Introduction
Partial or radical nephrectomy for primary renal
cell carcinoma (RCC) achieves excellent rates of cure (1), yet the procedure is invasive and often
results in loss of normal renal parenchyma leading to the
development of renal insufficiency with its associated long-term
morbidity and even mortality (1).
Radiation treatment of primary RCC is rarely employed for curative
intent as RCC is generally believed to be radiation-resistant. Of
note, in clinical practice, particular care is taken to keep kidney
radiation doses within acceptable tolerance limits as normal renal
parenchyma is considered relatively sensitive to radiation
(2). One possible explanation for
this conundrum may be the differential expression of proteins, such
as carbonic anhydrase IX (CA9), involved in radiation resistance in
RCC cells compared to normal renal cells. CA9 is not expressed in
normal kidney cells (3), yet its
expression is ubiquitous in clear cell RCC (ccRCC), most likely due
to the fact that expression of CA9 is transcriptionally regulated
by hypoxia-inducible factor-1α (4),
which accumulates in ccRCC cells as a result of frequent
inactivating mutations in the von Hippel-Lindau tumor-suppressor
gene (5).
The family of carbonic anhydrase enzymes catalyzes
the dissolution of CO2 in water as carbonic acid and
protons (6). CA9 contributes to the
acidification of the local tumor environment and guards tumor cells
against acidosis. We hypothesized that upregulation of CA9 in RCC
cells may account, at least in part, for the radiation resistance
of RCC, and thus targeting CA9 expression or enzymatic activity may
sensitize RCC to ionizing radiation.
Materials and methods
Cell culture and transfection
Mycoplasma-free human ccRCC 786-O and human prostate
adenocarcinoma LNCaP cells (ATCC, Manassas, VA, USA) were
propagated in RPMI-1640 medium supplemented with 10% FBS
(Invitrogen, Burlington ON, Canada). The 786-O cell identity was
verified by STR analysis (ATCC). Murine RCC RAG cells and human
glioblastoma LN-18 cells (ATCC) were maintained in Eagle's MEM and
Dulbecco's MEM, respectively, supplemented with 10% FBS.
The shRNA vector for human CA9 (CA9 shRNA) and the
non-effective negative scrambled control were purchased from
Origene (Rockville, MD, USA). Transfection of human ccRCC 786-O
cells was performed using 12% Fugene (Promega, Madison, WI, USA).
Cells stably transfected with shCA9 or the scrambled control were
selected with 1.0 µg/ml puromycin (Sigma-Aldrich, Oakville,
ON, Canada).
Clonogenic survival experiments
RAG, 786-O, and LN-18 cells (250 per well) were
seeded onto 6-well plates in 3 ml of medium and allowed to adhere
by incubation at 37°C and 5% CO2 for 4 h. For LNCaP,
1000 cells were seeded per well and allowed to adhere for 24 h.
For the AEBS treatment, a stock solution of
4-(2-aminoethyl)benzene sulfonamide (AEBS, 33 mM, Sigma-Aldrich)
was prepared fresh with H2O and filter-sterilized using
a 0.2 µm syringe filter. AEBS at concentrations ranging from
3.3 µM to 3300 µM was added to 100 µl of
H2O to 3 ml media. Solvent controls were also included.
The medium was aspirated and replaced with 3 ml of the appropriate
drug solutions in media in duplicate wells. After 24 h of
incubation, the media were aspirated, and 3 ml of fresh medium was
added to each well.
Ionizing radiation (IR) of cells was performed using
a Varian Linear Accelerator (LINAC) generating six MV X-rays
(Varian Medical Systems, Inc., Palo Alto, CA, USA). Cells plated in
duplicate 6-well plates were irradiated at a distance of 100 cm in
a 16 cm by 20 cm field. A 19-mm-thick acrylamide sheet was placed
on the plates as a build-up region. Thermoluminescent dosimeters
were used to measure and calibrate the dose. The cells received
from 1 to 8 Gy of 6 MV X-ray radiation.
Following treatment, the cells were incubated for an
additional 6 days (786-O), 7 days (RAG), or 12 days (LNCaP and
LN-18), after which the medium was aspirated and the colonies were
stained with crystal violet (0.25% in 95% ethanol) for 10 min.
Colonies of 50 cells or more were counted. Survival was expressed
as a percentage of the corresponding untreated controls, and
IC50 values were calculated using CalcuSyn software
version 1.2 (Biosoft, Cambridge, UK). Experiments were repeated
three times.
CA9 activity assay
Confluent RCC cells in 6-well plates were washed
twice with 3 ml of PBS. One ml of 0.9% saline (adjusted to pH 8.0
with NaOH) containing 0.15 mg/ml phenol red was added to each well.
Deionized water (100 µl) with or without AEBS at various
concentrations was added to the wells. Starting 5 sec after the
addition of saline to the cells, the absorbance at 565 nm was
measured using a Powerwave HT spectrophotometer (BioTek, Winooski,
VT, USA) at 1 sec intervals for 20 sec. The relative absorbance of
a particular well throughout the 20 sec was determined as a percent
of the no cell control average at that time interval (A/A no
cells).
