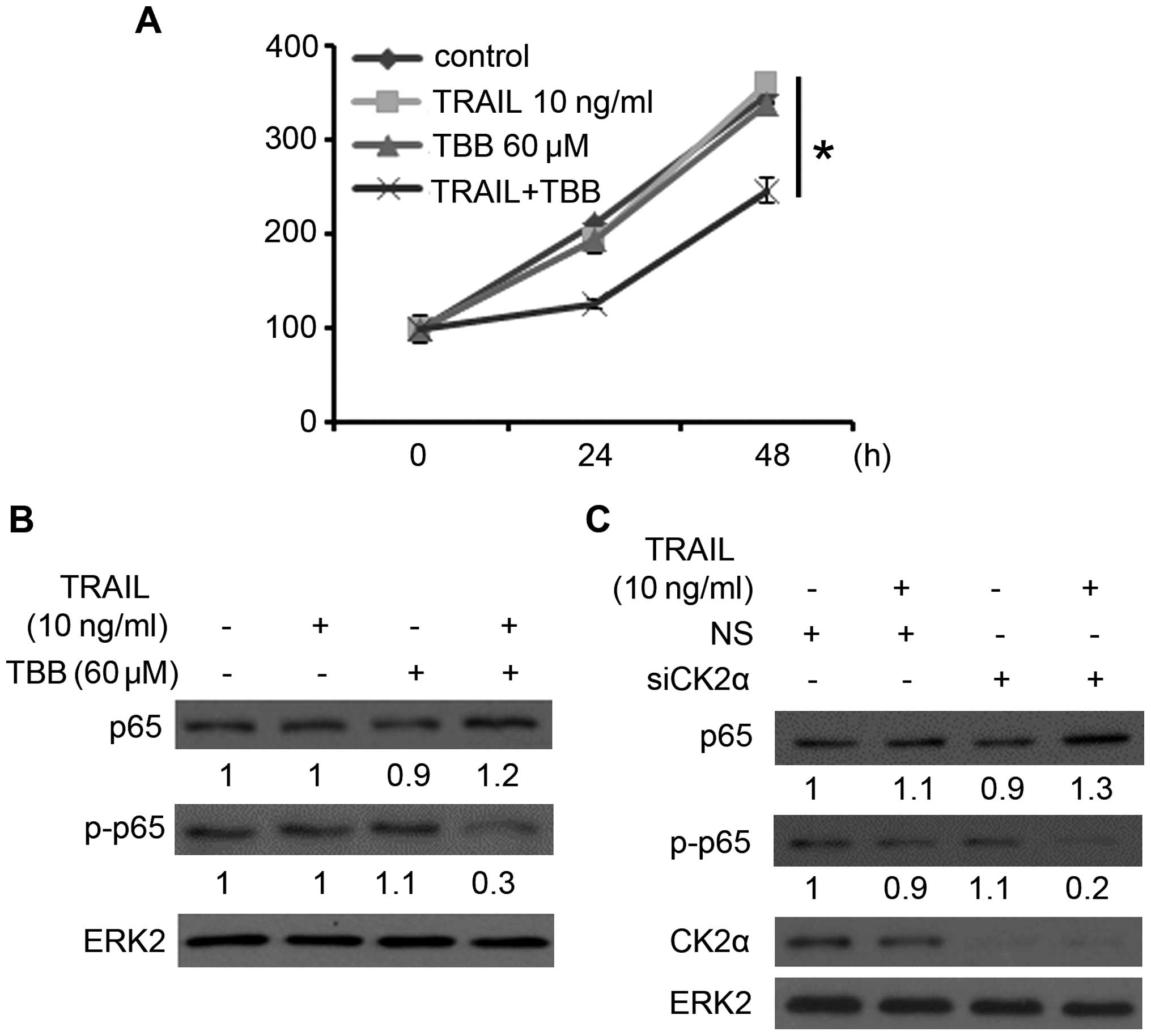 | Figure 1Downregulation of CK2 suppresses
TRAIL-induced p65 phosphorylation. (A) Cell viability assay was
carried out in the PC-3 cells as described in Materials and
methods. Error bars, SD; *P<0.05. (B) PC-3 cells were
treated with 60 μM of TBB for 3 h, and then maintained for
21 h with or without 10 ng/ml of TRAIL. Protein expression of p65,
p-p65 (phosphorylation levels of p65 at serine 536), and ERK2 were
analyzed by western blotting. The levels of p65 and p-p65 were
quantified, normalized to ERK2 levels, and expressed relative to
mock-treated cells. (C) PC-3 cells were transfected with NS control
or CK2α siRNA. After 24 h of transfection, the cells were treated
with or without 10 ng/ml of TRAIL for 24 h. p65, p-p65, CK2α and
ERK2 protein expression levels were analyzed by western blotting.
