Introduction
Malignant gliomas are the most common primary
malignant tumors in brain. Although various therapeutic modalities
are available, the disease remains incurable. The median survival
rate in patients with newly diagnosed glioblastoma multiforme is
~15 months even when treated by surgery alone or combined with
radiotherapy and chemotherapy (1–3). The
most important reason for the failure of clinical management is the
infiltrative and migrating behavior of gliomas, which leads to
diffuse growth and/or recurrence of the tumor (4). Therefore, new therapeutic strategies
are required to prevent the invasion and migration of glioma cells
effectively.
The regulation of cell migration is critical to
normal and pathological processes, including development, immune
function, such as neutrophils and macrophages and tumor metastasis
(5). Lamellipodia have been
proven essential for the directional migration of cells (6). The formation of lamellipodia
depends on a highly branched network of polymerized actin filaments
at the barbed ends, which drives membrane extension at the leading
edge of cells (7). The
actin-related protein 2/3 (Arp2/3) complex is a key regulator of
the actin network. It binds to the side of a pre-existing
filamentous (F)-actin filaments and stimulates new filament
formation to create branched actin networks, a process termed the
'dendritic nucleation̓ model of cortical actin assembly (8–10).
Previous findings confirmed the role of the Arp2/3 complex in the
metastasis of many tumors, including glioma. The ability of
migration and invasion of tumor cells may be significantly reduced
after Arp2/3 complex disruption by RNA interference (11–13).
Cortactin is an Arp2/3 complex-activating and
F-actin-binding protein. It possess a multi-domain structure
consisting of an acidic domain at the amino terminus (NTA),
followed by six complete and one partial tandem repeating segments,
a proline-rich helical region and an Src homology SH3
domain located at the carboxyl terminus (14,15).
Cortactin was first identified as a prominent substrate of the Src
non-receptor tyrosine kinase. Subsequently, cortactin has been
shown to play an essential role in many actin-based cell processes,
including migration and invasion, axon guidance, neuronal
morphogenesis and tumor cell metastasis (15). In many of the processes, cortactin
regulates activation of the Arp2/3 complex and stabilizes actin
branch points in the dynamic assembly and disassembly of actin
polymerization at the cell periphery. The ability of cortactin to
promote actin polymerization requires the NTA domain, which binds
the Arp3 subunit of the Arp2/3 complex. Cortactin lacking the NTA
domain fails to localize at the cell periphery. The Sh3
domain of cortactin regulates its ability to activate the Arp2/3
complex synergized with some proteins, including Wiskott-Aldrich
syndrome protein (WASP) and WASP-interacting protein (WIP)
(16–18).
As mentioned above, cortactin facilitates migration
by increasing lamellipodia persistence and promotes adhesion
assembly through the binding and activation of the Arp2/3 complex.
In previous studies, amplification of segment 11q13 on chromosome
11, a region that includes the CTTN gene associated the over
expression of cortactin, has been associated with many types of
cancer, including oral squamous and head and neck squamous cell
carcinoma (HNSCC), lung, breast, colorectal cancer and melanoma
(15,19–22)
However, whether cortactin also plays a role in migration and
invasion of glioma cells remains to be determined. In the present
study, we investigated cortactin expression in human gliomas with
different WHO grade and how cortactin influenced the morphology and
motility of glioma cells by regulating the formation of
lamellipodia.
Materials and methods
Reagents and specimens
Cortactin antibody (Abcam, Burlingame, CA, USA);
p34-Arc antibody (Millipore, Billerica, CA, USA), which was
specific for the Arp2/3 complex; rhodamine phalloidin
(Invitrogen-Life Technologies, Carlsbad, CA, USA) used for actin
staining; Alexa Fluor 488 and 555-conjugated and Texas Red goat
anti-mouse secondary antibodies (Invitrogen-Life Technologies);
Triton X-100 (Solarbio, haidian, Beijing, China); 4%
paraformaldehyde (Solarbio), DAPI (Sigma, St. Louis, Mo, USA); and
Lipofectamine™ 2000 Reagent (Invitrogen) were used in the present
study.
Forty tumor specimens were obtained from patients
with glioma by surgical resection at the Department of
Neurosurgery, Tianjin Medical University General Hospital (Tianjin,
China) from July 2011 to November 2013. None of the patients had
undergone radiation or chemotherapy prior to surgical therapy. The
pathological diagnosis and grading for each glioma was assessed by
neuropathologists according to the 2007 World health organization
(WHO) Classification of Nervous System Tumors. Glioma specimens
included 6 cases of diffuse astrocytoma (WHO grade II), 9 of
oligoastrocytoma (WHO grade II), 9 of anaplastic oligodendroglioma
(WHO grade III) and 16 of glioblastoma (WHO grade IV). Eight
specimens of non-tumor brain tissues were obtained from patients
undergoing craniotomy for epilepsy as the control.
Immunostaining results were determined using 5 high
power fields of the specimens. To determine the intensity of the
immunohistochemical staining, scores were determined as: −
(negative staining for target cells), + (positive staining for 1–9%
target cells), ++ (positive staining for 10–49% target cells) and
+++ (positive staining for >49% target cells). Thus, +
represented weak staining, ++ was moderate staining, +++ was strong
staining and - was negative staining.