Western blot analysis
Cultured cells were lysed using RIPA lysis buffer.
Lysate (40 µg) was resolved on a 10% SDS-PAGE gel and
transferred onto nitrocellulose membrane. Primary antibodies used
were CA9 (1:1,000 Epitomics, Burlingame, CA, USA) and β-actin
(1:2,000, Sigma-Aldrich). Secondary HRP-conjugated antibody (1:200,
Dako, Carpinteria, CA, USA) was used in conjunction with
chemiluminescence detection.
Animal studies
All protocols for animal studies were reviewed and
approved by the institutional Animal Research Ethics Board (AUP#
12-09-37). Per group, 7–10 female inbred nude (Balb/c nu/nu) mice
(Charles River, St. Constant, QC, Canada) 5 weeks of age were used.
786-O parental, shCA9 or scrambled control 786-O cells
[1–3×106 in 50% (v/v) Matrigel] were injected
subcutaneously into the right flank of each mouse. Tumor size was
measured every three days using Vernier calipers, and the tumor
volume was determined using the formula π/6(length x width x
height) until the largest tumor reached 400 mm3. The
mice were sacrificed 7–12 weeks after tumor cell injection, when
the tumors were dissected, weighed, fixed in formalin and embedded
in paraffin.
For mice in the IR group, 21–27 days post injection,
when tumors were palpable, the animals were anaesthetized using
isoflurane and positioned in sterile, acrylamide cylinders
connected to a portable anaesthetic machine. Cylinders were
transported to the treatment area, where they were positioned at a
distance of 100 cm to the source and irradiated with 6 Gy in a 2 cm
by 2 cm field using a Varian Linear Accelerator generating 6 MV
X-rays. A 5-mm-thick sheet of superflab bolus material served as a
build-up region. Animals in non-irradiated control groups were
anaesthetized for a similar time period.
Animals in the AEBS-treatment groups received 50 or
200 µg/ml AEBS in the drinking water supplied fresh every
two days starting two days before IR. No adverse effects of the
treatment were observed.
The serum levels of VEGF were determined using a
mouse VEGF ELISA kit (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer's instructions on a BMG Labtech
SpectroStar Nano multi-well plate reader. Immunostaining of 4
µm-thick tumor xenograft sections for CD31 and subsequent
image analysis was performed as previously described (7) resulting in the microvessel density
expressed as endothelial length (in µm) per
mm2.
AEBS mass spectrometry
Trifluoroacetic acid (2 µl) and methanol (500
µl) were added to 100 µl mouse serum, vortexed for 10
sec and centrifuged at 5000 rpm for 10 min. Resulting supernatant
(300 µl) was removed and blown down to dryness with
nitrogen. The sample was reconstituted in 200 µl of
methanol/water (1:1) containing 25 µg/ml phenylalanine
(internal standard) and filtered using a 13-mm syringe filter (0.2
µm GHP membrane). The sample (5 µl) was run on the
LC-MS (Agilent 6340 Ion Trap coupled to an Agilent 1200 HPLC,
Agilent Technologies Inc, Mississauga, ON, Canada) at the McMaster
regional centre for mass spectrometry. Analysis was performed using
multiple reaction monitoring on AEBS and phenylalanine with the
transition at 201-184 (m/z) and 166-120 (m/z), respectively.
Control serum was spiked with AEBS at 1–16 µg/ml.
Statistical analysis
Values are expressed as the mean ± the standard
error of the mean. Where appropriate, results are presented with
95% confidence intervals (CI). Dependent on whether the data were
normally distributed or not, parametric (Student's t-test) or
nonparametric methods (Mann-Whitney U-test) were used with a
p-value <0.05 indicative of statistical significance.