The levels of p65 and p-p65 were quantified, normalized to ERK2
levels, and expressed relative to mock-treated cells. TRAIL,
TNF-related apoptosis-inducing ligand; NS, non-specific. |
In order to study the effect of CK2 on the
TRAIL-induced NF-κB pathway, PC-3 cells were treated with 60
μM of TBB for 3 h, and then maintained for 21 h with or
without 10 ng/ml of TRAIL (15).
After 24 h, the cells were harvested for western blot assay to
assess the expression level of NF-κB subunit p65 (p65) and the
phosphorylation levels of p65 at serine 536 (p-p65), which play an
important role in the activation of the NF-κB pathway (19,20).
As shown in Fig. 1B, the treatment
of cells with TBB followed by TRAIL resulted in a slight increase
in p65 expression but a marked decrease in the phosphorylation
levels of p65. PC-3 cells were then treated with non-specific or
CK2α-specific siRNA. After 24 h of transfection, the cells were
treated with or without 10 ng/ml of TRAIL. After another 24 h, the
cells were harvested for western blot assay. After knockdown of
CK2α, the level of p-p65 (Ser536) was significantly downregulated
following the treatment of TRAIL (Fig.
1C). Thus, downregulation of CK2α combined with a sub-dose of
TRAIL suppressed the phosphorylation of p65 at serine 536 in the
PCa cells.
The combination treatment of TRAIL and
TBB regulates p65 nuclear translocation
p65 nuclear localization is the rate-limiting step
in the activation of the NF-κB pathway, thus we investigated the
effect of CK2 on TRAIL-induced p65 nuclear localization. PC-3 cells
were treated with 60 μM of TBB for 3 h, and then maintained
for 21 h with or without 10 ng/ml of TRAIL. The cytoplasmic and
nuclear separation technology was employed to assess the expression
of p65. As shown in Fig. 2,
treatment of TBB followed by TRAIL resulted in an increase in p65
expression in the cytoplasm. Yet, the expression of p65 in the
nucleus was decreased following the combination treatment of a
sub-dose of TRAIL and TBB. These data indicate that the combination
treatment decreased the nuclear translocation of p65.
Downregulation of CK2 combined with a
sub-dose of TRAIL suppresses NF-κB transcriptional activity
To further understand the changes associated with
CK2 and the TRAIL-induced NF-κB pathway, we investigated the
transcriptional activity of NF-κB regulated by CK2 following the
treatment of a sub-dose of TRAIL. PC-3 cells were treated with
non-specific or CK2α-specific siRNA. After 24 h of transfection,
the cells were treated with or without 10 ng/ml of TRAIL for
another 24 h. The knockdown effect of CK2α is shown in Fig. 3A. Treatment of 10 ng/ml of TRAIL or
knockdown of CK2α alone did not affect the NF-κB luciferase
activity. Yet, when CK2α was knocked down and a sub-dose of TRAIL
was added, the NF-κB luciferase activity decreased significantly
(Fig. 3B). Consistent results were
found in the combination of TRAIL and TBB treatment. The treatment
of 10 ng/ml of TRAIL or 60 μM of TBB alone did not affect
the NF-κB luciferase activity, while the NF-κB luciferase activity
decreased significantly following treatment with TBB followed by
TRAIL (Fig. 3C). Thus, following
treatment of a sub-dose of TRAIL and downregulation of CK2α, using
both genetic and pharmacological approaches, the transcriptional
activity of NF-κB was decreased in the PCa cells.
 | Figure 3Effect of CK2 on the TRAIL-induced
NF-κB transcriptional activity. (A) PC-3 cells were treated with NS
or CK2α-specific siRNA. After 48 h of transfection, CK2α and ERK2
protein expression levels were analyzed by western blotting. (B)
PC-3 cells were treated with NS or CK2α-specific siRNA. After 24 h
of transfection, the cells were transfected with NF-κB firefly
luciferase reporter, and Renilla luciferase as a control
using Lipofectamine 2000, and then treated with or without 10 ng/ml
of TRAIL for another 24 h. Firefly was normalized to Renilla
luciferase activity for every measurement in all experiments.