The tissue samples were collected in accordance with
the institutional review board-approved protocols. After surgical
resection, tissue specimens were immediately frozen and stored in
liquid nitrogen until use. The present study was approved by the
Ethics Committee of the institutional review boards of Tianjin
Medical University General Hospital. Written informed consent was
obtained from all patients.
Cell culture
Human U251, LN229 and SNB19 glioma cell lines, were
purchased from the Chinese Academy of Sciences Cell Bank. U251,
LN229 and SNB19 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS)
(Solarbio) and maintained at 37°C in an atmosphere of 5%
CO2 and routinely passaged at 2–3 day intervals.
Immunohistochemistry
For immunohistochemistry, tissue sections were
incubated with cortactin primary antibody (Abcam, 1:100 dilution)
overnight at 4°C. Biotinylated secondary antibody at a dilution of
1:100 was then added at room temperature for 30 min, followed by
incubation with ABC-peroxidase for an additional 30 min. After
washing with Tris-buffer, the sections were incubated with
3,3′-diaminobenzidine (DAB, 30 mg dissolved in 100 ml Tris-buffer
containing 0.03% H2O2) for 5 min, rinsed in
water and counter stained with hematoxylin.
RNA interference
RNA interference reagent (GenePharma, Hi-Tech Park,
Shanghai, China) include cortactin-siRNA sequence
(5′-CAAGCUUCGAGAGAAUGUCUUTT-3′) and negative control sequence
(5′-UUCUCCGAACGUGUCACGUTT-3′). The two type siRNA sequence
dissolved in DEPC water, respectively. Cortactin-siRNA sequence (5
µl), 5 µl negative control sequence or equal empty
vector was mixed with 5 µl transfection Lipofectamine™ 2000
reagent in 500 µl serum-free medium for the siRNA-cortactin
group, siRNA-NC and siRNA-N groups, respectively.
RT-qPCR
The different treated glioma cells were lysed and
RNA extracts were collected after 48 h. Total RNA was isolated
using the RNeasy kit (Tiangene Biotech Co., Ltd., Beijing, China).
Reverse transcription-PCR (RT-PCR) reaction was implemented using
the RT-PCR kit.
PCR amplification was performed under the
conditions: Denaturation at 94°C for 5 min, 94°C for 30 sec,
annealing at 49°C for 30 sec, total of 35 cycles; with a final
extension at 72°C for 5 min. Primer sequences for cortactin used
were: forward, 5′-GAACAAGACCGAATGGATAAG-3′ and reverse,
5′-TTCAAAGCCTACAGCAGAC-3′. Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) served as the internal standard, with the annealing
temperature at 54°C, whereas the other conditions were identical to
the previous ones. Primer sequences used for GAPDH were: forward,
5′-TCTCTGCTCCTCCTGTTC-3′ and reverse 5′-ATCCGTTGACTCCGACCT-3′.
Western blot analysis
For each specimen, 50 mg of tissue was dissected
into small sections and transferred into a 1.5 ml microcentrifuge
tube. A total of 500 µl cell lysis buffer was added to the
tube. The tissue was homogenized on ice with 10–15 strokes (3–4
sec/stroke) of a mini-homogenizer and plastic pestle. The sample
was centrifuged at 12,000 × g for 15 min at 4°C and the supernatant
was transferred to a fresh tube. A total of 50 µg protein
and an equal volume of 2X sample buffer were heated at 94°C for 5
min.
Following treatment of glioma cells for 48 h, blots
of whole-cell lysates were prepared. Briefly an equal number of
cells were directly lysed in SDS-PAGE loading buffer [0.1 mol/l
Tris (pH 6.8), 20% SDS, 0.2% glycerol, 0.2 mol/l DTT] and boiled at
94°C for 5 min.
Proteins were separated on 10% SDS-polyacrylamide
gel and then transferred onto a polyvinylidene difluoride (PVDF)
membrane. The blot was blocked in PBST and 5% skimmed dried milk at
37°C for 1 h. The membrane was then incubated in primary antibody
(cortactin, rabbit, 1:1,000) at 4°C overnight, followed by
treatment with mouse anti-rabbit secondary antibody (1:5,000).
Blots were developed using enhanced chemiluminescence (ECL)
reagents (Amersham Pharmacia, Buckinghamshire, UK) and visualized
using the gene genius Imaging System (Frederick, MD, USA).
Cortactin antibody was used to detect the expression of cortactin
and β-actin was used as the internal standard.
Wound-healing and Transwell invasion
assays
For the wound-healing assay, the glioma cells of the
treated groups were seeded in 6-well plates at a density of
2.0×105 cells/ml and allowed to reach confluency. A
confluent monolayer was obtained and wounds were created using a
200 µl sterile pipette tip. Subsequently, cell debris was
removed by washing the plates twice with PBS and fresh DMEM
supplemented with 3% FBS was added to each well. The cells were
then cultivated for up to 24 h. The wound-healing area was recorded
by taking photomicrographs at different time-points.
For the Transwell invasion assay, after the glioma
cells were treated for 24 h, the top chambers were coated with a
layer of 25 mg/cm2 matrigel (Millipore). Cells
(5.0×104) in serum-free medium were seeded in each
chamber for each group after the Matrigel freezing. Serum medium
(500 µl) was added in the lower chambers as a
chemoattractant. Following incubation for 48 h, non-invading cells
were removed from the top chamber with a cotton swab. The cells on
the lower surface were fixed by replacing the culture medium in the
bottom with 4% paraformaldehyde. After fixation for 15 min at room
temperature, the chambers were rinsed in PBS and stained with 0.2%
crystal violet for 10 min. For each experimental condition, 10
image fields were photographed and quantified.