Results
CA9 is present in the radiation-resistant
786-O and RAG RCC cells
Protein expression of CA9 was determined in the
lysates of human prostate adenocarcinoma LNCaP, human ccRCC 786-O,
murine RCC RAG and immortalized human embryonic kidney HEK-293
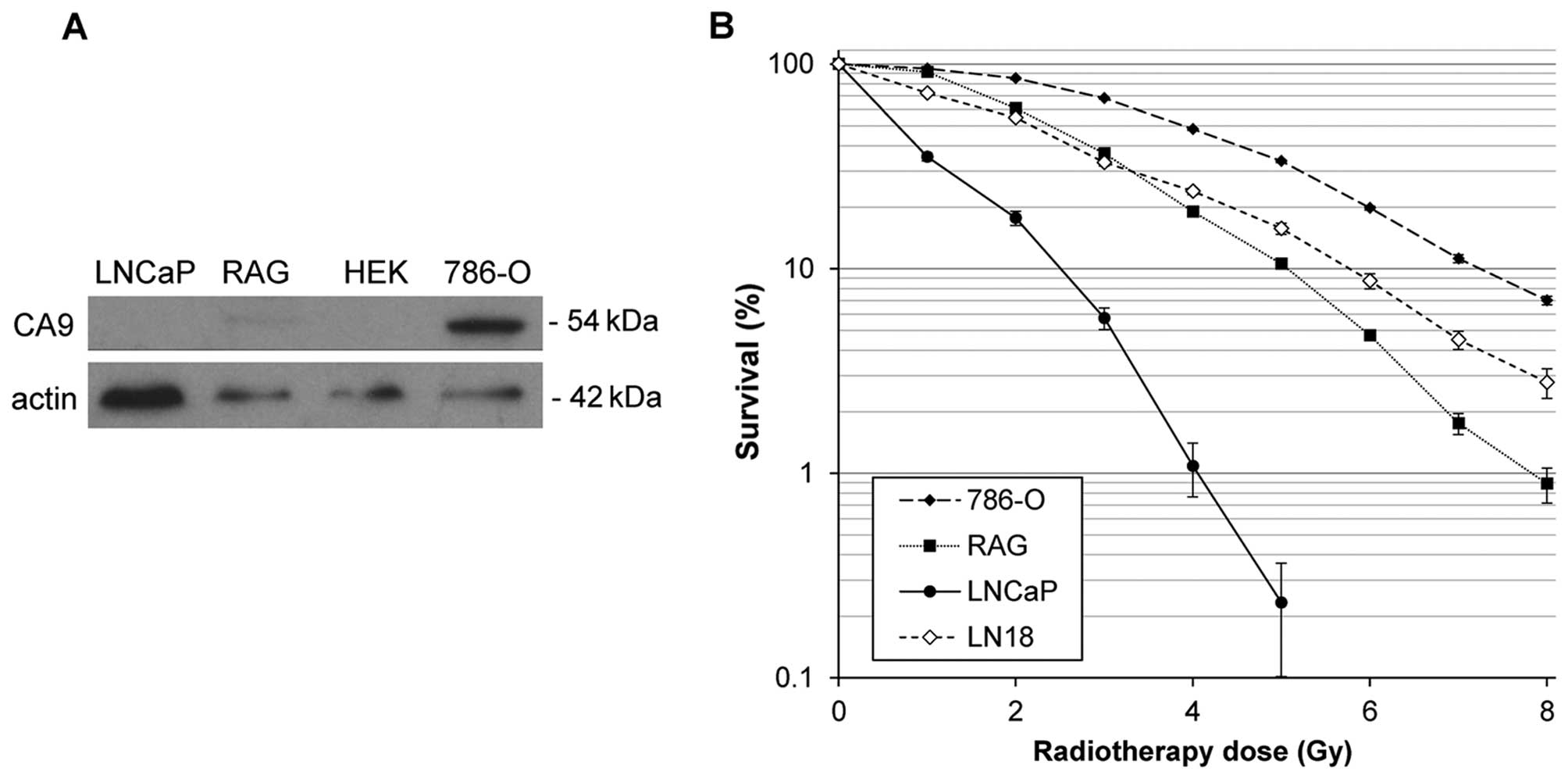
cells by western blot analysis (Fig.
1A). CA9 was detectable in the 786-O and RAG cells, but not in
the LNCaP or HEK-293 cells. Clonogenic survival experiments
demonstrated that RAG cells were more sensitive to IR than 786-O
cells (p<0.05), whereas LNCaP cells were the most sensitive
among the cell lines tested (Fig.
1B). Human glioblastoma LN-18 cells exhibited similar tolerance
to IR as the RAG cells. The 786-O cells displayed significantly
decreased survival at a dose of 2 Gy and above (p<0.001),
whereas LNCaP cells displayed significantly decreased survival at
all radiation doses (p<0.001). The calculated IC50
values for each cell line are presented in Table I.
 | Table ISensitivity of the different cell
lines to ionizing radiation in vitro as determined by the
IC50 value measured by clonogenic survival. |
Table I
Sensitivity of the different cell
lines to ionizing radiation in vitro as determined by the
IC50 value measured by clonogenic survival.
| Cell line | IC50
(Gy) | 95% confidence
interval (Gy) |
|---|
| Human ccRCC
786-O | 3.52 | 3.22–3.85 |
| Murine RCC RAG | 2.29 | 2.05–2.56 |
| Human prostate
adenocarcinoma LNCaP | 1.01 | 0.65–1.57 |
| Human glioblastoma
LN18 | 1.94 | 1.61–2.34 |
Knockdown of CA9 expression by shRNA in
786-O cells leads to radiosensitivity
To investigate the significance of CA9 expression on
RCC radiosensitivity, human ccRCC 786-O subclones stably expressing
shRNA specific for CA9 were generated. These shCA9 cells showed 92%
knockdown of CA9 protein expression compared to the respective
control cells (scrambled shRNA) (Fig.
2A). Clonogenic survival of the 786-O scrambled control and
shCA9 cells was determined after IR with increasing doses of 1–8 Gy
and clearly demonstrated that knockdown of CA9 confers sensitivity
to IR (p<0.001). The IC50 value decreased by >50%,
from 3.64 Gy (95% CI: 3.27–4.05) in the scrambled control cells to
1.81 Gy (95% CI: 1.44–2.27) in the shCA9 cells (Fig. 2B). To further demonstrate the effect
of CA9 knockdown in vitro, the activity of the CA9 enzyme
was measured using phenol red as an indicator. In comparison to the
scrambled control cells, shCA9 cells had significantly decreased
acidification capacity (p<0.001) and experienced 51% of the
absorbance change observed in the scrambled control cells (Fig. 2C).