Columns, mean values among three replicates; error bars, SD;
*P<0.05. (C) PC-3 cells were transfected with NF-κB
firefly luciferase reporter, and Renilla luciferase as
control using Lipofectamine 2000, treated with 60 μM of TBB
for 3 h, and then maintained for 21 h with or without 10 ng/ml of
TRAIL. Firefly was normalized to Renilla luciferase activity
for every measurement in all experiments. Columns, mean values
among three replicates; error bars, SD; *P<0.05.
TRAIL, TNF-related apoptosis-inducing ligand; NS, non-specific;
siRNA, small interfering RNA. |
Downregulation of CK2 combined with a
sub-dose of TRAIL suppresses NF-κB downstream apoptosis-related
gene expression
Furthermore, we assessed the mRNA expression level
of NF-κB downstream anti-apoptotic genes (21), BCL-XL and TRAF2. The
PC-3 cells were treated with non-specific or CK2α-specific siRNA.
After 24 h of transfection, the cells were treated with or without
10 ng/ml of TRAIL for another 24 h. When we knocked down CK2 and
the sub-dose TRAIL was added, the expression of BCL-XL and
TRAF2 was decreased significantly (Fig. 4A and B). When the cells were treated
with TBB followed by TRAIL, the expression of these genes was also
significantly decreased (Fig. 4C and
D). Thus, following treatment of a sub-dose of TRAIL,
downregulation of CK2 decreased the expression of the NF-κB
downstream anti-apoptotic genes in the PCa cells.
Discussion
Since the mid-1990′s, TRAIL has been used as a
target of several anticancer therapies. To improve the
effectiveness of TRAIL therapy, efforts should be focused on the
understanding of how the TRAIL pathway is disrupted in individual
cancers and which combination therapies can be utilized most
effectively. Similar to previous studies (22,23),
we found that the cell viability was significantly reduced
following the combination therapy of a sub-dose of TRAIL and TBB.
Suppression of CK2 sensitized the PCa cells to the antitumor effect
of TRAIL.
CK2, a highly conserved and ubiquitous protein
Ser/Thr kinase, plays important roles in tumorigenesis (24,25).
Previous studies found that CK2 downregulation sensitized cells to
TRAIL-induced cell apoptosis, but the mechanism was mostly related
to the TRAIL-induced activation of intrinsic and extrinsic cell
death pathways (15). NF-κB is a
dimeric transcription factor consisting of p50, p52, p65/relA, relB
and c-rel subunits. Constitutive NF-κB activation is a common event
in cancer (26,27), and has been implicated in resistance
to TRAIL (7). CK2 acts at multiple
levels in NF-κB activation, such that it targets not only IκB, but
also IKKi/IKKe and p65 (28–30).
In the present study, we found that following treatment of a
sub-dose of TRAIL in combination with downregulation of CK2 the
transcriptional activity of NF-κB was decreased and the expression
of the NF-κB downstream anti-apoptotic genes was also decreased in
the PCa cells. These results revealed that the downregulation of
CK2 sensitized the tumor cells to the antitumor therapy of TRAIL at
least partially due to the suppression of NF-κB transcriptional
activity and the downregulation of NF-κB downstream anti-apoptotic
genes. Reduction in the expression of anti-apoptotic genes allowed
the tumor cells to be more sensitive to TRAIL-induced apoptosis,
thereby alleviating TRAIL resistance to some extent.
CK2-regulated phosphorylation and localization of
p65 are important for the sensitization of tumor cells to TRAIL.
Phosphorylation of the p65 subunit at Ser-536 is an alternative to
classical NF-κB activation. It serves as an integrator for multiple
signaling pathways during NF-κB activation (31,32).
In the present study, downregulation of CK2 combined with a
sub-dose of TRAIL almost blocked the phosphorylation of p65 at
Ser-536. This is an important mechanism of the inhibition of p65
activity.
An indispensable mechanism involved in the
regulation of NF-κB transcriptional activity and NF-κB downstream
antiapoptotic gene expression is NF-κB nucleus translocation. The
import of NF-κB from the cytoplasm to the nucleus is the key step
in the activation of the NF-κB pathway. Based on our data, the
combination treatment of TRAIL and the CK2 inhibitor decreased p65
nuclear import. The reduction in p65 nuclear translocation caused a
decrease in NF-κB downstream anti-apoptotic gene expression
resulting in a pro-apoptotic effect.
In summary, we provide molecular insight into the
mechanisms by which CK2 regulates the sensitivity of PCa cells to
the antitumor effect of TRAIL in a completely new perspective.