Immunofluorescence
The glioma cells under different treatments were
grown on glass coverslips for 24 h. The cells were washed and then
fixed with 4% paraformaldehyde for 25 min. Fixed cells were
permeabilized by treatment with 0.5% TritonX-100 for 5 min and
blocked by incubation with 5% BSA in PBS for 1 h. The cells were
then incubated overnight at 4°C with cortactin (rabbit) and p34-Arc
(mouse) antibodies at a dilution of 1:100. The cells were washed
three times with PBS and then incubated for 1 h with Alexa
488-conjugated goat anti-rabbit secondary antibody at a dilution of
1:1,000 for 1 h at 37°C. The cells were washed with PBS and then
counterstained with rhodamine phalloidin for 20 min to stain actin
filaments and DAPI to stain DNA. The cells were imaged under a
confocal microscope (Olympus FV1000; Research Center of Basic
Medical Science of Tanjin Medical University Olympus, Tokyo,
Japan).
Statistical analysis
Data were analyzed using SPSS 17.0 software
(Chicago, IL, USA). One-way analysis of variance (ANOVA), least
significant difference and Pearson's correlation tests were used.
Values are presented as means ± standard error of measurement
(SEM). P<0.05 was considered statistically significant. In
vitro experiments were repeated three times.
Results
Expression of cortactin in human glioma
specimens
Histological assessment for glioma specimens and
normal brain tissues was performed by neuropathologists. To
determine cortactin expression in human gliomas,
immunohistochemistry and western blot analysis were performed.
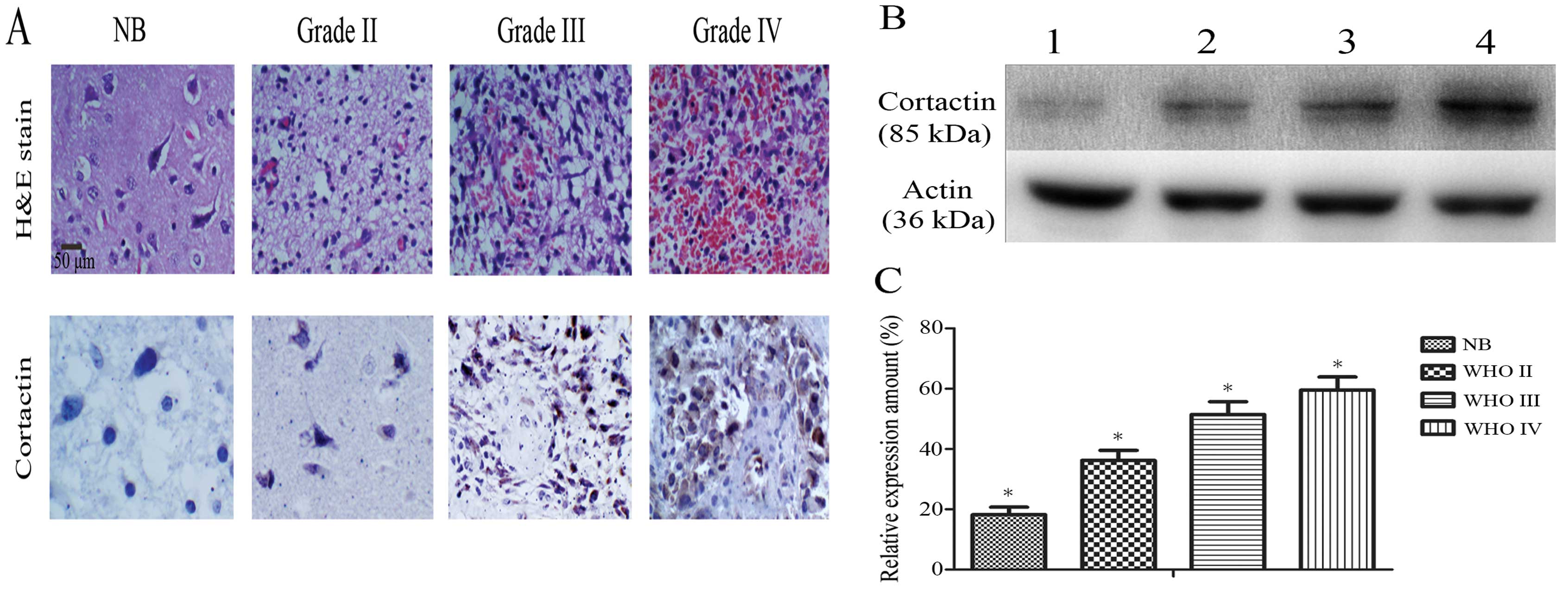
Immunohistochemistry of the tissue sections revealed that cortactin
was localized at the cell cytoplasm and its signal intensity
increased with the increasing tumor malignancy (Fig. 1A). A comparison of cortactin
staining intensity in different pathological grade gliomas and
non-tumor brain tissue is shown in Table I. Relative to β-actin, the level of
cortactin in tissue specimens was 18.20±2.52% in non-tumor brain
tissue (NB, n=8), 36.20±3.34% in WHO grade II (n=15), 51.40±4.3% in
WHO grade III (n=9) and 59.6±4.31% in WHO grade Iv (n=16) (Fig. 1B and C). The expression of cortactin
in the glioma specimens was significantly higher than in non-tumor
brain tissue (P<0.05) and positively correlated with the
malignancy of glioma specimens (r=0.912, P=0.00).