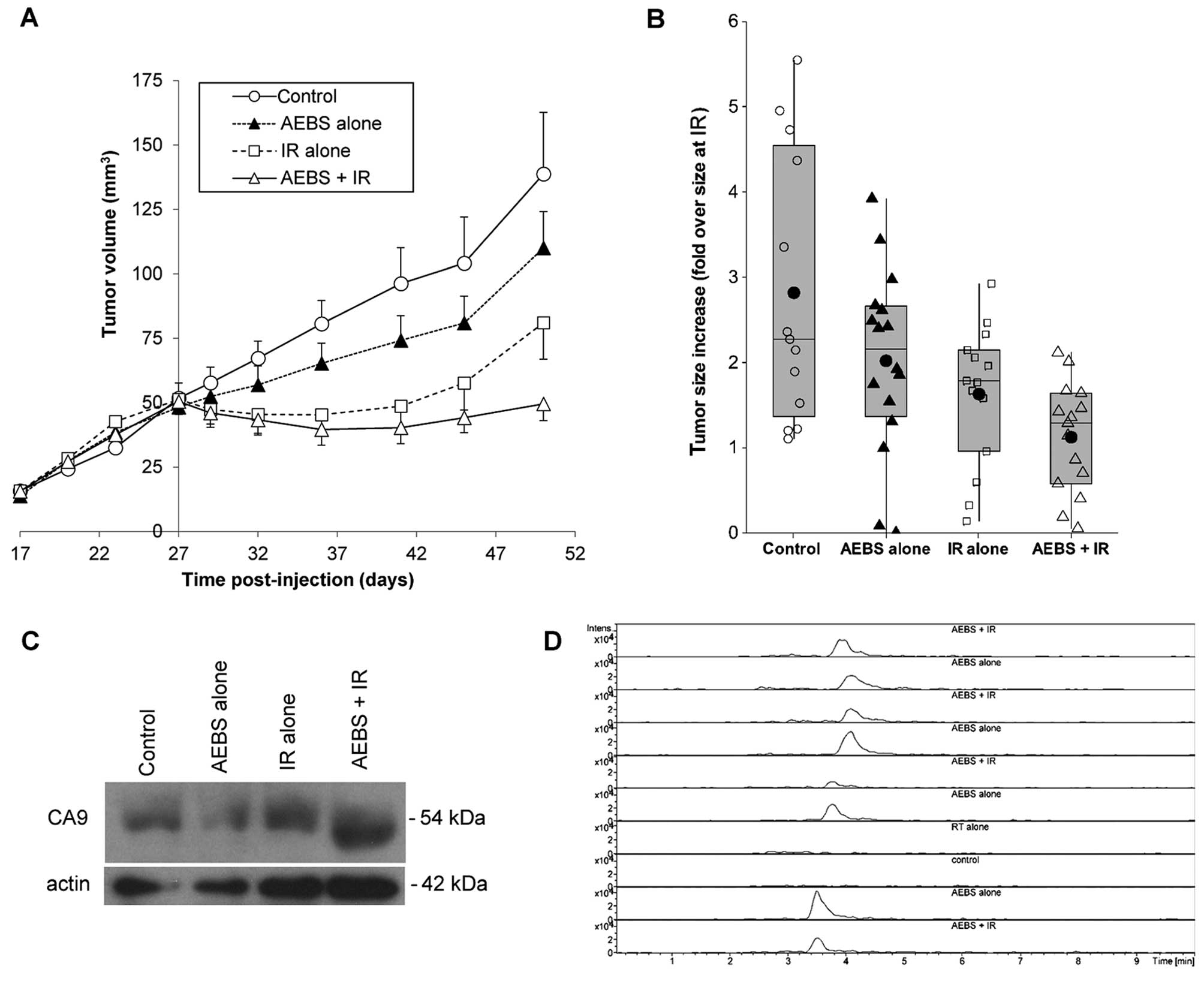
The effect of CA9 knockdown on the in vivo
growth after subcutaneous injection of shCA9 or scrambled control
cells (1×106) into nude mice (n=7/group) was determined.
A tumor take rate of 64% was achieved. The tumor volume from mice
injected with the shCA9 cells was not significantly different from
mice injected with cells transfected with the scrambled control
shRNA (Fig. 3A and B). However,
when the subcutaneous tumors were also irradiated 21 days after
cell injection, we observed that IR of the shCA9-transfected 786-O
cells led to decreased tumor growth (p<0.001) and a 78.7%
decrease in tumor volume at sacrifice (Fig. 3B). IR of the scrambled
control-injected mice led to a 20.7% reduction in tumor volume
after 12 weeks (Fig. 3A). Western
blot analysis of the tumor homogenates showed that CA9 remained
reduced in the shCA9-injected mice (53%) at sacrifice, 12 weeks
after tumor cell injection (Fig.
3C).
AEBS inhibits CA9 activity in vitro and
leads to radio-sensitivity
AEBS is a known inhibitor of CA9's enzymatic
reaction with a Ki of 33 nM (8).