Downregulation of CK2 combined with a sub-dose of
TRAIL suppressed the phosphorylation of p65 at Ser-536. The
combination treatment of TRAIL and a CK2 inhibitor decreased p65
nuclear translocation. These effects finally led to the suppression
of NF-κB transcriptional activity and the downregulation of
expression of NF-κB downstream anti-apoptotic genes, thereby
promoting TRAIL-induced apoptosis. Understanding the molecular
mechanisms may lead to the identification of a novel therapeutic
strategy to overcome the resistance of TRAIL in the treatment of
PCa.
Acknowledgments
This study was supported in part by grants from the
Natural Science Foundation of China (no. 81172541 to G.W.) and the
Natural Science Foundation of Jilin Province of China (no.
201015139 to G.W.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allen JE and El-Deiry WS: Regulation of
the human TRAIL gene. Cancer Biol Ther. 13:1143–1151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morales JC, Ruiz-Magaña MJ and Ruiz-Ruiz
C: Regulation of the resistance to TRAIL-induced apoptosis in human
primary T lymphocytes: Role of NF-kappaB inhibition. Mol Immunol.
44:2587–2597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YS, Schwabe RF, Qian T, Lemasters JJ
and Brenner DA: TRAIL-mediated apoptosis requires NF-kappaB
inhibition and the mitochondrial permeability transition in human
hepatoma cells. Hepatology. 36:1498–1508. 2002.PubMed/NCBI
|
|
6
|
Keane MM, Rubinstein Y, Cuello M,
Ettenberg SA, Banerjee P, Nau MM and Lipkowitz S: Inhibition of
NF-kappaB activity enhances TRAIL mediated apoptosis in breast
cancer cell lines. Breast Cancer Res Treat. 64:211–219. 2000.
View Article : Google Scholar
|
|
7
|
Jani TS, DeVecchio J, Mazumdar T, Agyeman
A and Houghton JA: Inhibition of NF-kappaB signaling by quinacrine
is cytotoxic to human colon carcinoma cell lines and is synergistic
in combination with tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) or oxaliplatin. J Biol Chem.
285:19162–19172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozören N and El-Deiry WS: Defining
characteristics of types I and II apoptotic cells in response to
TRAIL. Neoplasia. 4:551–557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stuckey DW and Shah K: TRAIL on trial:
Preclinical advances in cancer therapy. Trends Mol Med. 19:685–694.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalvez F and Ashkenazi A: New insights
into apoptosis signaling by Apo2L/TRAIL. Oncogene. 29:4752–4765.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Voelkel-Johnson C: TRAIL-mediated
signaling in prostate, bladder and renal cancer. Nat Rev Urol.
8:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Edelstein LC and Gélinas C: The
Rel/NF-kappaB family directly activates expression of the apoptosis
inhibitor Bcl-x(L). Mol Cell Biol. 20:2687–2695. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo W, Yu WD, Ma Y, Chernov M, Trump DL
and Johnson CS: Inhibition of protein kinase CK2 reduces Cyp24a1
expression and enhances 1,25-dihydroxyvitamin D(3) antitumor
activity in human prostate cancer cells. Cancer Res. 73:2289–2297.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agarwal M, Nitta RT and Li G: Casein
kinase 2: A novel player in glioblastoma therapy and cancer stem
cells. J Mol Genet Med. 8:82013.
|
|
15
|
Wang G, Ahmad KA and Ahmed K: Role of
protein kinase CK2 in the regulation of tumor necrosis
factor-related apoptosis inducing ligand-induced apoptosis in
prostate cancer cells. Cancer Res. 66:2242–2249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orzechowska E, Kozłowska E, Staroń K and
Trzcińska-Danielewicz J: Time schedule-dependent effect of the CK2
inhibitor TBB on PC-3 human prostate cancer cell viability. Oncol
Rep. 27:281–285. 2012.
|
|
17
|
Chen S, Bohrer LR, Rai AN, Pan Y, Gan L,
Zhou X, Bagchi A, Simon JA and Huang H: Cyclin-dependent kinases
regulate epigenetic gene silencing through phosphorylation of EZH2.