 | Table IComparison of cortactin staining
intensity in different pathological grade gliomas and non-tumor
brain tissue (Case). |
Table I
Comparison of cortactin staining
intensity in different pathological grade gliomas and non-tumor
brain tissue (Case).
| Tissue type | Sample cases | Results
|
|---|
| − | + | ++ | +++ |
|---|
| Control brain
tissue | 8 | 8 | 0 | 0 | 0 |
| WHO II grade | 15 | 0 | 12 | 3 | 0 |
| WHO III grade | 9 | 0 | 0 | 4 | 5 |
| WHO IV grade | 16 | 0 | 0 | 2 | 14 |
| Total | 48 | 8 | 12 | 9 | 19 |
Effect of cortactin-siRNA on the
expression of cortactin in glioma cells
For the RT-qPCR, the expression of cortactin in
glioma cells was inhibited significantly in the siRNA-cortactin
group compared to the siRNA-N and siRNA-NC groups. The cortactin
expression of the siRNA-cortactin group in the SNB19 (21.6±3.9%),
LN229 (16.6±3.7%) and U251 (18.6±4.2%) glioma cell lines was
markedly decreased compared to the siRNA-N and siRNA-NC groups
(P<0.05) (Fig. 2B). The
expression of cortactin between the siRNA-NC and siRNA-N groups
indicated no significant differences (P>0.05).
The western blot analysis revealed that for all
three glioma cell lines, the expression of cortactin was
significantly knocked down in the siRNA-cortactin group compared to
the siRNA-N and siRNA-NC groups. Compared to β-actin, the level of
cortactin in the siRNA-cortactin group in SNB19 (15.00±1.14%),
LN229 (13.00±1.58%) and U251 (13.80±1.66%) glioma cells was
significantly decreased compared to the siRNA-N and siRNA-NC groups
(P<0.05). The expression of cortactin between the siRNA-NC and
siRNA-N groups indicated no significant differences (P>0.05)
(Fig. 2A).
Knocked down expression of cortactin
reduces motility of human glioma cells
The wound-healing assay was one of the first methods
to be developed to study cell migration in vitro. Although
not an exact duplication of cell migration in vivo, this
method mimics to some extent the migration of cells in wound
healing. To assess the inhibition of cortactin by siRNA on
migration, we performed the assay in the different treated groups
of human glioma cells. Wound closure was monitored by capturing
photomicrographs at 0 and 24 h after wound creation. The result
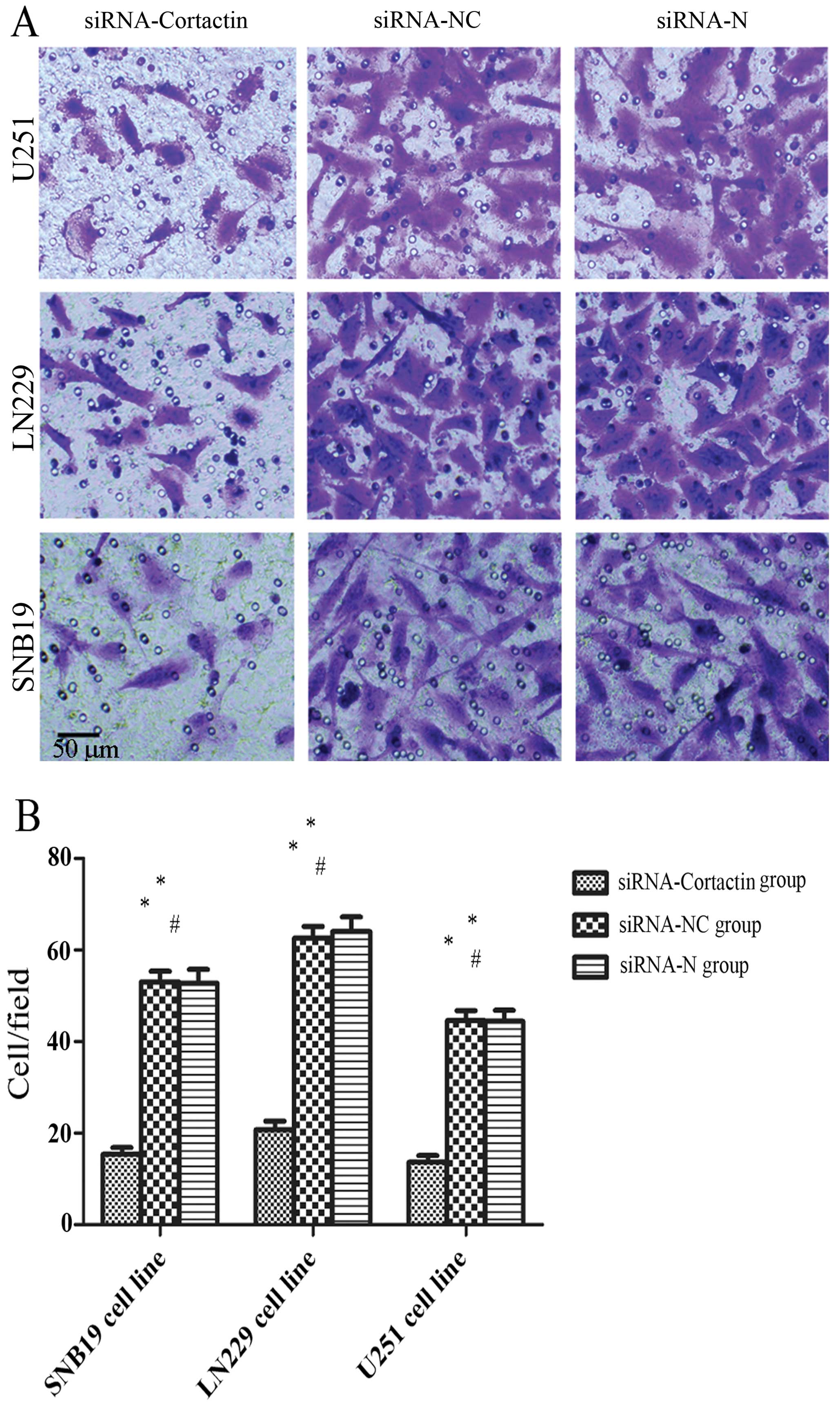
showed that the wound-healing area in the siRNA-cortactin group
(16.80±1.53 in SNB19, 19.80±1.77 in LN229 and 20.60±1.21% in U251
cells) was smaller than that in the siRNA-NC group (52.60±2.84 in
SNB19, 62.80±3.14 in LN229 and 56.00±3.21% in U251 cells) and the
siRNA-N group (52.00±3.11 in SNB19, 63.80±2.75 in LN229 and