However, to the best of our knowledge, AEBS has not been used
previously to inhibit CA9 in vivo, in contrast to
acetazolamide, a similar sulfonamide. In the presence of 33
µM AEBS, the human ccRCC 786-O cells exhibited a significant
decrease in clonogenic survival after IR when compared to the
untreated control (Fig. 4A,
p<0.001). Similarly, the more radiation-sensitive murine RAG
cells also exhibited a decrease in clonogenic survival after IR
when treated with the same concentration of AEBS for 24 h (Fig. 4B, p=0.018). To further demonstrate
the effectiveness of AEBS in vitro, the acidification of the
extracellular environment of 786-O cells was determined (Fig. 4C). In comparison to the untreated
control 786-O cells, incubation with either 33 µM or 33 nM
AEBS caused significantly less acidification (p<0.001), leading
to a decrease of 70 and 36%, respectively. AEBS was not cytotoxic;
by clonogenic survival the IC50 values for AEBS amounted
to 1,360 and >5,000 µM in the 786-O and RAG cells,
respectively (data not shown).
Radiosensitization is more pronounced
when the radiation is hypofractionated
To investigate whether a hypofractionated regimen of
radiation delivery could increase the survival difference between
CA9-inhibited and non-inhibited RCC cells, 786-O cells expressing
scrambled control shRNA or shCA9 or with and without the addition
of AEBS to the medium were subjected to either one treatment of 6
Gy or three treatments of 2 Gy for three consecutive days. Cells
receiving AEBS or expressing shCA9 had significantly reduced
survival (p<0.001) after receiving a single dose treatment of 6
Gy compared to the fractionated radiation treatment of three doses
of 2 Gy (Fig. 5).
AEBS administration increases
radiosensitivity in vivo
We determined the effect of CA9 inhibition on the
in vivo growth after subcutaneous injection of 786-O cells
(3×106) into nude mice (n=20/group). A tumor take rate
of 85% was achieved. Mice were treated with either AEBS starting 25
days after cell injections or received IR on day 27 and were
sacrificed 24 days later. One untreated control group was included
and one group of mice received both IR and AEBS. The tumor volume
in mice treated with AEBS was significantly smaller than that in
the control mice (Fig. 6A; p=0.03
ANOVA). When the subcutaneous tumors were also irradiated 27 days
after cell injection, we observed that the combination treatment
led to decreased tumor growth compared to either AEBS or IR alone
(p<0.0005 and p=0.04, respectively). At sacrifice, this led to
an average increase of a mere 12% in tumor volume from the time of
IR on day 27 to sacrifice on day 51 in the combination group
(Fig. 6B). In comparison, the tumor
volumes of the untreated control mice had increased in size by
almost 3-fold. Protein levels of CA9 in the mouse tumors did not
differ between treatment groups (Fig.
6C). Mass spectrometry showed that measurable and
CA9-inhibitory levels of AEBS were achieved in mouse serum
(Fig. 6D). Levels reached 26.5
µM 18 h after starting the treatment and remained similar
throughout the experiment (data not shown).
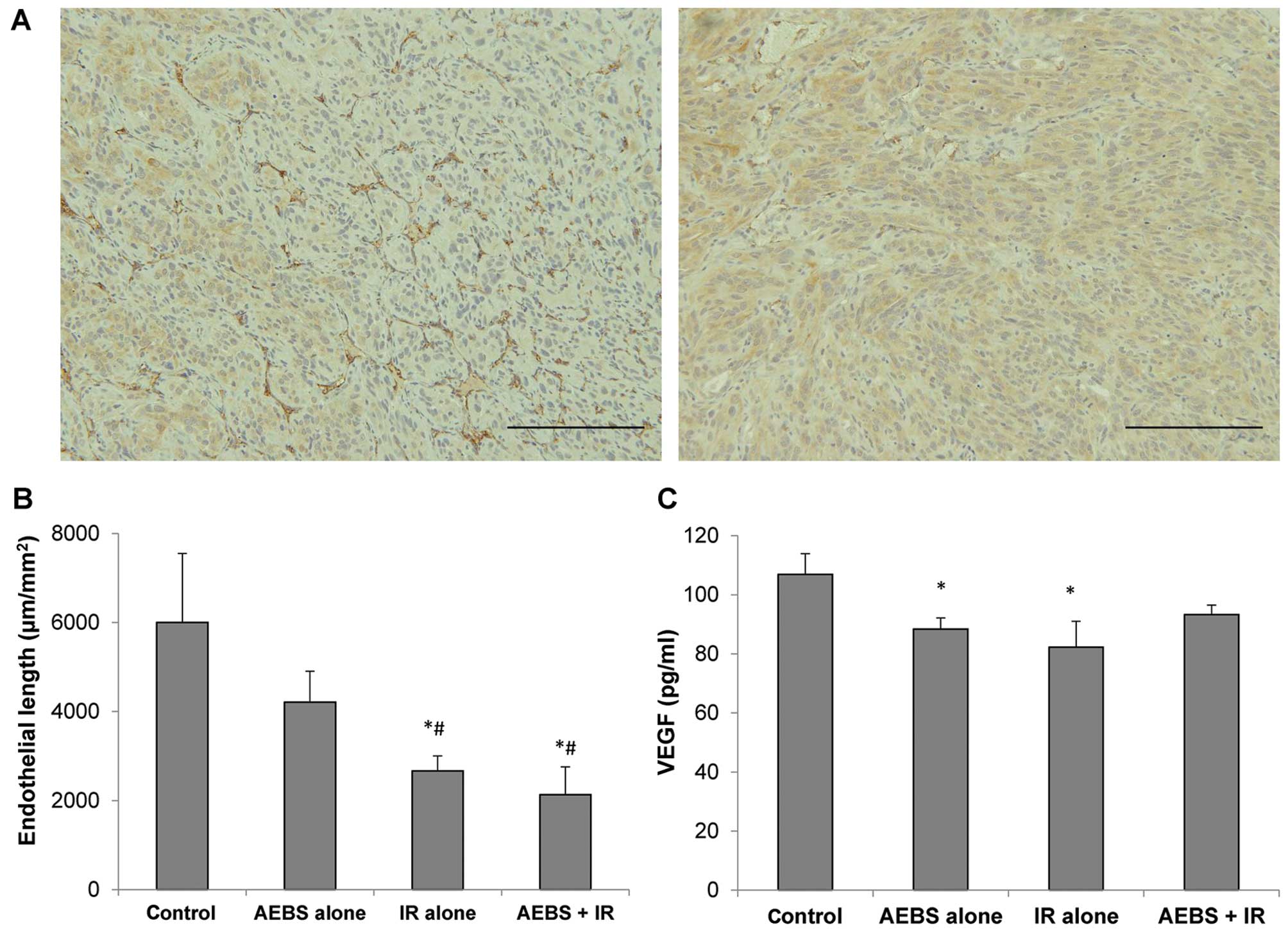
Using immunohistochemical staining of the
subcutaneous tumors for CD31, an endothelial marker, we also
demonstrated a decrease in the microvessel density in the
irradiation-treated mice, with or without AEBS, with a linear
microvessel length decrease of 55.6 and 64.5% compared to the