Nat Cell Biol. 12:1108–1114. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva L, Oh HS, Chang L, Yan Z,
Triezenberg SJ and Knipe DM: Roles of the nuclear lamina in stable
nuclear association and assembly of a herpesviral transactivator
complex on viral immediate-early genes. MBio. 3:e00300–e00311.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Ma J, Wu Z, Li W, Zhang D, Han L,
Wang F, Reindl KM, Wu E and Ma Q: Arginine deiminase augments the
chemosensitivity of argininosuccinate synthetase-deficient
pancreatic cancer cells to gemcitabine via inhibition of NF-κB
signaling. BMC Cancer. 14:6862014. View Article : Google Scholar
|
|
20
|
Hu J, Nakano H, Sakurai H and Colburn NH:
Insufficient p65 phosphorylation at S536 specifically contributes
to the lack of NF-kappaB activation and transformation in resistant
JB6 cells. Carcinogenesis. 25:1991–2003. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harikumar KB, Kunnumakkara AB, Ahn KS,
Anand P, Krishnan S, Guha S and Aggarwal BB: Modification of the
cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by
xanthohumol leads to suppression of NF-kappaB-regulated gene
products and potentiation of apoptosis in leukemia cells. Blood.
113:2003–2013. 2009. View Article : Google Scholar
|
|
22
|
Kim HR, Kim K, Lee KH, Kim SJ and Kim J:
Inhibition of casein kinase 2 enhances the death ligand- and
natural killer cell-induced hepatocellular carcinoma cell death.
Clin Exp Immunol. 152:336–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Llobet D, Eritja N, Encinas M, Llecha N,
Yeramian A, Pallares J, Sorolla A, Gonzalez-Tallada FJ, Matias-Guiu
X and Dolcet X: CK2 controls TRAIL and Fas sensitivity by
regulating FLIP levels in endometrial carcinoma cells. Oncogene.
27:2513–2524. 2008. View Article : Google Scholar
|
|
24
|
Hundsdörfer C, Hemmerling HJ, Hamberger J,
Le Borgne M, Bednarski P, Götz C, Totzke F and Jose J: Novel
indeno[1,2-b] indoloquinones as inhibitors of the human protein
kinase CK2 with antiproliferative activity towards a broad panel of
cancer cell lines. Biochem Biophys Res Commun. 424:71–75. 2012.
View Article : Google Scholar
|
|
25
|
Wang G, Ahmad KA, Unger G, Slaton JW and
Ahmed K: CK2 signaling in androgen-dependent and -independent
prostate cancer. J Cell Biochem. 99:382–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tong L, Yuan Y and Wu S: Therapeutic
microRNAs targeting the NF-kappa B signaling circuits of cancers.
Adv Drug Deliv Rev. 81:1–15. 2015. View Article : Google Scholar
|
|
27
|
Jin R, Yamashita H, Yu X, Wang J, Franco
OE, Wang Y, Hayward SW and Matusik RJ: Inhibition of NF-kappa B
signaling restores responsiveness of castrate-resistant prostate
cancer cells to anti-androgen treatment by decreasing androgen
receptor-variant expression. Oncogene. Sep 15–2014.(Epub ahead of
print).http://dx.doi.org/10.1038/onc.2014.302.
|
|
28
|
Eddy SF, Guo S, Demicco EG, Romieu-Mourez
R, Landesman-Bollag E, Seldin DC and Sonenshein GE: Inducible
IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2
and promotes aberrant nuclear factor-kappaB activation in breast
cancer cells. Cancer Res. 65:11375–11383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manni S, Brancalion A, Mandato E, Tubi LQ,
Colpo A, Pizzi M, Cappellesso R, Zaffino F, Di Maggio SA, Cabrelle
A, et al: Protein kinase CK2 inhibition down modulates the NF-κB
and STAT3 survival pathways, enhances the cellular proteotoxic
stress and synergistically boosts the cytotoxic effect of
bortezomib on multiple myeloma and mantle cell lymphoma cells. PLoS
One. 8:e752802013. View Article : Google Scholar
|
|
30
|
Kim KJ, Cho KD, Jang KY, Kim HA, Kim HK,
Lee HK and Im SY: Platelet-activating factor enhances tumour
metastasis via the reactive oxygen species-dependent protein kinase
casein kinase 2-mediated nuclear factor-κB activation. Immunology.
143:21–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Douillette A, Bibeau-Poirier A, Gravel SP,
Clément JF, Chénard V, Moreau P and Servant MJ: The proinflammatory
actions of angiotensin II are dependent on p65 phosphorylation by
the IkappaB kinase complex. J Biol Chem. 281:13275–13284. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gutierrez H, O'Keeffe GW, Gavaldà N,
Gallagher D and Davies AM: Nuclear factor kappa B signaling either
stimulates or inhibits neurite growth depending on the
phosphorylation status of p65/RelA. J Neurosci. 28:8246–8256. 2008.
View Article : Google Scholar : PubMed/NCBI
|


