57.20±2.76% in U251 cells) (P<0.05). These results suggested
that inhibition of cortactin by siRNA effectively reduced the
migration ability of glioma cells (Fig.
3A and B).
Part of the invasion cascade involves tumor cells
attaching to and penetrating basement membranes. Therefore,
basement membranes are critical barriers to the passage of
disseminating tumor cells. The Transwell chamber with Matrigel has
been used to assess the invasive ability of tumor cells. Since cell
migration and invasion are critical properties for the diffuse
growth of glioma, we investigated the role of inhibition of
cortactin by siRNA on tumor cell invasion using the Transwell
invasion assay. The number of cells migrating through the membrane
of the siRNA-cortactin group (15.4±1.43 in SNB19, 20.80±1.85 in
LN229 and 13.60±1.50% in U251 cells) was less than that in the
siRNA-NC group (53.00±2.39 in SNB19, 62.60±2.58 in LN229 and
44.60±2.16% in U251 cells) and the siRNA-N group (52.80±3.94 in
SNB19 cells, 64.00±3.24%) in LN229 cells, (44.40±2.44% in U251
cells) (P<0.05). The invasion of U251, LN229 and SNB19 glioma
cells across the Transwell chamber were significantly impaired by
siRNA compared to the siRNA-NC and siRNA-N groups (Fig. 4A and B) (P<0.05).
Knocked down expression of cortactin
alters the morphology of glioma cells
To assess the inhibition of cortactin by siRNA on
the lamellipodia of glioma cells, glioma cells in the
various treated groups were stained for actin with rhodamine
phalloidin, while IF was used for cortactin and contactin antibody
and DAPI, respectively, for the cell nucleus. The result showed
cortactin was localized in the F actin-enriched area. The
lamellipodia were smaller in the cells treated with siRNA
specific to cortactin compared to the other two groups (Fig. 5). This result explains that
cortactin plays a key role in the formation of lamellipodia
in glioma cells.
Knocked down expression of cortactin
reduces the persistence time of lamellipodia of glioma cells
To examine the persistence time of
lamellipodia after the expression of cortactin in glioma
cells was knocked down, glioma cells in each treated group were
placed under an inverted microscope to observe the transformation
of lamellipodia at 0, 15 and 30 min for the three cell
lines. The result showed that the size of lamellipodia was
smaller (0 min) and the persistence time was reduced (15 and 30
min) in the cells of the siRNA-cortactin group than the remaining
two groups (Fig. 6). This result
showed that cortactin maintained the persistence time of
lamellipodia in glioma cells.