control (Fig. 7A and B, p=0.04 and
p=0.03). Serum VEGF levels at endpoint were significantly decreased
in the AEBS-treated and irradiated mice compared to the control
mice (Fig. 7C).
Discussion
This study presents proof-of-concept for CA9 being a
target for inhibition to increase the sensitivity of RCC cells to
radiation. We elected CA9 as a target as it regulates intracellular
pH which has been suggested to play a key protective role in
irradiated cells (9). Moreover, CA9
is highly expressed on the majority of ccRCC cells, but absent in
normal kidney (3). In the present
study, we employed two different methods to show that CA9 confers
radiation resistance in RCC; we used knockdown of CA9 expression
via transfection with specific shRNA and we inhibited the enzymatic
activity of CA9 using AEBS, a sulfonamide that has previously been
shown to efficiently inhibit CA9 by competition for the active site
(8). We demonstrated, for the first
time, that by adding AEBS to the drinking water (50–200
µg/ml), we achieved serum concentrations that were several
magnitudes (26.5 µM) higher than its Ki (Fig. 6D). While AEBS is relatively specific
for CA9, it can also efficiently inhibit carbonic anhydrase XII
(CA12) with a 10-fold lower Ki (8).
Employing an agent that efficiently blocks the enzymatic activity
of both CA9 and CA12 could be of therapeutic advantage, as CA12
functions similarly to CA9, and is also significantly expressed in
RCC (10). Similar sulfonamides
have been used as anti-bacterial agents before the discovery of
antibiotics and are currently used as diuretics and anti-glaucoma
agents due to their ability to mediate water transport and pressure
in various tissues. Sulfonamides are well tolerated and are usually
associated with few side effects other than potential allergic
reactions (11).
The treatment of primary RCC is currently limited to
surgical removal of the tumor via partial or radical nephrectomy or
thermal ablation. Radiation therapy has typically been dismissed as
a curative therapeutic option since RCC is generally regarded as a
radiation-resistant tumor, even though normal kidney is considered
radiation-sensitive. For this reason, nephropathy is a complication
observed in gastrointestinal and retroperitoneal non-Hodgkin's
lymphoma patients receiving abdominal radiotherapy as their primary
treatment (12). Moreover, the
historical landmark trial by the Copenhagen Renal Cell Cancer Study
group which randomized RCC patients at high risk for recurrence
post-nephrectomy to receive 50 Gy vs. no radiation found no
survival benefit and closed prematurely due to a toxicity-related
mortality rate of 20% (13). With
the utilization of more accurate CT-based image-guided delivery of
radiation, a retrospective study demonstrated more acceptable
complication rates (14).
Nevertheless, radiation treatment of RCC is currently limited to
the treatment of oligometastases or inoperable disease (15).
Comparing two CA9-positive RCC cell lines (human
786-0 and murine RAG) with tumor cell lines that are considered
radiation-sensitive (LNCaP) (16)
and radiation-resistant (LN-18) (17), we confirmed by clonogenic survival
that these RCC cells are indeed radiation resistant (Fig. 1B). Both 786-O and RAG RCC cells were
significantly more resistant to radiation than glioblastoma LN-18
cells with IC50 values of 3.52, 2.29 and 1.94 Gy,
respectively.