Cortactin and the Arp2/3 complex are
co-localized in glioma cells
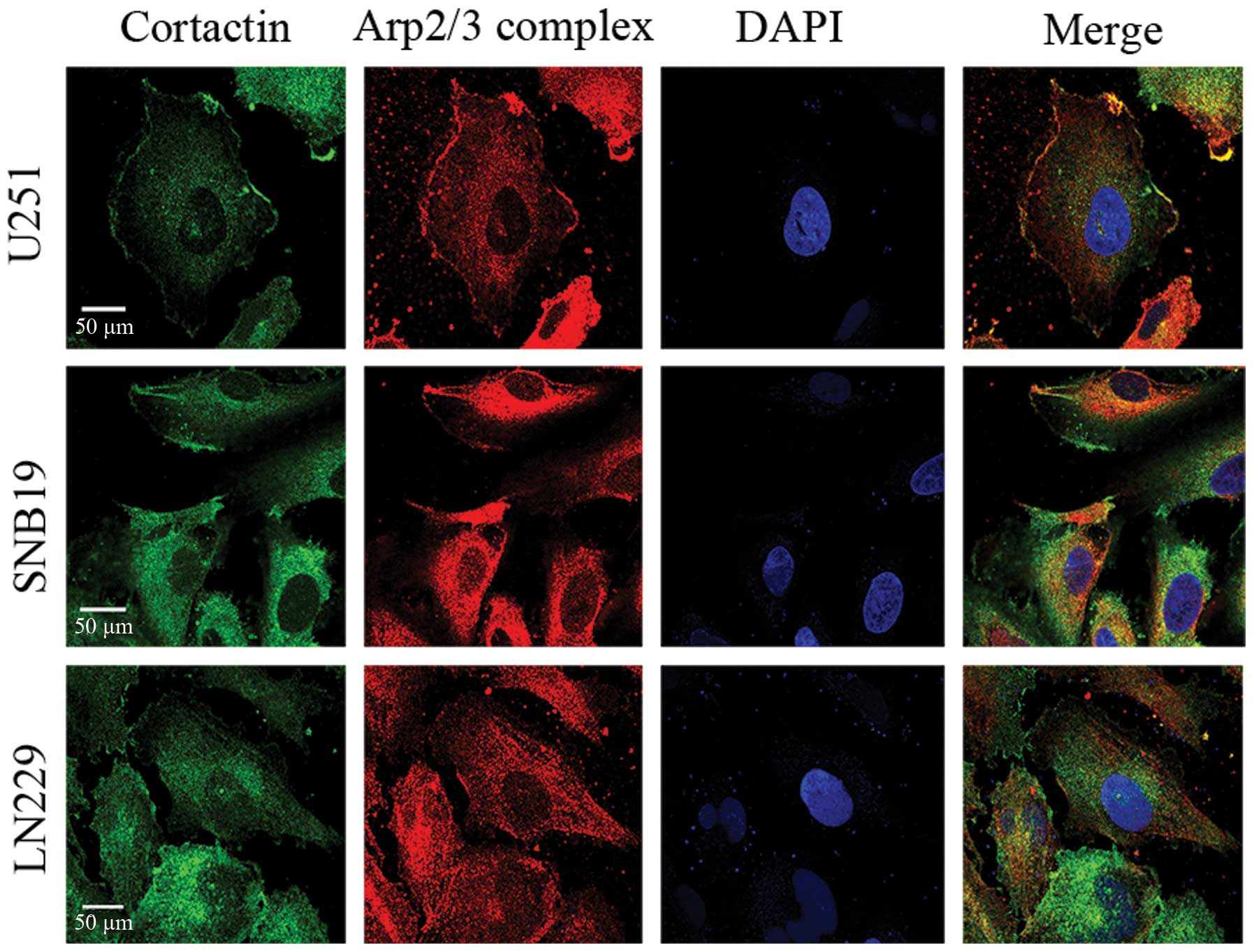
To investigate the relationship between cortactin
and the Arp2/3, IF of cortactin and the Arp2/3 was detected in
U251, LN229 and SNB19 human glioma cells. Glioma cells were stained
with cortactin antibody, p34-Arc subunit antibody specific for the
Arp2/3 complex, rhodamine phalloidin for actin filaments and DAPI
for nucleus. The result showed that cortactin co-localized with the
actin-related protein Arp2/3 complex (Fig. 8) at sites of actin polymerization
within the lamellipodia (Fig.
7).
Discussion
A hallmark of malignant gliomas is their ability to
disperse through neural tissue (24,25).
Cortactin plays a positive role in the migration and invasion of
many other tumors (17,26–29),
however, its role in gliomas remain to be determined. In the
initial phase of the present study, we explored the expression of
cortactin in different grade gliomas and non-tumor brain tissues.
We found that cortactin was expressed weakly in non-tumor brain
tissues, but strongly expressed in gliomas and the expression level
of cortactin was positively correlated with the malignancy of
gliomas. This result showed that cortactin plays a key role in
gliomas and can clarify higher-grade glioma infiltration into the
surrounding brain tissue. The result also encouraged us to
investigate the mechanism of cortactin in glioma motility. Studies
were performed in three human glioma cell lines in vitro and
we observed the effect of the reduction of cortactin in the
migration and invasion of glioma cells. Following treatment with
specific cortactin siRNA, the expression of cortactin was decreased
at the transcription and translation level in glioma cells. The
wound-healing and Transwell invasion assays, respectively, revealed
that migration and invasion was decreased markedly after glioma
cells were treated. Therefore it suggests that the possible
mechanism of the above results is the inhibition of cortactin,
which reduces its ability to regulate lamellipodia formation
in glioma cells.
Cortactin promotes cell motility by regulating the
characteristics of lamellipodia including their stability or
persistence, and actin dynamics within the lamellipodia
(16). The main migration movement
pattern of glioma cells is interstitial movement, which has four
continuous processes: tumor cells detach from the solid tumors,
tumor cell adhesion to extracellular matrix (ECM), the degradation
of ECM and tumor cells movement and contraction (4,29,30).
Lamellipodia is the organization of membrane domains and the
primary sites of actin incorporation, and plays an important role
in cell movement (6,18). To some extent, regulation of the
formation and persistence of lamellipodia, results in the
restriction of the interstitial movement of glioma cells. For this
reason, we stained glioma cells using IF and observed the variation
of lamellipodia in glioma cells after the down regulation of
cortactin. The result showed that the size and persistence time of
lamellipodia was reduced. These findings suggest a reduction
of cortactin can decrease the formation of lamellipodia and
the movement ability of glioma cells.
Cortactin and the Arp2/3 complex are closely
associated with the regulation of cell motility (8). The Arp2/3 complex is an evolutionarily
conserved actin nucleation factor localized in the
lamellipodia. The dendritic nucleation model has been
rigorously evaluated in several computational studies experimental
studies demonstrating a critical role for Arp2/3 in the generation
of protrusive actin structures and cell motility (9,31).
Previous findings have shown that the Arp2/3 complex plays a key
role in the regulation of lamellipodia in glioma cells
(13). In the present study, we
found that cortactin is pivotal in the formation and persistence of
lamellipodia, the former of which is inconsistent with
findings of previous studies (20,21).
The decrease of the ability to regulate other related proteins,
especially the Arp2/3 complex, was the main reason for the result.
On the other hand, the interaction between cortacin and actin was
also decreased following the inhibition of cortactin. In another
result of IF, it was found that cortactin, actin and the Arp2/3
complex were located in the membrane surrounding the site where
lamellipodia formed. This result suggests that, actin as the
material of lamellipodia is regulated by many molecules,
including cortactin and the Arp2/3 complex. Additionally,
consistent with other studies, cortactin and the Arp2/3 complex
play a role in actin polymerization, and the two proteins may
exhibit collaborative action in glioma cells. To confirm the
result, we used the double staining of cortactin and the Arp2/3
complex. The result showed that cortactin and the Arp2/3 complex
were co-localized in the front of glioma cells, which explains our
results.
The main reason gliomas are incurable is the wide
dissemination of these cells as opposed to the anti-glioma invasion
(34). Cilengitide, an inhibitor of
αvβ3 and αvβ5 integrin receptor did not affect the promotion of the
median survival rate in patients with malignant gliomas (33–35).
Cell movement is important to identifying treatment for various
types of cancer. Cortactin and the Arp2/3 complex as the main
molecules associated with cell movement, have been demonstrated to
play a key role in glioma cell migration and invasion (26,38).
Thus, they may serve as new targets and contribute to the
identification of appropriate anti-glioma treatment.
In summary, cortactin plays a crucial role in the
migration and invasion of glioma. Our results indicate that
cortactin promotes the motility of glioma cells by adjusting
lamellipodia and this process requires the combination of
cortactin and the Arp2/3 complex. Future studies should be
conducted to examine cortactin promoting lamellipodia
formation in vivo and the interaction with its binding
proteins such as N-WASP and cofilin (20,36).
Investigation of cortactin with regard to migration and invasion
may lead to identification of a treatment for inhibiting glioma
infiltrative growth.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81272782) and the
Research Fund for the Doctoral program of Higher Education of China
(no. 20131202110006).
References
|
1
|
Alqudah MA, Agarwal S, Al-Keilani MS,
Sibenaller ZA, Ryken TC and Assem M: NOTCH3 is a prognostic factor
that promotes glioma cell proliferation, migration and invasion via
activation of CCND1 and EGFR. PLoS One. 8:e772992013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sciumè G, Santoni A and Bernardini G:
Chemokines and glioma: Invasion and more. J Neuroimmunol. 224:8–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agudelo-Garcia PA, De Jesus JK, Williams
SP, Nowicki MO, Chiocca EA, Liyanarachchi S, Li PK, Lannutti JJ,
Johnson JK, Lawler SE, et al: Glioma cell migration on
three-dimensional nanofiber scaffolds is regulated by substrate
topography and abolished by inhibition of STAT3 signaling.
Neoplasia. 13:831–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Godlewski J, Bronisz A, Nowicki MO,
Chiocca EA and Lawler S: microRNA-451: A conditional switch
controlling glioma cell proliferation and migration. Cell Cycle.
9:2742–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Small JV, Stradal T, Vignal E and Rottner
K: The lamellipodium: Where motility begins. Trends Cell Biol.
12:112–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar
|
|
8
|
Wu C, Asokan SB, Berginski ME, Haynes EM,
Sharpless NE, Griffith JD, Gomez SM and Bear JE: Arp2/3 is critical
for lamellipodia and response to extracellular matrix cues but is
dispensable for chemotaxis. Cell. 148:973–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suraneni P, Rubinstein B, Unruh JR, Durnin
M, Hanein D and Li R: The Arp2/3 complex is required for
lamellipodia extension and directional fibroblast cell migration. J
Cell Biol. 197:239–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goley ED and Welch MD: The ARP2/3 complex:
An actin nucleator comes of age. Nat Rev Mol Cell Biol. 7:713–726.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koestler SA, Steffen A, Nemethova M,
Winterhoff M, Luo N, Holleboom JM, Krupp J, Jacob S, Vinzenz M,
Schur F, et al: Arp2/3 complex is essential for actin network
treadmilling as well as for targeting of capping protein and
cofilin. Mol Biol Cell. 24:2861–2875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwaya K, Norio K and Mukai K: Coexpression
of Arp2 and WAVE2 predicts poor outcome in invasive breast
carcinoma. Mod Pathol. 20:339–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Yang X, Chen C, Liu B, Ren B, Wang
L, Zhao K, Yu S and Ming H: Expression of the Arp2/3 complex in
human gliomas and its role in the migration and invasion of glioma
cells. Oncol Rep. 30:2127–2136. 2013.PubMed/NCBI
|
|
14
|
Croucher DR, Rickwood D, Tactacan CM,
Musgrove EA and Daly RJ: Cortactin modulates RhoA activation and
expression of Cip/Kip cyclin-dependent kinase inhibitors to promote
cell cycle progression in 11q13-amplified head and neck squamous
cell carcinoma cells. Mol Cell Biol. 30:5057–5070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Macgrath SM and Koleske AJ: Cortactin in
cell migration and cancer at a glance. J Cell Sci. 125:1621–1626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weaver AM, Karginov AV, Kinley AW, Weed
SA, Li Y, Parsons JT and Cooper JA: Cortactin promotes and
stabilizes Arp2/3-induced actin filament network formation. Curr
Biol. 11:370–374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weed SA, Karginov AV, Schafer DA, Weaver
AM, Kinley AW, Cooper JA and Parsons JT: Cortactin localization to
sites of actin assembly in lamellipodiarequires interactions with
F-actin and the Arp2/3 complex. J Cell Biol. 29–40. 2000.