We performed both in vitro and in vivo
investigations to test the potential radiation sensitization
effects of CA9 inhibition at the level of RCC cells in vitro
and using human RCC tumor xenografts where the effect on tumor
vascularization can be evaluated. Our study found, for the first
time, that in ccRCC both the pharmacological inhibition of CA9
activity and knockdown of the expression of CA9 sensitized RCC
cells to radiation in vitro (Figs. 2B, 4A
and B) and in vivo (Figs.
3B, 6A and B). Inhibition of
CA9 activity by treatment of mice with AEBS in combination with IR
had an inhibitory effect on the growth rate of the RCC xenografts
and had a significantly larger effect compared with radiation alone
(p=0.04, Fig. 6A and B). Similarly,
using a xenograft model of human colorectal adenocarcinoma cells,
Mclntyre et al demonstrated that knockdown of CA9 reduced
the growth rate of xenografts (18). Doyen et al showed that
silencing of CA9 significantly increased radiation-induced cell
death in another human colorectal adenocarcinoma cell line, while
ectopic expression of CA9 in fibroblasts lacking CA9 expression
improved survival following radiation in an acidic environment
(9). Treatment of mice bearing
human colon carcinoma xenografts with acetazolamide, another
sulfonamide similar to AEBS, which has been demonstrated to inhibit
CA9 in 786-O cells via induced apoptosis (19), also led to sensitization to
radiation (20). This lends
credibility to the concept that CA9 is associated with radiation
tolerance by limiting a cell's ability to avoid apoptosis after
irradiation.
Cells treated with AEBS or expressing shCA9
exhibited significantly reduced survival (p<0.001) after
receiving a single dose treatment of 6 Gy compared to the
fractionated radiation treatment of three doses of 2 Gy. This
reduction in survival underscores the importance of CA9 in the
radiation resistance of 786-O cells and is important in the context
of RCC. Whereas conventional 1.8–3.0 Gy fractions do not cause
sufficient endothelial apoptosis, high-dose radiotherapy
efficiently induces endothelial apoptosis via increased ceramide
production and is expected to be detrimental in RCC, which is
typically highly vascularized (21). While clinical evidence for the
efficacy of stereotactic body radiotherapy of primary RCC is
currently sparse (15), a renewed
interest in this treatment option using novel CT-based image-guided
radiation delivery for primary RCC has recently been expressed
which awaits confirmation in the setting of prospective randomized
trials (22). In our mice, IR
delivered as one radiotherapy dose of 6 Gy significantly reduced
the microvessel density within the tumors, alone and in combination
with AEBS (p=0.03 and p=0.04, respectively), whereas the mouse
serum VEGF levels were decreased after IR and after inhibition of
CA9 by AEBS compared with the control untreated animals (p=0.04,
Fig. 7).
In conclusion, our study presents experimental
proof-of-concept for the potential role of CA9 inhibition as a
means to sensitize RCC to radiation. Specifically, it offers the
concept of pharmacological inhibition of CA9 by sulfonamides, such
as AEBS and acetazolamide, which have already been in clinical use
for decades (11). While further
mechanistic studies are underway, our data warrant consideration to
perform phase I clinical trials.
Acknowledgments
This research was financially supported by McMaster
Surgical Associates (W.C.M.D. and J.H.P.). We are grateful for the
assistance by Dr Kirk Green and Sujan Fernando of the McMaster
Regional Centre for Mass Spectrometry.
Abbreviations:
|
AEBS
|
4-(2-aminoethyl)benzene
sulfonamide
|
|
CA9
|
carbonic anhydrase IX
|
|
ccRCC
|
clear cell renal cell carcinoma
|
|
IR
|
ionizing radiation
|
|
RCC
|
renal cell carcinoma
|
References
|
1
|
Buchou T, Vernet M, Blond O, Jensen HH,
Pointu H, Olsen BB, Cochet C, Issinger OG and Boldyreff B:
Disruption of the regulatory beta subunit of protein kinase CK2 in
mice leads to a cell-autonomous defect and early embryonic
lethality. Mol Cell Biol. 23:908–915. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mountford PJ and Temperton DH:
Recommendations of the International Commission on Radiological
Protection (ICRP) 1990. Eur J Nucl Med. 19:77–79. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nyhan MJ, El Mashad SM, O'Donovan TR,
Ahmad S, Collins C, Sweeney P, Rogers E, O'Sullivan GC and McKenna
SL: VHL genetic alteration in CCRCC does not determine
de-regulation of HIF, CAIX, hnRNP A2/B1 and osteopontin. Cell Oncol
(Dordr). 34:225–234. 2011. View Article : Google Scholar
|
|
4
|
Grabmaier K, A de Weijert MC, Verhaegh GW,
Schalken JA and Oosterwijk E: Strict regulation of CAIX(G250/MN) by
HIF-1alpha in clear cell renal cell carcinoma. Oncogene.