View Article : Google Scholar
|
|
18
|
Bryce NS, Clark ES, Leysath JL, Currie JD,
Webb DJ and Weaver AM: Cortactin promotes cell motility by
enhancing lamellipodial persistence. Curr Biol. 15:1276–1285. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dedes KJ, Lopez-Garcia MA, Geyer FC,
Lambros MB, Savage K, Vatcheva R, Wilkerson P, Wetterskog D,
Lacroix-Triki M, Natrajan R, et al: Cortactin gene amplification
and expression in breast cancer: A chromogenic in situ
hybridisation and immunohistochemical study. Breast Cancer Res
Treat. 124:653–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desmarais V, Yamaguchi H, Oser M, Soon L,
Mouneimne G, Sarmiento C, Eddy R and Condeelis J: N-WASP and
cortactin are involved in invadopodium-dependent chemotaxis to EGF
in breast tumor cells. Cell Motil Cytoskeleton. 66:303–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hofman P, Butori C, Havet K, Hofman V,
Selva E, Guevara N, Santini J and Van Obberghen-Schilling E:
Prognostic significance of cortactin levels in head and neck
squamous cell carcinoma: Comparison with epidermal growth factor
receptor status. Br J Cancer. 98:956–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada S, Yanamoto S, Kawasaki G, Mizuno A
and Nemoto TK: Overexpression of cortactin increases invasion
potential in oral squamous cell carcinoma. Pathol Oncol Res.
16:523–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perrin BJ, Amann KJ and Huttenlocher A:
Proteolysis of cortactin by calpain regulates membrane protrusion
during cell migration. Mol Biol Cell. 17:239–250. 2006. View Article : Google Scholar :
|
|
24
|
Giese A, Bjerkvig R, Berens Me and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westermark B: Glioblastoma - a moving
target. Ups J Med Sci. 117:251–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weaver AM: Cortactin in tumor
invasiveness. Cancer Lett. 265:157–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tehrani S, Faccio R, Chandrasekar I, Ross
FP and Cooper JA: Cortactin has an essential and specific role in
osteoclast actin assembly. Mol Biol Cell. 17:2882–2895. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou H, Chen W, Zhao L, Zuo Q, Zhang G,
Zhang X, Wang H, Gong H, Li X, Wang M, et al: Cortactin is
associated with tumour progression and poor prognosis in prostate
cancer and SIRT2 other than HADC6 may work as facilitator in situ.
J Clin Pathol. 65:1088–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jovčevska I, Kočevar N and Komel R: Glioma
and glioblastoma - how much do we (not) know? Mol Clin oncol.
1:935–941. 2013.
|
|
30
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pfaendtner J, Volkmann N, Hanein D,
Dalhaimer P, Pollard TD and Voth GA: Key structural features of the
actin filament Arp2/3 complex branch junction revealed by molecular
simulation. J Mol Biol. 416:148–161. 2012. View Article : Google Scholar :
|
|
32
|
Chang L, Su J, Jia X and Ren H: Treating
malignant glioma in Chinese patients: Update on temozolomide. Onco
Targets Ther. 7:235–244. 2014.PubMed/NCBI
|
|
33
|
Reardon DA, Fink KL, Mikkelsen T,
Cloughesy TF, O'Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ,
Rich KM, et al: Randomized phase II study of cilengitide, an
integrin-targeting arginine-glycine-aspartic acid peptide, in
recurrent glioblastoma multiforme. J Clin Oncol. 26:5610–5617.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eisele G, Wick A, Eisele A-C, Clément PM,
Tonn J, Tabatabai G, Ochsenbein A, Schlegel U, Neyns B, Krex D, et
al: Cilengitide treatment of newly diagnosed glioblastoma patients
does not alter patterns of progression. J Neurooncol. 117:141–145.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng NC, van Zandwijk N and Reid G:
Cilengitide inhibits attachment and invasion of malignant pleural
mesothelioma cells through antagonism of integrins αvβ3 and αvβ5.
PLoS One. 9:e903742014. View Article : Google Scholar
|
|
36
|
Yu X, Zech T, McDonald L, Gonzalez EG, Li
A, Macpherson I, Schwarz JP, Spence H, Futó K, Timpson P, et al:
N-WASP coordinates the delivery and F-actin-mediated capture of
MT1-MMP at invasive pseudopods. J Cell Biol. 199:527–544. 2012.
View Article : Google Scholar : PubMed/NCBI
|