23:5624–5631. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rini BI and Small EJ: Biology and clinical
development of vascular endothelial growth factor-targeted therapy
in renal cell carcinoma. J Clin Oncol. 23:1028–1043. 2005.
View Article : Google Scholar
|
|
6
|
Swietach P, Hulikova A, Vaughan-Jones RD
and Harris AL: New insights into the physiological role of carbonic
anhydrase IX in tumour pH regulation. Oncogene. 29:6509–6521. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kleinmann N, Duivenvoorden WC, Hopmans SN,
Beatty LK, Qiao S, Gallino D, Lhotak S, Daya D, Paschos A, Austin
RC, et al: Underactivation of the adiponectin-adiponectin receptor
1 axis in clear cell renal cell carcinoma: Implications for
progression. Clin Exp Metastasis. 31:169–183. 2014. View Article : Google Scholar
|
|
8
|
Akurathi V, Dubois L, Lieuwes NG, Chitneni
SK, Cleynhens BJ, Vullo D, Supuran CT, Verbruggen AM, Lambin P and
Bormans GM: Synthesis and biological evaluation of a 99mTc-labelled
sulfonamide conjugate for in vivo visualization of carbonic
anhydrase IX expression in tumor hypoxia. Nucl Med Biol.
37:557–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doyen J, Parks SK, Marcié S, Pouysségur J
and Chiche J: Knock-down of hypoxia-induced carbonic anhydrases IX
and XII radiosensitizes tumor cells by increasing intracellular
acidosis. Front Oncol. 2:1992012.
|
|
10
|
Ivanov S, Liao SY, Ivanova A,
Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ,
Proescholdt MA, Oldfield EH, Lee J, et al: Expression of
hypoxia-inducible cell-surface transmembrane carbonic anhydrases in
human cancer. Am J Pathol. 158:905–919. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neuman MG, Shear NH, Malkiewicz IM, Taeri
M, Shapiro LE, Krivoy N, Haber J, Gomez M, Fish J, Cartotto R, et
al: Immunopathogenesis of hypersensitivity syndrome reactions to
sulfonamides. Transl Res. 149:243–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim TH, Somerville PJ and Freeman CR:
Unilateral radiation nephropathy - the long-term significance. Int
J Radiat Oncol Biol Phys. 10:2053–2059. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kjaer M, Frederiksen PL and Engelholm SA:
Postoperative radiotherapy in stage II and III renal
adenocarcinoma. A randomized trial by the Copenhagen Renal Cancer
Study Group. Int J Radiat Oncol Biol Phys. 13:665–672. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kao GD, Malkowicz SB, Whittington R,
D'Amico AV and Wein AJ: Locally advanced renal cell carcinoma: Low
complication rate and efficacy of postnephrectomy radiation therapy
planned with CT. Radiology. 193:725–730. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Svedman C, Karlsson K, Rutkowska E,
Sandström P, Blomgren H, Lax I and Wersäll P: Stereotactic body
radiotherapy of primary and metastatic renal lesions for patients
with only one functioning kidney. Acta Oncol. 47:1578–1583. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hensley HH, Hannoun-Levi JM, Hachem P, Mu
Z, Stoyanova R, Khor LY, Agrawal S and Pollack A: PKA knockdown
enhances cell killing in response to radiation and androgen
deprivation. Int J Cancer. 128:962–973. 2011. View Article : Google Scholar
|
|
17
|
Barazzuol L, Jena R, Burnet NG, Jeynes JC,
Merchant MJ, Kirkby KJ and Kirkby NF: In vitro evaluation of
combined temozolomide and radiotherapy using X rays and high-linear
energy transfer radiation for glioblastoma. Radiat Res.
177:651–662. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
McIntyre A, Patiar S, Wigfield S, Li JL,
Ledaki I, Turley H, Leek R, Snell C, Gatter K, Sly WS, et al:
Carbonic anhydrase IX promotes tumor growth and necrosis in vivo
and inhibition enhances anti-VEGF therapy. Clin Cancer Res.
18:3100–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cianchi F, Vinci MC, Supuran CT, Peruzzi
B, De Giuli P, Fasolis G, Perigli G, Pastorekova S, Papucci L, Pini
A, et al: Selective inhibition of carbonic anhydrase IX decreases
cell proliferation and induces ceramide-mediated apoptosis in human
cancer cells. J Pharmacol Exp Ther. 334:710–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dubois L, Peeters S, Lieuwes NG, Geusens
N, Thiry A, Wigfield S, Carta F, McIntyre A, Scozzafava A, Dogné
JM, et al: Specific inhibition of carbonic anhydrase IX activity
enhances the in vivo therapeutic effect of tumor irradiation.
Radiother Oncol. 99:424–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuks Z and Kolesnick R: Engaging the
vascular component of the tumor response. Cancer Cell. 8:89–91.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Meerleer G, Khoo V, Escudier B, Joniau
S, Bossi A, Ost P, Briganti A, Fonteyne V, Van Vulpen M, Lumen N,
et al: Radiotherapy for renal-cell carcinoma. Lancet Oncol.
15:e170–e177. 2014. View Article : Google Scholar : PubMed/NCBI
|